Seasonal Distribution of Key Small-Sized Fish in the South Inshore of Zhejiang, China
Abstract
1. Introduction
2. Materials and Methods
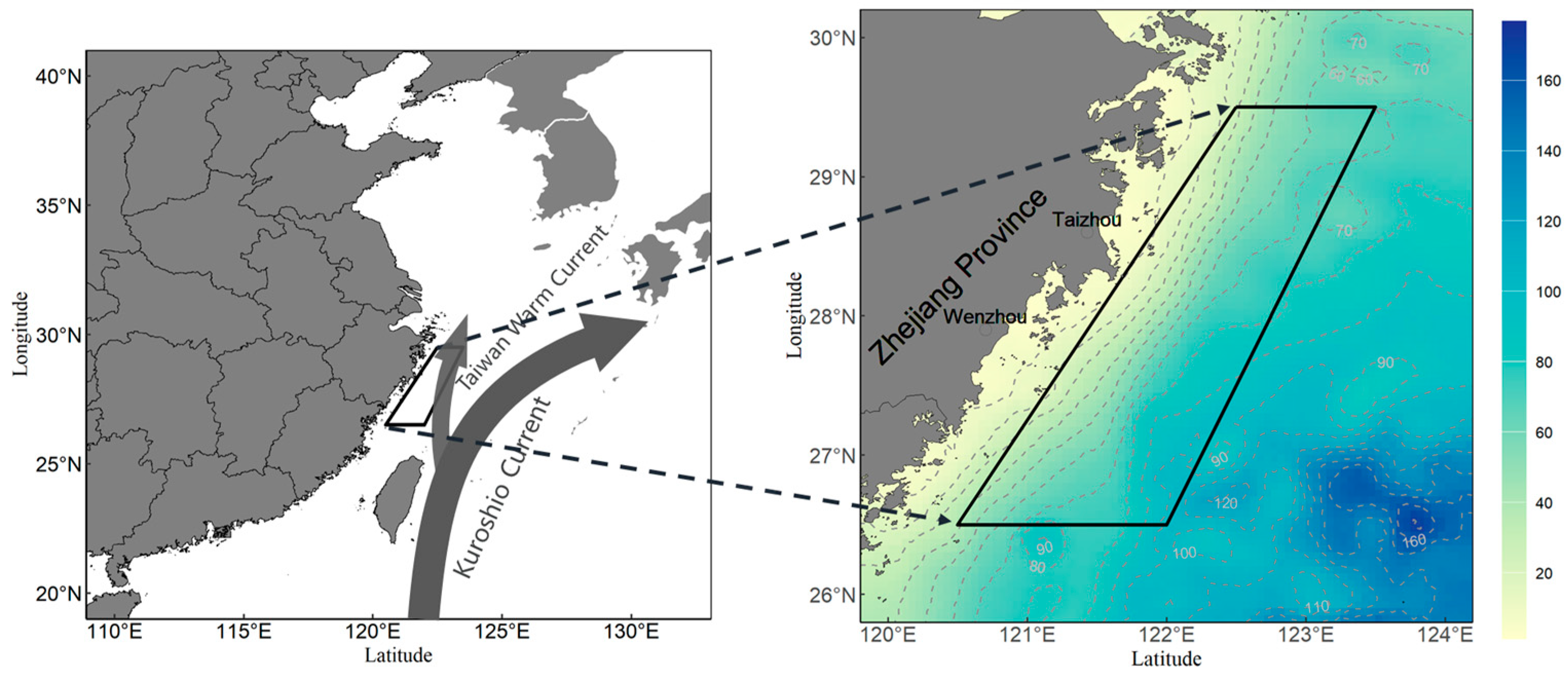
2.1. Data Collection
2.2. Species Distribution Model
3. Results
3.1. Model Performance
3.2. Environmental Preference
3.3. Spatiotemporal Distribution
4. Discussion
4.1. Dominant Environmental Variables
4.2. Differences in Spatial and Temporal Distribution
4.2.1. Amblychaeturichthys hexanema (AMH)
4.2.2. Apogon lineatus (or Jaydia lineata, APL)
4.2.3. Benthosema pterotum (BEP)
4.2.4. Bregmaceros mcclellandi (BRM)
4.3. Area-Based Marine Management Consideration
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Konar, M.; Qiu, S.; Tougher, B.; Vause, J.; Tlusty, M.; Fitzsimmons, K.; Barrows, R.; Cao, L. Illustrating the Hidden Economic, Social and Ecological Values of Global Forage Fish Resources. Resour. Conserv. Recycl. 2019, 151, 104456. [Google Scholar] [CrossRef]
- Nissar, S.; Bakhtiyar, Y.; Arafat, M.Y.; Andrabi, S.; Bhat, A.A.; Yousuf, T. A Review of the Ecosystem Services Provided by the Marine Forage Fish. Hydrobiologia 2023, 850, 2871–2902. [Google Scholar] [CrossRef]
- Pikitch, E.K.; Rountos, K.J.; Essington, T.E.; Santora, C.; Pauly, D.; Watson, R.; Sumaila, U.R.; Boersma, P.D.; Boyd, I.L.; Conover, D.O. The Global Contribution of Forage Fish to Marine Fisheries and Ecosystems. Fish Fish. 2014, 15, 43–64. [Google Scholar] [CrossRef]
- Hilborn, R.; Amoroso, R.O.; Bogazzi, E.; Jensen, O.P.; Parma, A.M.; Szuwalski, C.; Walters, C.J. When Does Fishing Forage Species Affect Their Predators? Fish. Res. 2017, 191, 211–221. [Google Scholar] [CrossRef]
- Halpern, B.S.; Walbridge, S.; Selkoe, K.A.; Kappel, C.V.; Micheli, F.; D’Agrosa, C.; Bruno, J.F.; Casey, K.S.; Ebert, C.; Fox, H.E.; et al. A Global Map of Human Impact on Marine Ecosystems. Science 2008, 319, 948–952. [Google Scholar] [CrossRef]
- Cheng, J.; Ding, F.; Li, S.F.; Yan, L.; Li, J.; Liang, Z. Changes of Fish Community Structure in the Coastal Zone of the Northern Part of East China Sea in Summer. J. Nat. Resour. 2006, 21, 775–781. [Google Scholar]
