Abstract
Agonistic behavior is a common behavior among agonistic Chinese mitten crabs (Eriocheir sinensis). Such behavior often leads to limb loss or physical impairment, and significantly affects the survival, growth, and quality of the crabs, and even the yield and economic value for E. sinensis. Agonistic behavior often occurs in agonistic crabs, which is closely related to personality traits and interactive behavior of animals. E. sinensis has personality traits such as boldness, aggression, and exploration as evidenced by the partition-crossing experiment, mirror experiment, and shelter experiment. Agonistic crabs were identified as individuals with boldness, high aggression, and high exploration. The interactive behavior spectrum of E. sinensis was first obtained, consisting of 15 behaviors. This spectrum described and summarized all interactive behaviors of E. sinensis during fights. The interactive behavior characteristics of agonistic crabs were identified as darting, intimidating, grabbing, pushing, stretching, and visitation. These results lay a theoretical basis for in-depth behavioral research on E. sinensis in the future. The technique of identifying agonistic crabs by personality traits and interactive behaviors not only allows for the elimination of agonistic crabs from the aquaculture process and the reduction in negative impacts caused by aggressive crabs, but also allows for the breeding of non-agonistic crabs and the further reduction in the economic losses caused by fighting behaviors.
Key Contribution:
E. sinensis has personality traits that include boldness, aggression, and exploration; there is a significant correlation between personality traits. Personality traits can be used to distinguish agonistic crabs. Agonistic crabs are individuals with bold, strong aggressiveness, and strong exploration. The interactive behavior spectrum of E. sinensis has been established for the first time. Interactive behavioral characteristics can be used to distinguish agonistic crabs.
1. Introduction
The Chinese mitten crab (Eriocheir sinensis) is an important freshwater aquaculture species in China. Valued for its culinary appeal within Chinese cultural and dietary traditions, it is a favored delicacy among many consumers. The interactive behavior of E. sinensis becomes increasingly intense in the context of high-density pond culture. Agonistic interactions often result in high energy expenditure and missing limbs [1]. An increase in agonistic crabs correlates with higher economic losses. Notably, agonistic behavior is closely linked to personality traits. Research has shown that personality traits can affect an individual’s health, growth, reproduction, metabolism, survival, and evolution [2,3,4,5,6]. Although many studies have identified personality traits in other crustaceans, such as Portunus trituberculatus, Procambarus clarkii, and members of the Paguridae family, information on personality traits in E. sinensis is still limited due to the large variation in personality among aquatic species [7,8,9].
Animal personality traits include boldness, aggression, and exploration, which have been studied extensively in recent years [10,11,12,13,14,15]. Boldness refers to an individual’s propensity to take risks [16]. Aggression plays a crucial role in both intra- and inter-species competition; it may be crucial to obtain resources such as spouses, food, and territory, and to establish a dominance hierarchy in social populations [16,17,18]. Exploration describes the tendency to seek information in new environments [19]. Due to the complexity of animal personality traits, researchers have designed various personality trait tests to assess the personality traits of different species. For example, boldness, aggression, and exploration have been assessed using obstacle, mirror, and shelter experiments [8,18,20]. Furthermore, in order to explore animal personality traits, it is necessary to study the correlation and repeatability of personality traits [21]. Some animals, such as green sea turtles (Chelonia mydas) and wasps (Vespidae), exhibit the correlation of personality traits [22,23]. Repeatability of personality traits refers to the fact that personality traits will remain the same through time and environment [24].
Interactive behavior is common in animals and there is a wide range of interactive behaviors in social interactions, predator–prey relationships, and interactions with the environment [3,25,26]. The interactive behavior of animals can be influenced by personality traits, leading to varied interactive behaviors among individuals. For example, the bold and active Eurasian perch (Dicentrarchus labrax) tended to hunt active prey [27]. Bold swallow-tailed gulls (Creagrus) tended to hunt unfamiliar prey in nature [28]. Boldness and exploration affected biological invasions of peacockfish (Poecilia reticulata) and spinyback fish (Notacanthus abbotti) [29]. In crustaceans, bolder swimming crabs exhibited more frequent fighting behavior [30]. Crabs exhibited significant variations in interactive behavior during fights, notably between agonistic and non-agonistic individuals [30]. However, it was difficult to accurately describe the interactive behavior of the agonistic crabs due to the limited research on the interactive behavior of E. sinensis.
Certain morphological characteristics of animals can help to differentiate individuals with different personality traits. For example, guppy (Poecilia reticulata) coloration and head patch size in hazel dormice (Fukomys anselli) were associated with personality traits [24,31]. In crustaceans, chelipeds and pereiopods possess highly heritable morphological characteristics and play crucial roles in interactive behavior [32]. These morphological characteristics can be used to differentiate between individuals with different personality traits. For example, larger cheliped size in crayfish is correlated with higher levels of aggression [20]. As a result, differences in morphological characteristics between agonistic and non-agonistic E. sinensis may exist, potentially facilitating the identification of agonistic crabs.
The primary objective of this research was to assess the personality traits and interactive behaviors of E. sinensis and to explore strategies for identifying agonistic individuals. A Principal Component Analysis (PCA) was used to study the interaction behavior of agonistic and non-agonistic crabs with a view for finding the characteristics of the interaction behavior of both. The findings from this study are poised to enhance our understanding of personality traits in E. sinensis, and refine approaches to behavioral studies in crabs. Two methods are provided to differentiate between agonistic crabs, and effectively reduce the adverse effects of agonistic crabs in the aquaculture process and improve the production and quality of E. sinensis.
