Pigmented planktonic microalgae (phytoplankton) are primary producers that form the basis of marine trophic webs. Phytoplankton blooms are natural phenomena, which sustain bivalves and small pelagic fish production. Over fifty years ago, Reuben Lasker observed that the formation of dense layers of phytoplankton (thin layers) under certain climatic conditions, i.e., 4 days with wind velocities not exceeding 5 m/s, now called a “Lasker event”, ensured the success of anchovy (Engraulis mordax) recruitment in the Californian upwelling system. However, the same beneficial bloom, under certain circumstances (e.g., an imbalance between growth and grazing), may lead to eutrophication, environmental distress, or even anoxia and mass mortalities of marine fauna, becoming what we know as a high biomass harmful algal blooms (HBHABs). Furthermore, some microalgae produce potent toxins that are transferred through the food web, mainly through filter-feeding bivalves, and cause illnesses such as paralytic (PSP), diarrheic (DSP), amnesic (ASP) and neurotoxic (NSP) shellfish poisoning. These toxin producers, even at low cell concentrations, are filtered and their toxins accumulate in bivalve mollusks, posing a serious threat to public health and shellfish exploitations. More recently, a new toxic syndrome, azaspiracid shellfish poisoning (AZP), has been added to the toxic syndromes list.
In recent decades, the number of HAB reports and their geographic extension have increased dramatically, a fact partly explained by a parallel increment in the exploitation of coastal resources, such as from aquaculture and the tourism industry, and by an exponential growth in the observations carried out in monitoring programs [1,2]. The irrefutable contribution of anthropogenic factors (e.g., agricultural runoff, industrial and domestic waste, and tourism) added to the existing problems for the fisheries and aquaculture sectors [3,4,5,6].
Aquaculture and fisheries products are the staple food in the diet and the main source of employment and/or subsistence in coastal populations worldwide. In particular, in developing areas with no alternative sources income, their economic sustainability is of critical importance. The most recent report on the state of word fisheries and aquaculture showed that the global production of aquatic animals had risen to 178 million tons in 2020. In addition to aquatic animals, 36 million tons (wet weight) of algae were produced the same year (Figure 1A). Of the overall production of aquatic animals, 157 million tons (89%), mainly of bivalve mollusks, were used for human consumption [7]. However, these production activities are increasingly affected by the occurrence of a large variety of HAB species and their impacts (Figure 1B). Analyses of the IOC database of HAB events (IOC-HAEDAT) between 1972 and 2022 showed a significant increase in the total number of reports, mainly of paralytic (PSP) and diarrhetic syndromes (DSP), associated with blooms of the genera Alexandrium and Dinophysis, respectively [8]. Thus, in 2020, a historical maximum number of events were reported worldwide, i.e., 417 reports, 364 (87.3%) of which corresponded to PSP (Figure 1A).
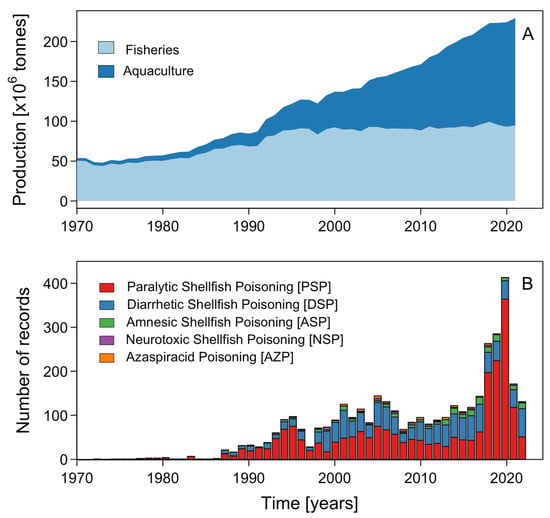
Figure 1.
(A) World fisheries’ catches and aquaculture production, and (B) number of global records of the five most common toxic HAB syndromes (PSP, DSP, ASP, NSP and AZP). Data are from FAO (https://www.fao.org) and the IOC HAEDAT (http://haedat.iode.org/).