- Jin, X. Long-Term Changes in Fish Community Structure in the Bohai Sea, China. Estuar. Coast. Shelf Sci. 2004, 59, 163–171. [Google Scholar] [CrossRef]
- Jiang, Y.Z.; Cheng, J.H.; Li, S.F. Temporal Changes in the Fish Community Resulting from a Summer Fishing Moratorium in the Northern East China Sea. Mar. Ecol. Prog. Ser. 2009, 387, 265–273. [Google Scholar] [CrossRef]
- Zhang, K.; Guo, J.; Xu, Y.; Jiang, Y.; Fan, J.; Xu, S.; Chen, Z. Long-Term Variations in Fish Community Structure under Multiple Stressors in a Semi-Closed Marine Ecosystem in the South China Sea. Sci. Total Environ. 2020, 745, 140892. [Google Scholar] [CrossRef]
- Zhai, L.; Pauly, D. Construction and Interpretation of Particle Size Distribution Spectra from 19 Ecopath Models of Chinese Coastal Ecosystems. Front. Mar. Sci. 2020, 7, 298. [Google Scholar] [CrossRef]
- Li, J.L.; Cao, K.; Ding, F.; Yang, W.B.; Shen, G.M.; Li, Y. Changes in Trophic-Level Structure of the Main Fish Species Caught by China and Their Relationship with Fishing Method. J. Fish. Sci. China 2017, 7, 109–119. [Google Scholar] [CrossRef]
- Wang, Y.; Kindong, R.; Gao, C.; Wang, J. Identification of Keystone Species in Ecological Communities in the East China Sea. Fishes 2023, 8, 224. [Google Scholar] [CrossRef]
- Wang, J.; Gao, C.; Tian, S.; Han, D.; Ma, J.; Dai, L.; Ye, S. Shifts in Composition and Co-Occurrence Patterns of the Fish Community in the South Inshore of Zhejiang, China. Glob. Ecol. Conserv. 2023, 44, e02502. [Google Scholar] [CrossRef]
- Su, J.L. Circulation Dynamics of the China Seas North 18°N. Glob. Coast. Ocean. Reg. Stud. Synth. 1998, 11, 483–506. [Google Scholar]
- Zheng, Y.; Chen, X.; Cheng, J.; Wang, Y.; Shen, X.; Chen, W.; Li, C. East China Sea Shelf Environment and Living Resources; Shanghai Scientific & Technical Publishers: Shanghai, China, 2003. [Google Scholar]
- GB/T 12763.6-2007; Specifications for Oceanographic Survey—Part 6: Marine Biological Survey. China National Standardization Management Committee: Beijing, China, 2007.