2. Materials and Methods
The E. sinensis crabs used in this study were obtained from Shanghai, Daohong Aquaculture Technology Co., Ltd. (Shanghai, China). The experimental protocols were thoroughly reviewed and approved by the Animal Bioethics Committee of Shanghai Ocean University, China. A total of 120 crabs were temporarily housed in a glass tank. Male crabs (33.90 ± 4.00 g) with intact appendages were selected for this study. The crabs were placed in an opaque circular tank 30 cm in diameter and kept individually for 7 days to avoid contact with each other’s physical, chemical, and biological information. The water depth was maintained at 10 cm and provided with independent aeration. Half of the water in the tank was changed regularly. The water temperature was maintained at a constant 20 ± 1 °C and the light/dark ratio was set at 12:12; we used a dissolved oxygen (DO) concentration ≥ 7.8 mg/L. The crabs were fed 5% of their body weight daily at 17:00 hrs. The crabs were fed using a commercial pellet feed between 17:00 and 18:00. Experiments began in October 2022 and ended in June 2023.
2.1. The Personality Traits and the Agonistic Crabs
In order to accurately obtain the personality traits of E. sinensis, several personality traits were tested to observe significant individual differences. The three most effective methods were identified as the partition-crossing experiment, mirror experiment, and shelter experiment. All crabs were individually tested for boldness, aggression, and exploration by these methods. There was a two-day interval between each method. A K-mean cluster analysis was tested to distinguish between agonistic and non-agonistic crabs.
2.1.1. The Personality Traits
(1) boldness
The boldness of the crabs was evaluated by the partition-crossing experiment [20]. The experimental setup consisted of a rectangular tank (23 × 18 × 28 cm) with a water depth of 10 cm and a partition (18 × 10 cm) placed in the middle of the bottom (Figure 1A). A camera was positioned directly above the tank to capture the behavior of the crabs. Each crab was placed on one side of the partition in the tank and observed for 30 min. Boldness was evaluated by recording the number of times the crab crossed the partition. For E. sinensis, climbing over the partition carried a risk. Therefore, the more times the crab climbed over the partition, the higher the boldness. More detailed information can be found in the Supplementary Videos.
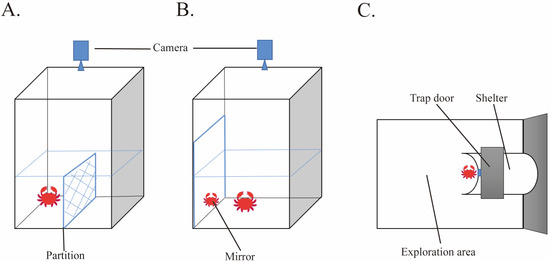
Figure 1.
Three personality-trait-testing devices ((A). boldness, (B). aggression, (C). exploration).
(2) aggression
Mirror experiments are widely used in animal aggression studies [18,33]. Crab aggression was assessed by recording the number of their interactions with mirrors [34]. The experimental setup consisted of a rectangular tank (23 × 18 × 28 cm) with a water depth of 10 cm and a flat mirror (20 × 20 cm) placed on one side of the tank and a camera placed directly above the tank (Figure 1B). A partition was used to cover the plane mirror before the start of the experiment to avoid premature contact between the individual crab and the mirror image. The partition was slowly removed after 10 min of adaptation, and then the number of aggressive interactions such as movement towards the mirror, cheliped displays, and physical contact between the crab and the mirror was recorded for 30 min using the camera. Previous studies have validated this test, demonstrating that these interactions mirror those against a ‘real’ opponent [35]. More detailed information can be found in the Supplementary Videos.
(3) exploration
Crab exploration was assessed by measuring their movement time in the exploration area during the shelter experiment [18]. The exploration area referred to the space outside the shelter, and the time the animals spent moving in this area was recorded to estimate their exploration. The experimental setup consisted of a tank (60 × 40 × 40 cm) with a shelter with a trap door. A camera was placed directly above the tank (Figure 1C). The crab was placed in shelters individually. After 10 min of adaptation, the trap door was opened, and the crab movement time in the exploration area was recorded. Longer time of movement indicated higher exploration.
2.1.2. Repeatability of Personality Traits
To ensure repeatability in the personality traits of E. sinensis, tests for boldness, aggression, and exploration were conducted on each crab twice, with a two-week interval between sessions [24]. Repeatability of E. sinensis boldness, aggression, and exploration was obtained from behavioral consistency between two tests.
2.1.3. Correlation Analysis between Personality Traits
The personality traits of crabs, including boldness, aggression, and exploration, were assessed using the partition-crossing experiment, mirror experiment, and shelter experiment, respectively. Pearson correlation tests were then employed to examine the relationships between these personality traits [24].
2.1.4. The Agonistic Crab Identification
The boldness, aggression, and exploration of E. sinensis were obtained through three personality trait tests, on the basis of which all individuals were differentiated into agonistic, non-agonistic, and ordinary crabs by the K-mean cluster analysis [18]. Ordinary crabs are individuals other than agonistic and non-agonistic crabs. Subsequently, differences in personality traits between agonistic and non-agonistic crabs were analyzed.