Understanding the complexity of HAB events requires a multidisciplinary approach. A good example was the international SCOR-IOC GEOHAB (Global Ecology and Oceanography of Harmful Algal Blooms) program. This program, initiated in 2001, was the umbrella of a long list of projects, working groups and workshops, which led to considerable progress in understanding the mechanisms underlying population dynamics of HABs within an ecological and oceanographic context [9]. Its continuation, the IOC-SCOR GlobalHAB program (www.globalhab.org) initiated in 2016, widened the scope of GEOHAB by incorporating a socioeconomic perspective, including epidemiology, toxicology and evaluation of economic impacts to the aquaculture and tourism industries.
In order to prevent the risks of human exposure to potent marine toxins, many countries have been implementing specific health and safety measures. Mardones et al. [10] estimated that the cost of microalgae and toxin monitoring on the Chilean coast was USD 6.9 million in 2019. This country, the world’s second largest producer of cultured blue mussels (Mytilus chilensis) and salmon (mainly Salmo salar and Oncorhynchus kisutch) with 400,000 and 1,000,000 tons per year, respectively, has been severely affected by HABs events in the last decade [11,12,13]. In summer 2016, a major HAB event in Southern Chile of the fish-killer Pseudochattonella verruculosa generated losses of USD 800 million [14]. This event, the world’s largest-ever recorded farmed-fish mortality, caused a severe economic and social crisis in the region. Five years later, a bloom of Heterosigma akashiwo generated a new massive salmon mortality in Comau Fjord, Chilean Patagonia [15]. During that event, high cell densities (>2 · 105 cells mL−1) discolored the sea surface waters with intense brown patches visible to the naked eye (Figure 2).

Figure 2.
Red tide caused by Heterosigma akashiwo. The aerial photo was taken over the Comau Fjord (Chilean Patagonia) in early April 2021. Courtesy of Pamela Urrutia.
The “visible” impacts of HABs, such as massive fish mortalities, extensive shellfish quarantines and human poisonings, are unquestionable and well documented [16]. However, there are important gaps in our knowledge of hidden or cryptic impacts of HAB toxins on marine organisms, which are often attributed without sufficient background knowledge to climatological (e.g., storms and heat waves) and oceanographic conditions (e.g., anoxia and hypoxia) [17] or infectious epidemics [18]. It was widely accepted that shellfish poisoning toxins cause no harm to the vector bivalve mollusks. Nevertheless, some marine biotoxins have been recently associated with massive mortalities, which affected wild invertebrate (bivalves, echinoderms and cephalopods) populations [19,20,21,22] and cultivated gastropods [23].
Paralytic shellfish toxins (PST) may cause impairment of adult bivalves by affecting their response mechanisms to physiological (e.g., filtration rates), immunological (e.g., immunocompetence of hemocytes) and behavioral (e.g., burrowing activity) processes [19,24,25,26]. Similarly, amnesic shellfish toxins (AST) may cause a slow-down of valvar closure and other physiological disturbances, such as, hemolymph acidosis, hypoxia, increased hemocytokine activity and DNA damage [27,28]. In the case of lipophilic toxins (LT) produced by Dinophysis species, okadaic acid (OA) causes a decline in filtration rates associated with a cytotoxic effect [29,30], while pectenotoxins (PTXs) induce hypersecretion of mucus and pseudo-phaeces, paralysis, alteration of the digestive gland tissues and reduced escape responses in adult scallops [31]. Other lipophilic toxins, such as YTX and homo-YTX, may cause mass mortalities in the adult stages of different marine organisms at low concentrations (<1 mg eq. YTX kg−1) [20,22,23]. To date, the specific mechanism of action of YTX is not known; however, some studies suggested that YTXs affect the digestive and immune systems [32,33] and cytoskeletal cell components [33,34].
Noxious effects of extracellular toxins on the early life stages of marine organisms have been described in laboratory studies. PST has been associated with decreased swimming activity, larval inactivity, aberrant development, decreased growth, lower settlement rates and mortality in different species, such as scallops, oysters and mussels [35,36,37,38,39,40,41,42]. Domoic acid (ASP toxins) may reduce swimming activity, survival and consequently, the settlement rate in scallops (Pecten maximus) [43]. Regarding LTs produced by Dinophysis, OA can reduce innate immune responses, hemocyte activity, and larval viability [44,45,46,47], while PTX2 produces larval inactivity and rapid mortality [42,48]. Finally, more research is needed to determine the negative effects of other LTs, such as YTX and homo-YTX.
