Selecting from the Fisheries Managers’ Tool-Box: Recreational Fishers’ Views of Stock Enhancement and Other Management Options
Abstract
1. Introduction
2. Materials and Methods
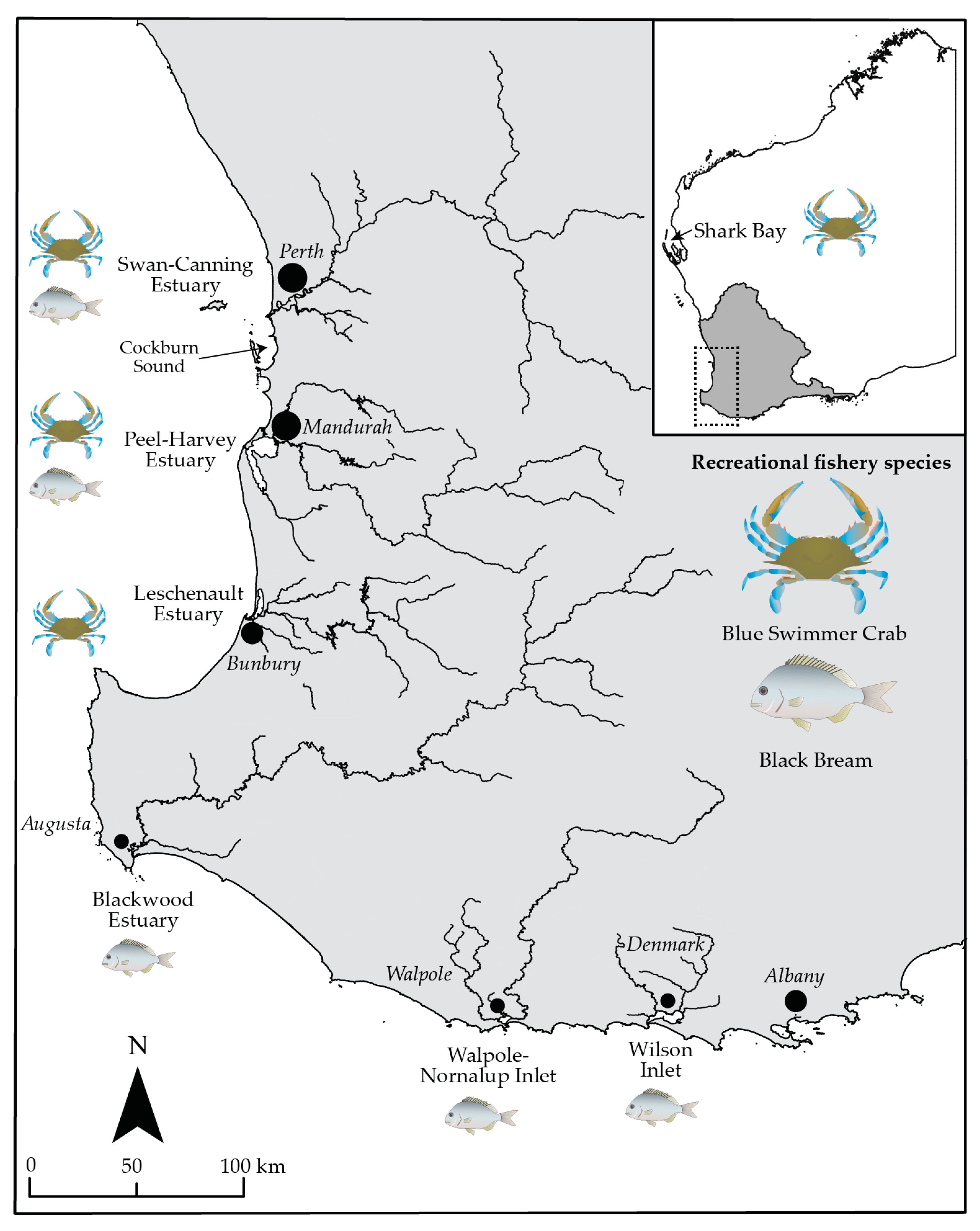
2.1. Study Area and Fisheries