2.2. Validation of Agonistic Crabs by Fighting Experiment
Based on the results of the cluster analysis, all individuals were divided into three groups: the (1) agonistic crab group; (2) non-agonistic crab group; (3) ordinary crab group. Separate fighting experiments were conducted for each group. The setup involved a cylindrical tank, divided by a partition, and equipped with a video recording system. A camera was positioned directly above the tank to capture the activity (Figure 2) [1]. Two crabs from the same group, with a weight difference of 1–4%, were paired and placed on opposite sides of the tank, separated by the partition. After 10 min of adaptation, the partition was removed and the high-definition camera recorded the crabs’ interactive behavior for 1 h. The recorded videos were analyzed in the laboratory and data were collected for a further analysis. In the fighting experiment, data such as fighting behavior, movement, contact behavior, and fighting time could be obtained to verify the agonistic crab [36].
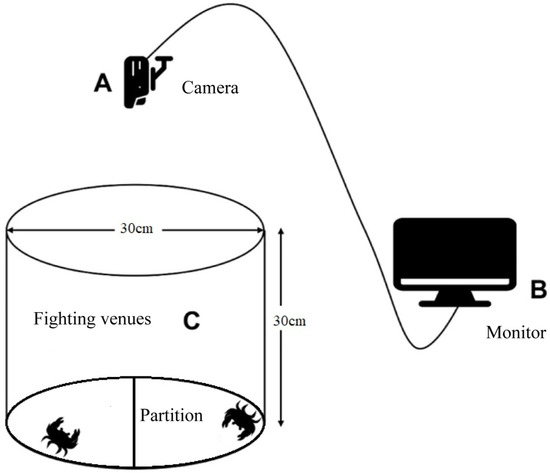
Figure 2.
Interactive behavior observation system.
2.3. The Interactive Behavior and the Agonistic Crab
To enhance the analysis of interactive behavior in E. sinensis, this study incorporated insights from descriptions of interactive behavior observed in underground naked mole rats (Heterocephalus glaber) [10]. The fighting experiments documented all interactive behaviors exhibited by agonistic crabs, non-agonistic crabs, and ordinary crabs. A Principal Component Analysis (PCA) was applied to these behaviors, facilitating the identification of distinct interactive behavior characteristics unique to agonistic crabs [10].
2.4. Morphological Characteristics and the Agonistic Crab
The morphological distinctions between agonistic and non-agonistic crabs were quantified using vernier calipers with a precision of 0.02 mm. This included measurements of carapace width (AB) and length (CD), cheliped width (EF), second pereiopod merus length (GH), and second pereiopod dactylus length (IJ). To enable comparison across individuals, the values for CD, EF, GH, and IJ for each crab were normalized by dividing them by the crab’s AB measurement (Figure 3) [37].
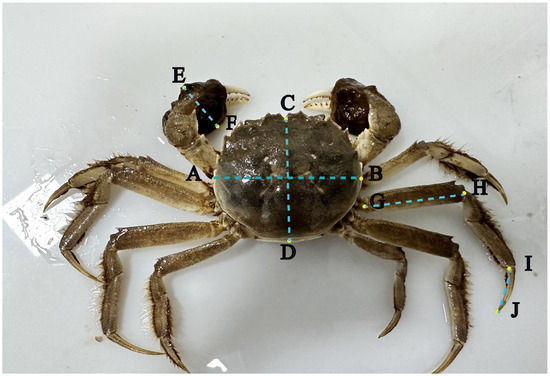
Figure 3.
Morphological indicators (AB: carapace width, CD: carapace length, EF: cheliped width, GH: second pereiopod merus length, IJ: second pereiopod dactylus length).
2.5. Statistical Analysis
SPSS Statistics 26.0 was used for all analyses. A Bivariate Correlation analysis was used to determine the correlation between the three personality traits. A cluster analysis was used to differentiate agonistic crabs. A single-factor analysis of variance was used to compare the movement towards the mirror, fighting time, contact behaviors, and fighting behaviors of fighting between different groups in each experiment. The Levene test was used to test the homogeneity of variance. Significant differences prompted the use of the Tukey test for multiple comparisons. A Principal Component Analysis on behaviors during the fighting process was performed using SPSS to examine attack and activity behaviors. For all analyses, a p-value < 0.05 was considered statistically significant.
3. Results
3.1. The Personality Traits
E. sinensis exhibited personality traits of boldness, aggression, and exploration, as assessed through the partition-crossing experiment, mirror experiment, and shelter experiment. Consistency in interactive behavior across the three personality tests for E. sinensis further suggested the boldness, aggression, and exploration of E. sinensis (Table 1). The only exception was the number of times the animal made contact with the mirror.

Table 1.
Recorded behaviors for n = 80 individuals in three tests, consistency over time (correlation between trial 1 and trial 2, Spearman: rho).
The correlation analysis revealed a significant positive correlation between boldness and aggression in E. sinensis (correlation coefficient = 0.639, p < 0.01, Figure 4). There was a significant positive correlation between boldness and exploration (correlation coefficient = 0.455, p < 0.01, Figure 4). Additionally, a positive correlation was noted between aggression and exploration (correlation coefficient = 0.622, p < 0.01, Figure 4).
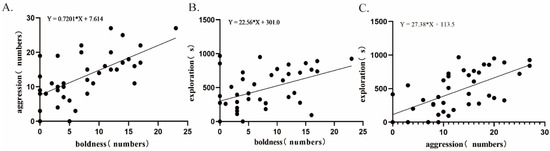
Figure 4.
Correlation analyses of three personality traits: (A). aggression and boldness; (B). exploration and boldness; (C). exploration and aggression. Notes: Aggression refers to the number of interactions with mirrors; boldness refers to the number of times the crab moves over the partition; and exploration refers to the movement time in the exploration area. Each dot represents a crab.