According to some climate predictions, several regions with valuable aquaculture exploitations will be exposed to an increased risk of HAB impacts [12,49]. Current tools for HAB prediction and mitigation lack accuracy, because the response of each HAB species to environmental stressors on multiple scales is species- and site-specific. This Special Issue (SI) seeks to compile contributions with new results aiming to bridge the gaps in our knowledge regarding the visible and hidden impacts of HABs on aquaculture and fisheries.
Author Contributions
Conceptualization, P.A.D. and G.Á.; writing—original draft preparation, P.A.D. and G.Á.; writing—review and editing, P.A.D. and G.Á. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
We thank Pamela Urrutia for her aerial photography of Heterosigma akashiwo bloom in Comau Fjord, Chilean Patagonia, in early autumn 2021.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hallegraeff, G.M. A review of harmful algal blooms and their apparent global increase. Phycologia 1993, 32, 79–99. [Google Scholar] [CrossRef]
- Hallegraeff, G.M.; Anderson, D.M.; Belin, C.; Bottein, M.-Y.D.; Bresnan, E.; Chinain, M.; Enevoldsen, H.; Iwataki, M.; Karlson, B.; McKenzie, C.H.; et al. Perceived global increase in algal blooms is attributable to intensified monitoring and emerging bloom impacts. Commun. Earth Environ. 2021, 2, 117. [Google Scholar] [CrossRef]
- Hallegraeff, G. Ocean climate change, phytoplankton community responses, and harmful algal blooms: A formidable predictive challenge. J. Phycol. 2010, 46, 220–235. [Google Scholar] [CrossRef]
- Heisler, J.; Glibert, P.M.; Burkholder, J.M.; Anderson, D.M.; Cochlan, W.; Dennison, W.C.; Dortch, Q.; Gobler, C.J.; Heil, C.A.; Humphries, E.; et al. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 2008, 8, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Glibert, P.M.; Allen, J.I.; Artioli, Y.; Beusen, A.; Bouwman, L.; Harle, J.; Holmes, R.; Holt, J. Vulnerability of coastal ecosystems to changes in harmful algal blooms distribution in response e to climate change: Projections based on model analysis. Glob. Chang. Biol. 2014, 20, 3845–3858. [Google Scholar] [CrossRef] [PubMed]
- Gobler, C.J.; Doherty, O.M.; Hattenrath-Lehamnn, T.K.; Griffith, A.W.; Kang, Y.; Litaker, R.W. Ocean warming since 1982 has expanded the niche of toxic algal blooms in the North Atlantic and North Pacific oceans. Proc. Natl. Acad. Sci. USA 2017, 114, 4975–4980. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2022. Towards Blue Transformation; FAO: Rome, Italy, 2022. [CrossRef]
- Hallegraeff, G. Global HAB Status Report. A Scienfic Summary for Policy Makers; Hallegraeff, G.M., Enevoldsen, H., Zingone, A., Eds.; UNESCO: Paris, France, 2021; (IOC Informa on Document, 1399). [Google Scholar]
- Kudela, R.M.; Berdalet, E.; Enevoldsen, H.; Pitcher, G.; Raine, R.; Urban, E. GEOHAB–The Global Ecology and Oceanography of Harmful Algal Blooms Program: Motivation, goals, and legacy. Oceanography 2017, 30, 12–21. [Google Scholar] [CrossRef]
- Mardones, J.I.; Holland, D.S.; Anderson, L.; Le Bihan, V.; Gianella, F.; Clément, A.; Davidson, K.; Sakamoto, S.; Yoshida, T.; Trainer, V.L. Estimating and mitigating the economic costs of harmful algal blooms on commercial and recreational shellfish harvesters. Economic costs of HABs on commercial and recreational shellfish harvesters. PICES Sci. Rep. 2020, 59, 66–83. [Google Scholar]
- Díaz, P.A.; Álvarez, G.; Pizarro, G.; Blanco, J.; Reguera, B. Lipophilic toxins in Chile: History, producers and impacts. Mar. Drugs 2022, 20, 122. [Google Scholar] [CrossRef] [PubMed]