2.2. Surveys of Recreational Fishers
2.3. Data Analyses
3. Results
3.1. Demographics
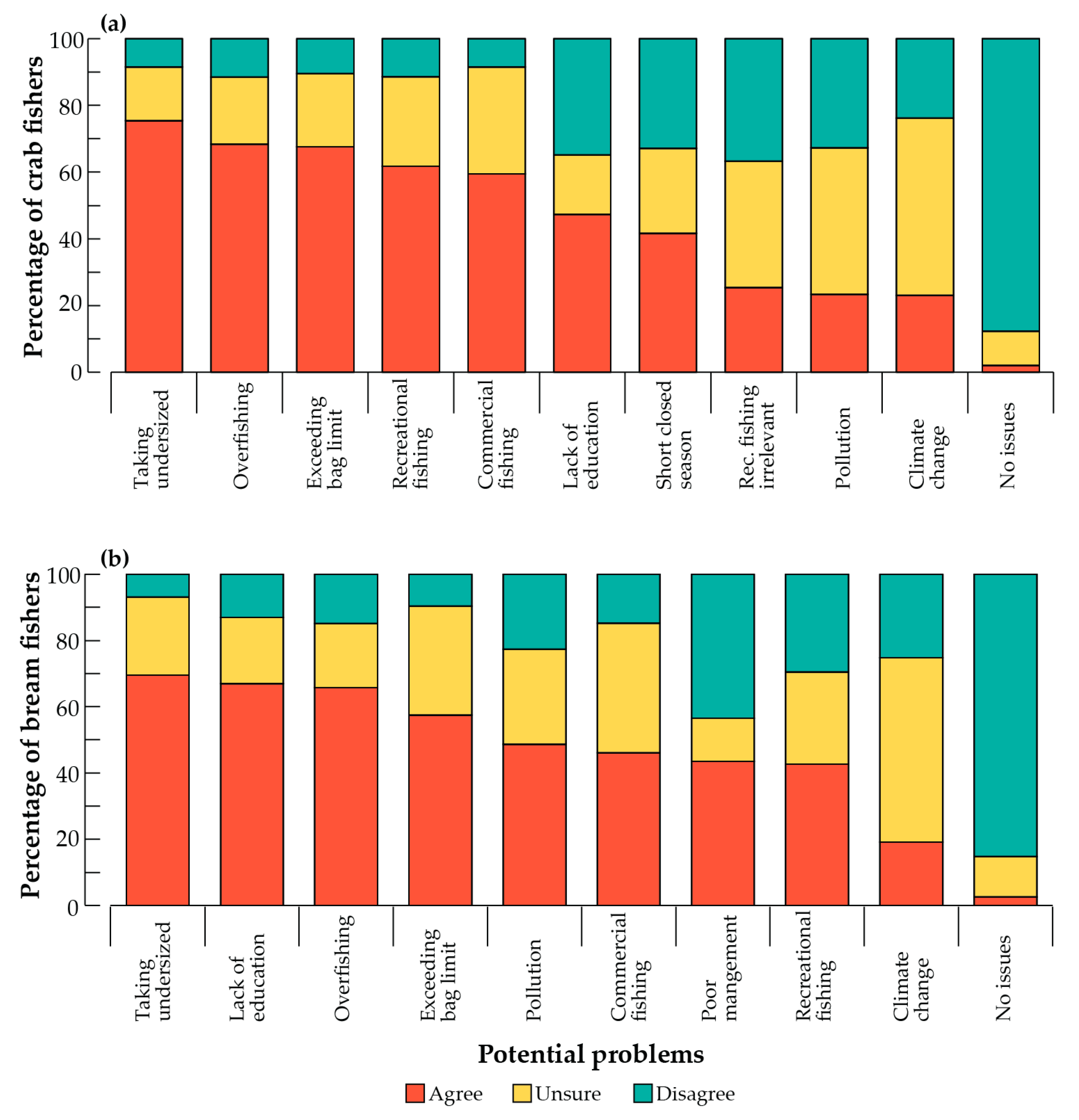