3.2. Identification and Validation of Agonistic Crabs Based on Personality Traits
The cluster analysis showed that out of a total of 120 individuals, 42 were classified as agonistic crabs, while 38 were identified as non-agonistic crabs. Agonistic crabs made up approximately 35% of the total population. These agonistic crabs displayed boldness, high aggression, and high exploration (p < 0.01, Figure 5). The agonistic crab group showed significantly more fighting behaviors (p < 0.01, Figure 6A), contact behaviors (p < 0.01, Figure 6B), movement (p < 0.05, Figure 6C), and fighting time (p < 0.01, Figure 6D) compared to the non-agonistic crab group.
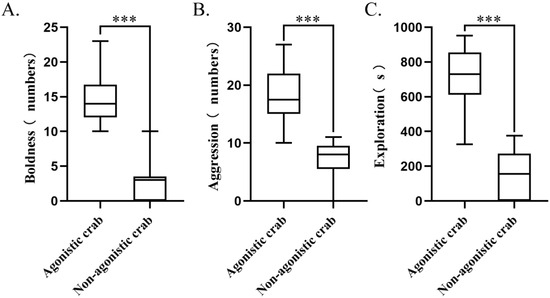
Figure 5.
Differences in personality traits between agonistic crabs and non-agonistic crabs: (A). aggression; (B). boldness; (C). exploration. Notes: “***” indicates a significant difference observed between agonistic and non-agonistic crabs (p < 0.001), N = 16.
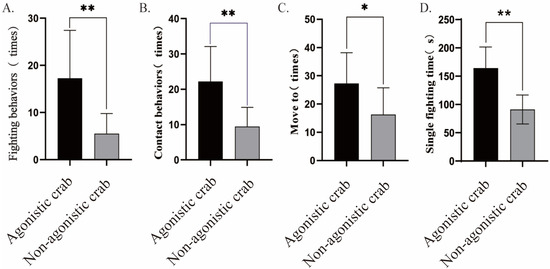
Figure 6.
Differences in interactive behavior between agonistic crabs and non-agonistic crabs: (A). fighting behaviors; (B). contact behaviors; (C). movement; (D). single fighting time. Notes: “*” “**” indicates a significant difference observed between agonistic and non-agonistic crabs (p < 0.05, p < 0.01).
3.3. The Interactive Behavior and the Agonistic Crab
During the observation of fighting experiments, a total of 15 interactive behaviors were recorded and described as the interactive behavior spectrum for E. sinensis (Table 2).

Table 2.
The interactive behavior spectrum of E. sinensis.
Victory posture, grooming, and counterattack in self-defense were found to be statistically insignificant from the interactive behavior spectrum. Consequently, the remaining 12 behaviors were utilized as variables in the Principal Component Analysis (PCA) (Table 3). These three components accounted for 74.7% of the total variance, with PC1 explaining 45.74% of the total variance. We noticed that PC1 was an agonistic type that included two opposing variables in the load plot (Figure 7A). Behaviors such as darting, intimidating, grabbing, pushing, stretching, and visitation were positively loaded on PC1, while freezing and curling up were negatively loaded on PC1. On the other hand, PC2 explained a total variance of 19.26% and scored the highest on provocation, crawling along the wall, and ignoring (Table 3). Of the 12 interactive behaviors, we found that there was a significant positive correlation between darting, intimidating, grabbing, pushing, stretching, and visitation (p < 0.05, Figure 7B). Meanwhile, we observed a significant positive correlation between freezing and curling up. However, these behaviors were negatively correlated with the first six behaviors (p < 0.05, Figure 7B). The other four behaviors showed no significant correlation.

Table 3.
PCA loadings for the first three principal components (PCs) of recorded behaviors.
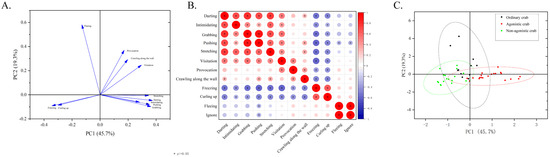
Figure 7.
Principal Component Analysis charts. (A). Load graph for Principal Component Analysis of interactive behavior. (B). Correlation heatmap of 12 interactive behaviors. (C). PCA scores of agonistic, non-agonistic, and ordinary crabs. Note: “*” indicates significant difference observed between interactive behaviors (p < 0.05). Each dot represents a crab in (C).
The PCA score plot revealed distinct differences in the interactive behaviors among agonistic, non-agonistic, and ordinary crabs during the fighting experiments. Agonistic crabs predominantly exhibited behaviors such as darting, intimidating, grabbing, pushing, stretching, and visitation. In contrast, non-agonistic crabs primarily engaged in freezing and curling up, while ordinary crabs showed tendencies towards fleeing, crawling along the wall, and provocation (Figure 7C).
3.4. Morphological Characteristics of Agonistic Crabs
No significant difference in morphological characteristics including carapace length, cheliped width, second pereiopod merus length, and second pereiopod dactylus length were observed between agonistic and non-agonistic crabs (p > 0.05, Figure 8).
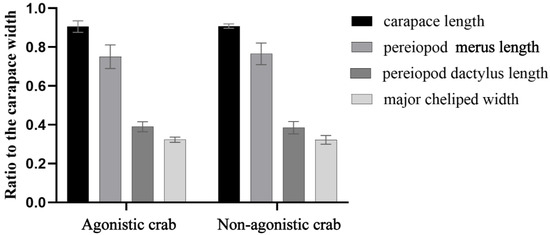
Figure 8.