- Díaz, P.A.; Figueroa, R.I. Toxic algal bloom recurrence in the era of global change: Lessons from the Chilean Patagonian fjords. Microorganisms 2023, 11, 1874. [Google Scholar] [CrossRef] [PubMed]
- Díaz, P.A.; Pérez-Santos, I.; Álvarez, G.; Garreaud, R.; Pinilla, E.; Díaz, M.; Sandoval, A.; Araya, M.; Álvarez, F.; Rengel, J.; et al. Multiscale physical background to an exceptional harmful algal bloom of Dinophysis acuta in a fjord system. Sci. Total Environ. 2021, 773, 145621. [Google Scholar] [CrossRef] [PubMed]
- Mardones, J.I.; Paredes, J.; Godoy, M.; Suarez, R.; Norambuena, L.; Vargas, V.; Fuenzalida, G.; Pinilla, E.; Artal, O.; Rojas, X.; et al. Disentangling the environmental processes responsible for the world’s largest farmed fish-killing harmful algal bloom: Chile, 2016. Sci. Total Environ. 2021, 766, 144383. [Google Scholar] [CrossRef] [PubMed]
- Díaz, P.A.; Pérez-Santos, I.; Basti, L.; Garreaud, R.; Pinilla, E.; Barrera, F.; Tello, A.; Schwerter, C.; Arenas-Uribe, S.; Soto-Riquelme, C.; et al. How local and climate change drivers shaped the formation, dynamics and potential recurrence of a massive fish-killer microalgal bloom in Patagonian fjord. Sci. Total Environ. 2023, 865, 161288. [Google Scholar] [CrossRef]
- Díaz, P.A.; Álvarez, A.; Varela, D.; Pérez-Santos, I.; Díaz, M.; Molinet, C.; Seguel, M.; Aguilera-Belmonte, A.; Guzmán, L.; Uribe, E.; et al. Impacts of harmful algal blooms on the aquaculture industry: Chile as a case study. Perspect. Phycol. 2019, 6, 39–50. [Google Scholar] [CrossRef]
- Rogers-Bennett, L.; Kudela, R.; Nielsen, K.; Paquin, A.; O’Kelly, C.; Langlois, G.W.; Crane, D.B.; Moore, J. Dinoflagellate bloom coincides with marine invertebrate mortalities in northern California. Harmful Algae News 2012, 46, 10–11. [Google Scholar]
- Harvell, C.D.; Kim, K.; Burkholder, J.; Colwell, R.; Epstein, P.R.; Grimes, D.J.; Hofmann, E.E.; Lipp, E.K.; Osterhaus, A.D.; Overstreet, R.M.; et al. Emerging marine diseases–climate links and anthropogenic factors. Science 1999, 285, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Landsberg, J.H. The effects of harmful algal blooms on aquatic organisms. Rev. Fish. Sci. 2002, 10, 113–390. [Google Scholar] [CrossRef]
- Jurgens, L.J.; Rogers-Bennett, L.; Raimondi, P.T.; Schiebelhut, L.M.; Dawson, M.N.; Grosberg, R.K.; Gaylord, B. Patterns of mass mortality among rocky shore Invertebrates across 100 km of Northeastern Pacific Coastline. PLoS ONE 2015, 10, e0126280. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, G.; Díaz, P.A.; Godoy, M.; Araya, M.; Ganuza, I.; Pino, R.; Álvarez, F.; Rengel, J.; Hernández, C.; Uribe, E.; et al. Paralytic Shellfish Toxins in Mesodesma donacium during an exceptional bloom of Alexandrium catenella associated to an intense mass mortality. Toxins 2019, 11, 188. [Google Scholar] [CrossRef]
- Álvarez, G.; Rengel, J.; Álvarez, F.; Pino, R.; Muñoz, P.; Rosales, S.; Hevia, V.; Araya, M.; Díaz, P.A.; Rivera, A.; et al. Mass mortality of marine invertebrates associated by the presence of yessotoxins in northern Chile. Harmful Algae News 2020, 64, 6–7. [Google Scholar]
- Pitcher, G.C.; Foord, C.J.; Macey, B.M.; Mansfield, L.; Mouton, A.; Smith, M.E.; Osmond, S.J.; van der Molen, L. Devastating farmed abalone mortalities attributed to yessotoxin-producing dinoflagellates. Harmful Algae 2019, 81, 30–41. [Google Scholar] [CrossRef] [PubMed]