- FRY, F.E.J. The Effect of Environmental Factors on the Physiology of Fish. In Fish Physiology; Elsevier: Amsterdam, The Netherlands, 1971; Volume 6, pp. 1–98. ISBN 1546-5098. [Google Scholar]
- Zhang, H.; Yuan, X.; Ling, J.; Jiang, Y. Larval Ecology of Gobiid Fishes in a Subtropical Embayment: Environmental Preferences and Spatiotemporal Habitat Partitioning. Front. Mar. Sci. 2022, 9, 929919. [Google Scholar] [CrossRef]
- Xu, M.; Liu, Z.; Wang, Y.; Jin, Y.; Yuan, X.; Zhang, H.; Song, X.; Otaki, T.; Yang, L.; Cheng, J. Larval Spatiotemporal Distribution of Six Fish Species: Implications for Sustainable Fisheries Management in the East China Sea. Sustainability 2022, 14, 14826. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R.; Hastie, T. A Working Guide to Boosted Regression Trees. J. Anim. Ecol. 2008, 77, 802–813. [Google Scholar] [CrossRef]
- Yu, H.; Cooper, A.R.; Infante, D.M. Improving Species Distribution Model Predictive Accuracy Using Species Abundance: Application with Boosted Regression Trees. Ecol. Model. 2020, 432, 109202. [Google Scholar] [CrossRef]
- Shabani, F.; Kumar, L.; Ahmadi, M. A Comparison of Absolute Performance of Different Correlative and Mechanistic Species Distribution Models in an Independent Area. Ecol. Evol. 2016, 6, 5973–5986. [Google Scholar] [CrossRef]
- Norberg, A.; Abrego, N.; Blanchet, F.G.; Adler, F.R.; Anderson, B.J.; Anttila, J.; Araújo, M.B.; Dallas, T.; Dunson, D.; Elith, J. A Comprehensive Evaluation of Predictive Performance of 33 Species Distribution Models at Species and Community Levels. Ecol. Monogr. 2019, 89, e01370. [Google Scholar] [CrossRef]
- Team, R.C.; Team, M.R.C.; Suggests, M.; Matrix, S. Package Stats; The R-4.3.1 Stats Package: Vienna, Austria, 2018. [Google Scholar]
- Ayers, J.M.; Lozier, M.S. Physical Controls on the Seasonal Migration of the North Pacific Transition Zone Chlorophyll Front. J. Geophys. Res. Oceans 2010, 115, C05001. [Google Scholar] [CrossRef]
- Rykaczewski, R.R. Changes in Mesozooplankton Size Structure along a Trophic Gradient in the California Current Ecosystem and Implications for Small Pelagic Fish. Mar. Ecol. Prog. Ser. 2019, 617, 165–182. [Google Scholar] [CrossRef]
- Feuilloley, G.; Fromentin, J.-M.; Stemmann, L.; Demarcq, H.; Estournel, C.; Saraux, C. Concomitant Changes in the Environment and Small Pelagic Fish Community of the Gulf of Lions. Prog. Oceanogr. 2020, 186, 102375. [Google Scholar] [CrossRef]
- Kudela, R.M.; Cochlan, W.P.; Peterson, T.D.; Trick, C.G. Impacts on Phytoplankton Biomass and Productivity in the Pacific Northwest during the Warm Ocean Conditions of 2005. Geophys. Res. Lett. 2006, 33. [Google Scholar] [CrossRef]
- Cushing, D.H. Plankton Production and Year-Class Strength in Fish Populations: An Update of the Match/Mismatch Hypothesis. In Advances in Marine Biology; Elsevier: Amsterdam, The Netherlands, 1990; Volume 26, pp. 249–293. ISBN 0065-2881. [Google Scholar]
- Lin, L.; Zheng, Y.; Liu, Y.; Zhang, H. The Ecological Study of Small Sized Fish in the East China Sea I-the Species Composition and Seasonal Variation of Small Sized Fish. Mar. Sci. 2006, 30, 58–63. (In Chinese) [Google Scholar]
- Zou, Q.; Wang, Z.; Zhang, S.; Cheng, X.; Chen, Y.; Zhou, Y. Characteristics of Fish Community Structure in the Sea Area of Dachen Island. Chin. J. Ecol. 2024, 43, 795–803. (In Chinese) [Google Scholar]
- Shen, D.; Zhang, Y.; Cui, Y.; Yu, H.; Zhang, C.; Xu, B.; Zhang, C.; Ji, Y.; Xue, Y. Study on the Influencing Factors of Fish Spatial Distribution Using Three Bayesian Models: A Case Study of Amblychaeturichthys hexanema in Haizhou Bay. Haiyang Xuebao 2023, 45, 88–100. (In Chinese) [Google Scholar] [CrossRef]