Comparison of morphological characteristics between agonistic and non-agonistic crabs.
4. Discussion
4.1. The Personality Traits
Boldness, aggression, and exploration are common personality traits in animals [10]. In order to accurately assess the personality traits of different animals, researchers have developed a variety of experiments to test personality traits. Among them, the obstacle experiment, mirror experiment, and shelter experiment have been widely used to study personality traits in crustaceans [34,38,39]. Therefore, in this study, the personality traits of E. sinensis were assessed by the above experiments.
Personality traits can influence the interaction behavior of mammals [40]. This study found that E. sinensis exhibiting boldness, high aggression, and high exploration demonstrated more fighting and contact behaviors. Similarly, in crayfish and swimming crabs, personality traits influenced interactive behaviors. Exploration has been linked to increased dispersal rates in invasive crayfish [8], and boldness to more frequent fighting behaviors in swimming crabs [30]. This suggests that the personality traits of crustaceans likewise have an impact on their interactive behavior. The correlation between different behavioral traits, also known as personality traits or behavioral syndromes, constitutes an important aspect of personality trait research [41]. Correlations among personality traits have been observed in numerous animals, such as the Iberian earthworm lizard (Blaus cinereus) and the warty-tailed lizard (Hemidactylus frenatus), with bold individuals being more exploratory than shy ones [42,43]. Similar results were found in this study: E. sinensis had a correlation between boldness, aggression, and exploration. The study of the correlations of personality traits can provide insights into the relationships between different interactive behaviors of animals and the response to predators, food resources, habitats, and social interactions with other individuals.
Personality traits have stability and plasticity [44,45]. Stability refers to the constancy of certain personality traits under the influence of environmental, physiological, and temporal factors [46]. This stability can help predict the behavior of target animals. For example, early personality traits in sows and piglets could predict their behavioral responses during pregnancy [47]. Based on the stability of personality traits, agonistic crabs may be predicted at early developmental stages. Identifying agonistic crabs as soon as possible may reduce the economic losses caused by their agonistic behaviors. Plasticity refers to the ability of certain personality traits to change under environmental, physiological, and temporal influences [45]. For example, light can influence animal personality traits [48]. Further research is needed to explore these relevant elements.
4.2. Interactive Behavior of E. sinensis
Limited research has been conducted on the interactive behavior of E. sinensis, particularly regarding its fighting intensity and behaviors. This study aims to fill this gap by focusing on the species’ interaction behaviors. Using a Principal Component Analysis (PCA) and correlation analysis, we constructed an interactive behavior spectrum describing 15 interactive behaviors observed during crab fights. The interactive behavior spectrum could more accurately estimate the intensity of fighting, desire to fight, and activity of E. sinensis [10]. In addition, several interesting behaviors have been found in the interactive behavior spectrum in this study, such as counterattack in self-defense and grooming. These behaviors were not characteristic of either agonistic or non-agonistic crabs, but were frequently observed during fights. However, the role of these behaviors in fights is not yet clear.
4.3. The Agonistic Crab Identification
Agonistic behavior in farm animals not only affects their economic value but also poses health risks to farmers [49,50,51,52]. For farm animals, non-agonistic individuals may be ideal. In this study, agonistic crabs constituted approximately 35% of the total population. Meanwhile, limb loss was only observed in agonistic crabs during fights. Therefore, it is important to differentiate agonistic E. sinensis.
Personality traits can influence the interactive behavior of individuals during fights. In this study, boldness, high-aggression, and high-exploration individuals demonstrated increased pushing and grabbing behaviors. This finding aligns with observations in swimming crabs, where bolder individuals displayed more frequent cheliped displays [30]. Researchers have attempted to identify agonistic individuals through aggression tests; however, these efforts lacked experimental validation via fighting scenarios [18]. In addition, it is inaccurate to differentiate agonistic crabs by just one personality trait. In this study, agonistic crabs were differentiated by three personality traits, and then verified by fighting experiments. The results indicated that the identified agonistic crabs exhibited boldness, high aggression, and high exploration. Thus, personality traits serve as effective markers for identifying agonistic crabs.
Agonistic crabs affect the economic value of crabs [53]. Fighting experiments were used to validate agonistic crabs. Researchers have found that agonistic crabs had more fighting behaviors than non-agonistic crabs [54]. There was no accurate way to differentiate agonistic from non-agonistic crabs. The interactive behavior spectrum may solve this problem. The interactive behavior spectrum of E. sinensis could be used to differentiate agonistic crabs, where darting, intimidating, grabbing, pushing, stretching, and visitation were identified as interactive behavior characteristics of agonistic crabs. Similarly, in the study of mammalian interactive behavior, researchers typically created the interactive behavior spectrum, which in turn accurately described mammalian interactive behaviors [10]. In addition, research on the interactive behavior of E. sinensis may help reduce limb loss and improve economic efficiency.
Morphological traits are highly heritable, while some morphological traits are linked to personality traits [32]. It is not clear whether agonistic E. sinensis has specific morphological characteristics. Although the morphological traits examined in this study did not differentiate agonistic crabs, individual carapace colors were observed to gradually lighten upon exposure to light. Furthermore, light-colored crabs displayed more intense agonistic behavior. Research has shown that crustacean carapace colors can change due to environmental influences [55]. The relationship between the color of carapace and agonistic behavior has yet to be explored.