- Bricelj, V.M.; Connell, L.; konoki, K.; MacQuarrie, S.P.; Scheuer, T.; Catterall, W.A.; Trainer, V.L. Sodium channel mutation leading to saxitoxin resistance in clams increases risk of PSP. Nature 2005, 434, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Navarro, J.M.; Widdows, J.; Chaparro, O.R.; Ortiz, A.; Mellado, C.; Villanueva, P.A. Pre-ingestive selection capacity and endoscopic analysis in the sympatric bivalves Mulinia edulis and Mytilus chilensis exposed to diets containing toxic and non-toxic dinoflagellates. PLoS ONE 2018, 12, e0193370. [Google Scholar] [CrossRef]
- Mello, D.F.; da Silva, P.M.; Barracco, M.A.; Soudant, P.; Hégaret, H. Effects of the dinoflagellate Alexandrium minutum and its toxin (saxitoxin) on the functional activity and gene expression of Crassostrea gigas hemocytes. Harmful Algae 2013, 26, 45–51. [Google Scholar] [CrossRef]
- Dizer, H.; Fischer, B.; Harabawy, A.S.A.; Hennion, M.C.; Hansen, P.D. Toxicity of domoic acid in the marine mussel Mytilus edulis. Aquat. Toxicol. 2001, 55, 149–156. [Google Scholar] [CrossRef]
- Jones, T.O.; Whyte, J.N.; Ginther, N.G.; Townsend, L.D.; Iwama, G.K. Haemocyte changes in the Pacific oyster, Crassostrea gigas, caused by exposure to domoic acid in the diatom Pseudonitzschia pungens cf. multiseries. Toxicon 1995, 33, 347–353. [Google Scholar] [CrossRef]
- Pillet, S.; Houvenaghel, G. Influence of Experimental Toxification by DSP Producing Microalgae, Prorocentrum lima, on Clearance Rate in Blue Mussels Mytilus edulis. In Harmful Marine Algal Blooms; Lassus, P., Arzul, G., Erard-Le Denn, E., Gentien, P., Marcaillou-Le Baut, C., Eds.; Lavoisier, Intercept Ltd: Paris, France, 1995; pp. 481–486. [Google Scholar]
- Pillet, S.; Pereira, A.; Braekman, J.-C.; Houvenaghel, G. Patterns in Long Term Accumulation of Okadaic Acid and DTX-1 in Blue Mussels, Mytilus edulis, Experimentally Fed with the DSP-Containing Alga Prorocentrum lima. In Harmful Marine Algal Blooms; Lassus, P., Arzul, G., Erard, E., Gentien, P., Marcaillou, C., Eds.; Lavoisier, Intercept Ltd.: Paris, France, 1995; pp. 487–492. [Google Scholar]
- Basti, L.; Uchida, H.; Kanamori, M.; Matsushima, R.; Suzuki, T.; Nagai, S. Mortality and Pathology of Japanese Scallop, Patinopecten (Mizuhopecten) yessoensis, and Noble Scallop, Mimachlamys nobilis, Fed Monoclonal Culture of PTX Producer, Dinophysis caudata. In Proceedings of the 16th International Conference on Harmful Algae, Wellington, New Zealand, 27–31 October 2014; MacKenzie, L.A., Ed.; Cawthron Institute: Nelson, New Zeland; International Society for the Study of Harmful Algae: Wellington, New Zealand, 2015; pp. 27–30. [Google Scholar]
- Franchini, A.; Milandri, A.; Poletti, R.; Ottaviani, E. Immunolocalization of yessotoxins in the mussel Mytilus galloprovincialis. Toxicon 2003, 41, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Franchini, A.; Malagoli, D.; Ottaviani, E. Targets and effects of yessotoxin, okadaic acid and palytoxin: A differential review. Mar. Drugs 2010, 8, 658–677. [Google Scholar] [CrossRef] [PubMed]
- Tubaro, A.; Dell’ Ovo, V.; Sosa, S.; Florio, C. Yessotoxins: A toxicological overview. Toxicon 2010, 56, 163–172. [Google Scholar] [CrossRef]
- Yan, T.; Zhou, M.; Fu, M.; Wang, Y.; Yu, R.; Li, J. Inhibition of egg hatching success and larvae survival of the scallop, Chlamys farreri, associated with exposure to cells and cell fragments of the dinoflagellate Alexandrium tamarense. Toxicon 2001, 39, 1239–1244. [Google Scholar] [CrossRef]
- Yan, T.; Zhou, M.; Fu, M.; Yu, R.; Wang, Y.; Li, J. Effects of the dinoflagellate Alexandrium tamarense on early development of the scallop Argopecten irradians concentricus. Aquaculture 2003, 217, 167–178. [Google Scholar] [CrossRef]