- Jiang, S.; Chen, J. Research Progress on Small-Scale Marine Fishes. Mar. Fish. 2006, 28, 336–341. (In Chinese) [Google Scholar]
- Liu, K.; Yu, N.; Yu, C.; Zheng, J.; Xu, Y.; Yan, W.; Han, L.; Liu, H.; Sun, B.; Dai, D. The Spatial Niche and Differentiation of Major Fish Species in the Waters East of the Zhoushan Islands in Spring And Autumn. J. Fish. Sci. China 2021, 28, 100–111. (In Chinese) [Google Scholar] [CrossRef]
- He, J.-N.; Xu, Y.-J.; Zhang, H.L. Study on the Relationship between Fish Diversity, Quantity and Environmental Factors in the Middle Area of Zhejiang Offshore. J. Zhejiang Ocean. Univ. (Nat. Sci.) 2017, 36, 283–288. (In Chinese) [Google Scholar]
- Tang, L.; Zhang, Y.; Xu, B.; Zhang, C.; Ren, Y.; Xue, Y. Hbitat Suitability Analysis of Apogon lineatus during Autumn in Haizhou Bay. Peridical Ocean. Univ. China 2021, 51, 154–160. (In Chinese) [Google Scholar]
- Li, J.; Hu, F.; Li, S.; Cheng, J. Quantity Distribution of Benthosema Pterotum and in Relationship with Surface Layer Water Temperature and Salinity in the East China Sea Region. Mar. Fish. 2006, 28, 105–110. (In Chinese) [Google Scholar]
- Lu, Z.H.; Miao, Z.Q.; Lin, N. The Structure and Diversity Fish Communities in Spring in the Middle Sea Area of Zhejiang Province and Adjacent Region. J. Zhejiang Ocean. Univ. (Nat. Sci.) 2009, 28, 51–56. (In Chinese) [Google Scholar]
- Liu, Z.L.; Yang, L.L.; Yan, L.P.; Yuan, X.W.; Chen, J. Fish Assemblages and Environmental Interpretation in the Northern Taiwan Strait and Its Adjacent Waters in Summer. J. Fish. Sci. China 2016, 23, 1399–1416. (In Chinese) [Google Scholar]
- Hilborn, R.; Agostini, V.N.; Chaloupka, M.; Garcia, S.M.; Gerber, L.R.; Gilman, E.; Hanich, Q.; Himes-Cornell, A.; Hobday, A.J.; Itano, D.; et al. Area-Based Management of Blue Water Fisheries: Current Knowledge and Research Needs. Fish Fish. 2021, 23, 492–518. [Google Scholar] [CrossRef]
- Peck, M.A.; Alheit, J.; Bertrand, A.; Catalán, I.A.; Garrido, S.; Moyano, M.; Rykaczewski, R.R.; Takasuka, A.; van Der Lingen, C.D. Small Pelagic Fish in the New Millennium: A Bottom-up View of Global Research Effort. Prog. Oceanogr. 2021, 191, 102494. [Google Scholar] [CrossRef]




| Species | Code | Number of Trees | Tree Complexity (tc) | Learning Rate (lr) | Bagging Fraction | Explained Variance% |
|---|---|---|---|---|---|---|
| Amblychaeturichthys hexanema | AMH | 1250 | 12 | 0.001 | 0.75 | 40.0 |
| Apogon lineatus (or Jaydia lineata) | APL | 2550 | 12 | 0.001 | 0.75 | 59.5 |
| Benthosema pterotum | BEP | 2150 | 12 | 0.001 | 0.75 | 52.3 |
| Bregmaceros mcclellandi | BRM | 1500 | 6 | 0.001 | 0.75 | 25.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, M.; Gao, X.; Liu, W.; Wang, J. Seasonal Distribution of Key Small-Sized Fish in the South Inshore of Zhejiang, China. Fishes 2024, 9, 412. https://doi.org/10.3390/fishes9100412
Xu M, Gao X, Liu W, Wang J. Seasonal Distribution of Key Small-Sized Fish in the South Inshore of Zhejiang, China. Fishes. 2024; 9(10):412. https://doi.org/10.3390/fishes9100412
Chicago/Turabian StyleXu, Minghao, Xiaodi Gao, Weicheng Liu, and Jiaqi Wang. 2024. "Seasonal Distribution of Key Small-Sized Fish in the South Inshore of Zhejiang, China" Fishes 9, no. 10: 412. https://doi.org/10.3390/fishes9100412
APA StyleXu, M., Gao, X., Liu, W., & Wang, J. (2024). Seasonal Distribution of Key Small-Sized Fish in the South Inshore of Zhejiang, China. Fishes, 9(10), 412. https://doi.org/10.3390/fishes9100412