5. Conclusions
This study represented the first evaluation of personality traits in E. sinensis, demonstrating their potential in differentiating agonistic crabs. For the first time, the interactive behaviors of E. sinensis were organized and described, and the interactive behavior characteristics of agonistic crabs and non-agonistic crabs were proposed. Morphological characteristics of E. sinensis, including carapace length, major cheliped width, second pereiopod merus length, and second pereiopod dactylus length, do not serve as reliable indicators for identifying agonistic crabs. These findings contribute new insights into the study of crab personality traits and offer practical methods for identifying agonistic crabs, which are valuable for the aquaculture and production of E. sinensis.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes9100408/s1. Video S1: Bold and Shy Crab. Video S2: Aggressive crab.
Author Contributions
The experiments were designed by X.Y., J.S., D.Z. and C.W. and they assisted J.S. to complete several of the animal experiments. The results analysis and the manuscript writing were carried out by J.S. and were reviewed and edited by X.Y., Y.H. and Y.P. The funding resources came from X.Y. and Y.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Key Research and Development Program of China (2023YFD2401902), The research and application of Key techniques for Super Large size of E. sinensis (CK2022-37), the capacity-promoting Project of the Shanghai Engineering and Technology Center from the Shanghai Municipal Science and Technology Commission (No. 19DZ2284300), the earmarked fund for CARS-48, the Shandong Joint Fund (grant number U1706209), the Aquaculture Engineering Research Platform in Shanghai, established by the Shanghai Science and Technology Commission (grant number 16DZ2281200), and supported by the China Agriculture Research System of MOF and MARA, the program for the Shanghai Collaborative Innovation Center for Cultivating Elite Breeds and Green-culture of Aquaculture animals (No. 2021-KJ-02-12), and the industry-leading talent project of the Yellow River Delta (DYRC20190210).
Institutional Review Board Statement
The experimental protocols were thoroughly reviewed and approved by the Animal Bioethics Committee of Shanghai Ocean University (SHOU-DW-2021-084). Data on personality traits and agonistic behavior of E sinensis during the trial were collected passively. There were no injuries or deaths of E sinensis during the experiment.
Informed Consent Statement
Not applicable.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Conflicts of Interest
The authors have no relevant financial or non-financial interests to disclose. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Pang, Y.-Y.; Zhang, J.-Y.; Chen, Q.; Niu, C.; Shi, A.-Y.; Zhang, D.-X.; Ma, X.-L.; Zhang, Y.; Song, Y.-M.; Hou, M.-N. Effects of dietary L-tryptophan supplementation on agonistic behavior, feeding behavior, growth performance, and nutritional composition of the Chinese mitten crab (Eriocheir sinensis). Aquac. Res. 2024, 35, 101985. [Google Scholar] [CrossRef]
- Masilkova, M.; Boukal, D.; Ash, H.; Buchanan-Smith, H.M.; Konečná, M. Linking personality traits and reproductive success in common marmoset (Callithrix jacchus). Sci. Rep. 2022, 12, 13341. [Google Scholar] [CrossRef]
- Merz, M.R.; Boone, S.R.; Mortelliti, A. Predation risk and personality influence seed predation and dispersal by a scatter-hoarding small mammal. Ecosphere 2023, 14, e4377. [Google Scholar] [CrossRef]
- Found, R.; St. Clair, C. Ambidextrous ungulates have more flexible behaviour, bolder personalities and migrate less. R. Soc. Open Sci. 2017, 4, 160958. [Google Scholar] [CrossRef] [PubMed]
- Velasque, M.; Denton, J.; Briffa, M. Under the influence of light: How light pollution disrupts personality and metabolism in hermit crabs. Environ. Pollut. 2023, 316, 120594. [Google Scholar] [CrossRef]
- Stamps, J.; Groothuis, T.G. The development of animal personality: Relevance, concepts and perspectives. Biol. Rev. 2010, 85, 301–325. [Google Scholar] [CrossRef]
- Zhu, B.; Wang, X.; Ren, Z.; Zhang, H.; Liu, D.; Wang, F. Each Personality Performs Its Own Function: Boldness and Exploration Lead to Differences in the Territoriality of Swimming Crabs (Portunus trituberculatus). Biology 2023, 12, 883. [Google Scholar] [CrossRef] [PubMed]
- Galib, S.M.; Sun, J.; Twiss, S.D.; Lucas, M.C. Personality, density and habitat drive the dispersal of invasive crayfish. Sci. Rep. 2022, 12, 1114. [Google Scholar] [CrossRef]
- Broadhurst, H.E.; Morrell, L.J. Group size and individual ‘personality’influence emergence times in hermit crabs. Biosci. Horiz. Int. J. Stud. Res. 2018, 11, hzy011. [Google Scholar] [CrossRef]
- Majelantle, T.L.; Ganswindt, A.; Pirk, C.W.W.; Bennett, N.C.; Hart, D.W. Aggression, boldness, and exploration personality traits in the subterranean naked mole-rat (Heterocephalus glaber) disperser morphs. Animals 2022, 12, 3083. [Google Scholar] [CrossRef]