- Hégaret, H.; Wikfors, G.H.; Soudant, P.; Lambert, C.; Shumway, S.E.; Bérard, J.B.; Lassus, P. Toxic dinoflagellates (Alexandrium fundyense and A. catenella) have minimal apparent effects on oyster hemocytes. Mar. Biol. 2007, 152, 441–447. [Google Scholar] [CrossRef]
- Mu, C.; Li, Q. Effects of the dinoflagellate Alexandrium catenella on the early development of the Pacific oyster Crassostrea gigas. J. Shellfish Res. 2013, 32, 689–694. [Google Scholar] [CrossRef]
- Basti, L.; Nagai, S.; Go, J.; Okano, S.; Nagai, K.; Watanabe, R.; Suzuki, T.; Tanaka, Y. Differential inimical effects of Alexandrium spp. and Karenia spp. on cleavage, hatching, and two larval stages of Japanese pearl oyster Pinctada fucata martensii. Harmful Algae 2015, 43, 1–12. [Google Scholar] [CrossRef]
- Banno, K.; Oda, T.; Nagai, K.; Nagai, S.; Tanaka, Y.; Basti, L. Deleterious effects of harmful dinoflagellates and raphidophytes on egg viability and spermatozoa swimming velocity in the Japanese pearl oyster Pinctada fucata martensii. J. Shellfish Res. 2018, 37, 41–48. [Google Scholar] [CrossRef]
- Supono, S.; Knowles, G.; Bolch, C. Toxicity and histopathological effects of toxic dinoflagellate, Alexandrium catenella exudates on larvae of blue mussel, Mytilus galloprovincialis, and Pacific oyster, Crassostrea gigas. J. Ilm. Perikan. Dan Kelaut. 2020, 12, 188–198. [Google Scholar] [CrossRef]
- Pease, S.K.D.; Brosnahan, M.L.; Sanderson, M.P.; Smith, J.L. Effects of two toxin-884 producing harmful algae, Alexandrium catenella and Dinophysis acuminata (Dinophyceae), on activity and mortality of larval shellfish. Toxins 2022, 14, 335. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Kelly, M.S.; Campbell, D.A.; Dong, S.L.; Zhu, J.X.; Wang, S.F. Exposure to domoic acid affects larval development of king scallop Pecten maximus (Linnaeus, 1758). Aquat. Toxicol. 2007, 81, 152–158. [Google Scholar] [CrossRef] [PubMed]
- De Rijcke, M.; Vandegehuchte, M.; Vanden Bussche, J.; Nevejan, N.; Vanhaecke, L.; De Schamphelaere, K.; Janssen, C. Common European harmful algal blooms affect the viability and innate immune responses of Mytilus edulis larvae. Fish Shellfish Immunol. 2015, 47, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Prado-Alvarez, M.; Florez-Barros, F.; Sexto-Iglesias, A.; Méndez, J.; Fernández-Tajes, J. Effects of okadaic acid on haemocytes from Mytilus galloprovincialis: A comparison between field and laboratory studies. Mar. Environ. Res. 2012, 81, 90–93. [Google Scholar] [CrossRef]
- Prado-Alvarez, M.; Florez-Barros, F.; Méndez, J.; Fernandez-Tajes, J. Effect of okadaic acid on carpet shell clam (Ruditapes decussatus) haemocytes by in vitro exposure and harmful algal bloom simulation assays. Cell Biol. Toxicol. 2013, 29, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Svensson, S.; Förlin, L. Intracellular effects of okadaic acid in the blue mussel Mytilus edulis, and rainbow trout Oncorhynchus mykiss. Mar. Environ. Res. 1998, 46, 449–452. [Google Scholar] [CrossRef]
- Gaillard, S.; Le Goiċ, N.; Malo, F.; Boulais, M.; Fabioux, C.; Zaccagnini, L.; Carpentier, L.; Sibat, M.; Réveillon, D.; Séchet, V.; et al. Cultures of Dinophysis sacculus, D. acuminata and pectenotoxin 2 affect gametes and fertilization success of the Pacific oyster, Crassostrea gigas. Environ. Pollut. 2020, 265, 114840. [Google Scholar] [CrossRef] [PubMed]
- Soto, D.; León-Muñoz, J.; Garreaud, R.; Quiñones, R.A. Scientific warnings could help to reduce farmed salmon mortality due to harmful algal blooms. Mar. Policy 2021, 133, 104705. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).