- Sih, A.; Bell, A.; Johnson, J.C. Behavioral syndromes: An ecological and evolutionary overview. Tree 2004, 19, 372–378. [Google Scholar] [CrossRef] [PubMed]
- Dingemanse, N.J.; Kazem, A.J.; Réale, D.; Wright, J. Behavioural reaction norms: Animal personality meets individual plasticity. Tree 2010, 25, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Sih, A.; Mathot, K.J.; Moiron, M.; Montiglio, P.-O.; Wolf, M.; Dingemanse, N.J. Animal personality and state–behaviour feedbacks: A review and guide for empiricists. Tree 2015, 30, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Roche, D.G.; Careau, V.; Binning, S.A. Demystifying animal ‘personality’(or not): Why individual variation matters to experimental biologists. J. Exp. Biol. 2016, 219, 3832–3843. [Google Scholar] [CrossRef] [PubMed]
- Gherardi, F.; Aquiloni, L.; Tricarico, E. Behavioral plasticity, behavioral syndromes and animal personality in crustacean decapods: An imperfect map is better than no map. Curr. Zool. 2012, 58, 567–579. [Google Scholar] [CrossRef]
- Wilson, A.D.; Godin, J.-G.J. Boldness and behavioral syndromes in the bluegill sunfish, Lepomis macrochirus. Behav. Ecol. 2009, 20, 231–237. [Google Scholar] [CrossRef]
- Reaney, L.T.; Backwell, P.R. Risk-taking behavior predicts aggression and mating success in a fiddler crab. Behav. Ecol. 2007, 18, 521–525. [Google Scholar] [CrossRef]
- Liang, Q.; Su, X.; Wang, F.; Zhu, B.; He, M. The developmental plasticity of boldness and aggressiveness in juvenile and adult swimming crab (Portunus trituberculatus). Front. Mar. Sci. 2020, 7, 608565. [Google Scholar] [CrossRef]
- Contala, M.L.; Krapf, P.; Steiner, F.M.; Schlick-Steiner, B.C. Foraging valor linked with aggression: Selection against completely abandoning aggression in the high-elevation ant Tetramorium alpestre? Insect Sci. 2023, 31, 953–970. [Google Scholar] [CrossRef]
- Pârvulescu, L.; Stoia, D.I.; Miok, K.; Ion, M.C.; Puha, A.E.; Sterie, M.; Vereş, M.; Marcu, I.; Muntean, M.D.; Aburel, O.M. Force and boldness: Cumulative assets of a successful crayfish invader. Front. Ecol. Evol. 2021, 9, 581247. [Google Scholar] [CrossRef]
- Barbosa, M.; Morrissey, M.B. The distinction between repeatability and correlation in studies of animal behaviour. Anim. Behav. 2021, 175, 201–217. [Google Scholar] [CrossRef]
- Kudo, H.; Nishizawa, H.; Uchida, K.; Sato, K. Boldness–exploration behavioral syndrome in wild sub-adult green sea turtles caught at Oita, Japan. Appl. Anim. Behav. Sci. 2021, 236, 105216. [Google Scholar] [CrossRef]
- Wright, C.M.; Hyland, T.D.; Izzo, A.S.; McDermott, D.R.; Tibbetts, E.A.; Pruitt, J.N. Polistes metricus queens exhibit personality variation and behavioral syndromes. Curr. Zool. 2018, 64, 45–52. [Google Scholar] [CrossRef]
- Begall, S.; Bottermann, L.; Caspar, K.R. Self-domestication underground? Testing for social and morphological correlates of animal personality in cooperatively-breeding Ansell’s mole-rats (Fukomys anselli). Front. Ecol. Evol. 2022, 10, 862082. [Google Scholar] [CrossRef]
- Tomoya, D.; Midori, I.; Kazuhiro, T. Decomposing the effects of ocean environments on predator–prey body-size relationships in food webs. R. Soc. Open Sci. 2018, 5, 180707. [Google Scholar] [CrossRef]
- Tarsitano, E. Interaction between the environment and animals in urban Settings: Integrated and participatory planning. Environ. Manag. 2006, 38, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Andersson, M. Linking behavioral type and cannibalism propensity in Eurasian Perch. PLoS ONE 2016, 16, e0260938. [Google Scholar] [CrossRef]
- Cárdenas, R.E.; Carrera-García, E.; Cornejo-Würfl, F. Swallow-tailed gull predation on a marine eel: Personality traits implied? Front. Ecol. Environ. 2022, 20, 139. [Google Scholar] [CrossRef]
- Lukas, J.; Kalinkat, G.; Miesen, F.W.; Landgraf, T.; Krause, J.; Bierbach, D. Consistent behavioral syndrome across seasons in an invasive freshwater fish. Front. Ecol. Environ. 2021, 8, 583670. [Google Scholar] [CrossRef]
- Su, X.; Zhu, B.; Wang, F. Feeding strategy changes boldness and agonistic behaviour in the swimming crab (Portunus trituberculatus). Aquac. Res. 2022, 53, 419–430. [Google Scholar] [CrossRef]
- Řežucha, R.; Reichard, M. The association between personality traits, morphological traits and alternative mating behaviour in male endler’s guppies, poecilia wingei. Ethology 2016, 122, 456–467. [Google Scholar] [CrossRef]
- Matzel, L.D.; Bendrath, S.; Herzfeld, M.; Crawford, D.W.; Sauce, B. Mouse twins separated when young: A history of exploration doubles the heritability of boldness and differentially affects the heritability of measures of learning. Intelligence 2019, 74, 34–42. [Google Scholar] [CrossRef]
- Baran, N.M.; Streelman, J.T. Ecotype differences in aggression, neural activity and behaviorally relevant gene expression in cichlid fish. Genes Brain Behav. 2020, 19, e12657. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zhang, Z.; Zhang, Z.; Shen, F.; Xu, X.; Li, Z.; Zhang, Y.; Zhang, X. Boldness predicts aggressiveness, metabolism, and activity in black rockfish Sebastes schlegelii. Front. Mar. Sci. 2021, 8, 770180. [Google Scholar] [CrossRef]
- Ariyomo, T.O.; Watt, P.J. The effect of variation in boldness and aggressiveness on the reproductive success of zebrafish. Anim. Behav. 2012, 83, 41–46. [Google Scholar] [CrossRef]
- Su, X.; Liu, J.; Wang, F.; Zhang, D.; Zhu, B.; Liu, D. Effect of temperature on agonistic behavior and energy metabolism of the swimming crab (Portunus trituberculatus). Aquaculture 2020, 516, 734573. [Google Scholar] [CrossRef]
- Cao, Z.; Feng, G.; Zhuang, P.; Hou, J. Morphological difference analysis between migratory population and cultured population of Eriocheir sinensis in the Yangtze River. Freshw. Fish. 2013, 43, 5. [Google Scholar] [CrossRef]
- Dammhahn, M.; Mazza, V.; Schirmer, A.; Göttsche, C.; Eccard, J.A. Of city and village mice: Behavioural adjustments of striped field mice to urban environments. Sci. Rep. 2020, 10, 13056. [Google Scholar] [CrossRef]
- Ferderer, A.; Davis, A.R.; Wong, M.Y. Temperature and body size influence personality and behavioural syndromes in an invasive crayfish. Anim. Behav. 2022, 190, 187–198. [Google Scholar] [CrossRef]
- Delacoux, M.; Guenther, A. Stressfulness of the design influences consistency of cognitive measures and their correlation with animal personality traits in wild mice (Mus musculus). Anim. Cogn. 2023, 26, 997–1009. [Google Scholar] [CrossRef]
- Dalos, J.; Royauté, R.; Hedrick, A.V.; Dochtermann, N.A. Phylogenetic conservation of behavioural variation and behavioural syndromes. J. Evol. Biol. 2022, 35, 311–321. [Google Scholar] [CrossRef] [PubMed]
- López, P.; Hawlena, D.; Polo, V.; Amo, L.; Martín, J. Sources of individual shy–bold variations in antipredator behaviour of male Iberian rock lizards. Anim. Behav. 2005, 69, 1–9. [Google Scholar] [CrossRef]
- Nordberg, E.; Denny, R.; Schwarzkopf, L. Testing measures of boldness and exploratory activity in native versus invasive species: Geckos as a model system. Anim. Behav. 2021, 177, 215–222. [Google Scholar] [CrossRef]
- van Oers, K.; van den Heuvel, K.; Sepers, B. The epigenetics of animal personality. Neurosci. Biobehav. R. 2023, 150, 105194. [Google Scholar] [CrossRef]
- Frost, A.J.; Winrow-Giffen, A.; Ashley, P.J.; Sneddon, L.U. Plasticity in animal personality traits: Does prior experience alter the degree of boldness? Proc. R. Soc. B-Biol. Sci. 2007, 274, 333–339. [Google Scholar] [CrossRef]
- Evans, T.; Krzyszczyk, E.; Frère, C.; Mann, J. Lifetime stability of social traits in bottlenose dolphins. Commun. Biol. 2021, 4, 759. [Google Scholar] [CrossRef]
- Horback, K.; Parsons, T. Ontogeny of behavioral traits in commercial sows. Animal 2018, 12, 2365–2372. [Google Scholar] [CrossRef]
- Kareklas, K.; Arnott, G.; Elwood, R.W.; Holland, R.A. Plasticity varies with boldness in a weakly-electric fish. Front. Zool. 2016, 13, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Gitto, L.; Bonaccorso, L.; Bryant, S.M.; Serinelli, S. Death caused by a domestic pig attack. Forensic Sci. Med. Pathol. 2021, 17, 469–474. [Google Scholar] [CrossRef]
- Travnik, I.d.C.; Machado, D.d.S.; Gonçalves, L.d.S.; Ceballos, M.C.; Sant’Anna, A.C. Temperament in domestic cats: A review of proximate mechanisms, methods of assessment, its effects on human—Cat relationships, and one welfare. Animals 2020, 10, 1516. [Google Scholar] [CrossRef]
- Henneberger, A.K.; Coffman, D.L.; Gest, S.D. The effect of having aggressive friends on aggressive behavior in childhood: Using propensity scores to strengthen causal inference. Soc. Dev. 2017, 26, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Boulton, M.J. Lunchtime supervisors’ attitudes towards playful fighting, and ability to differentiate between playful and aggressive fighting: An intervention study. Br. J. Educ. Psychol. 1996, 66, 367–381. [Google Scholar] [CrossRef]
- Fazhan, H.; Waiho, K.; Ikhwanuddin, M.; Shu-Chien, A.C.; Fujaya, Y.; Wang, Y.; Liew, H.J.; Chen, C.; Abualreesh, M.H.; Jaya-Ram, A. Limb loss and feeding ability in the juvenile mud crab Scylla olivacea: Implications of limb autotomy for aquaculture practice. Appl. Anim. Behav. Sci. 2022, 247, 105553. [Google Scholar] [CrossRef]
- Su, X.; Sun, Y.; Liu, D.; Wang, F.; Liu, J.; Zhu, B. Agonistic behaviour and energy metabolism of bold and shy swimming crabs Portunus trituberculatus. J. Exp. Biol. 2019, 222, jeb188706. [Google Scholar] [CrossRef]
- Takeshita, F. Color changes of fiddler crab between seasons and under stressful conditions: Patterns of changes in lightness differ between carapace and claw. J. Exp. Mar. Biol. Ecol. 2019, 511, 113–119. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).