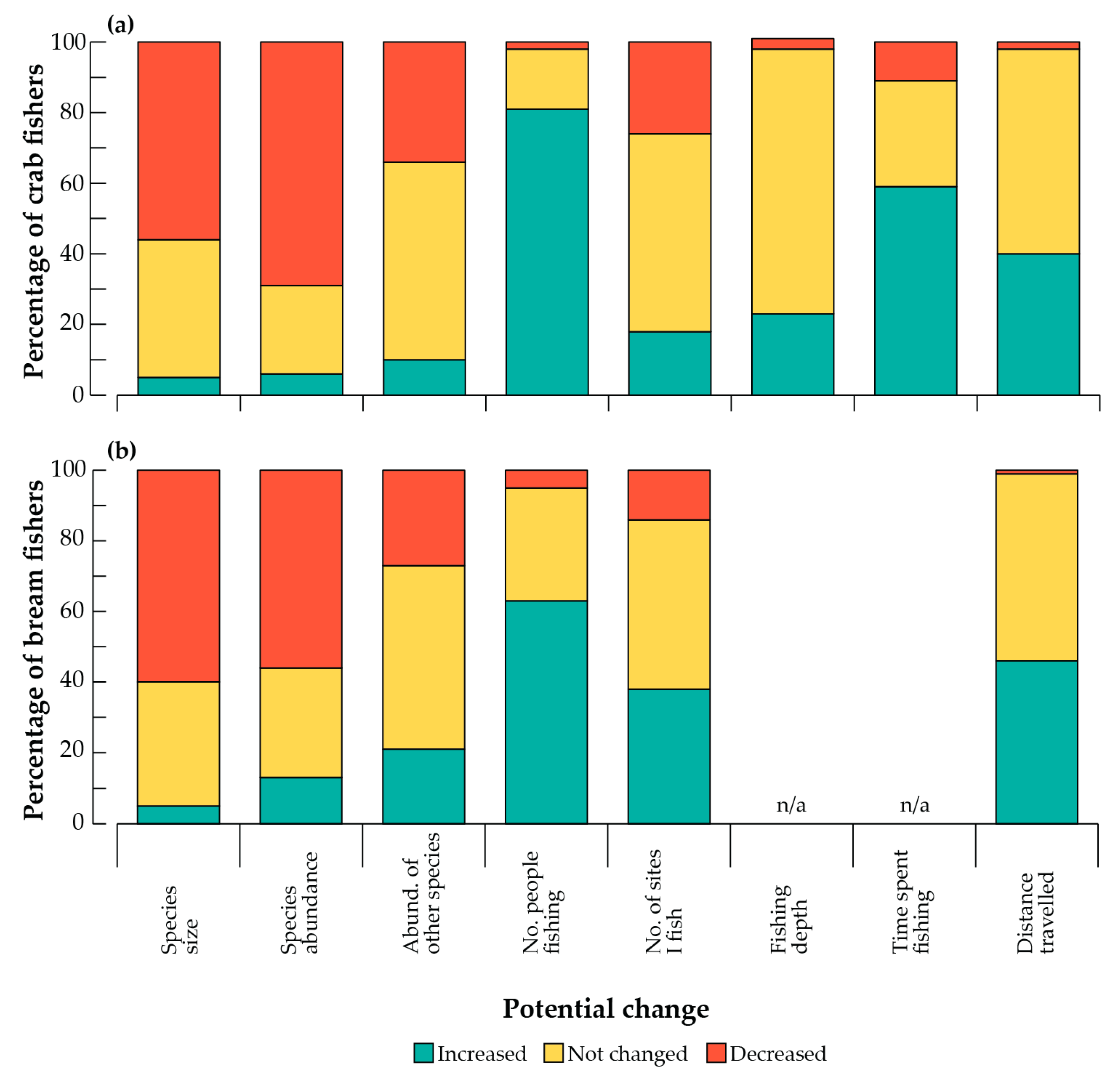
3.2. Perceived Fishery Issues and Effects on Catches
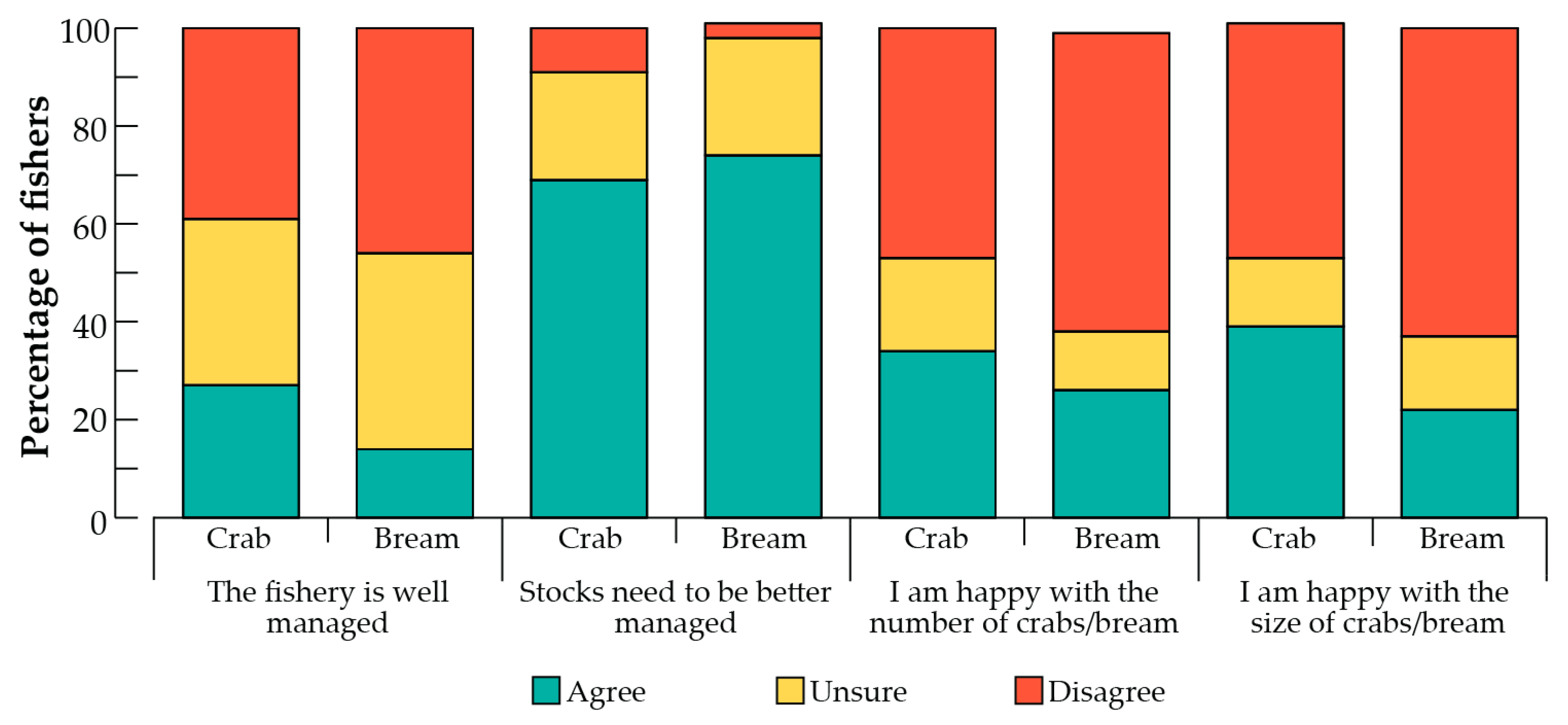
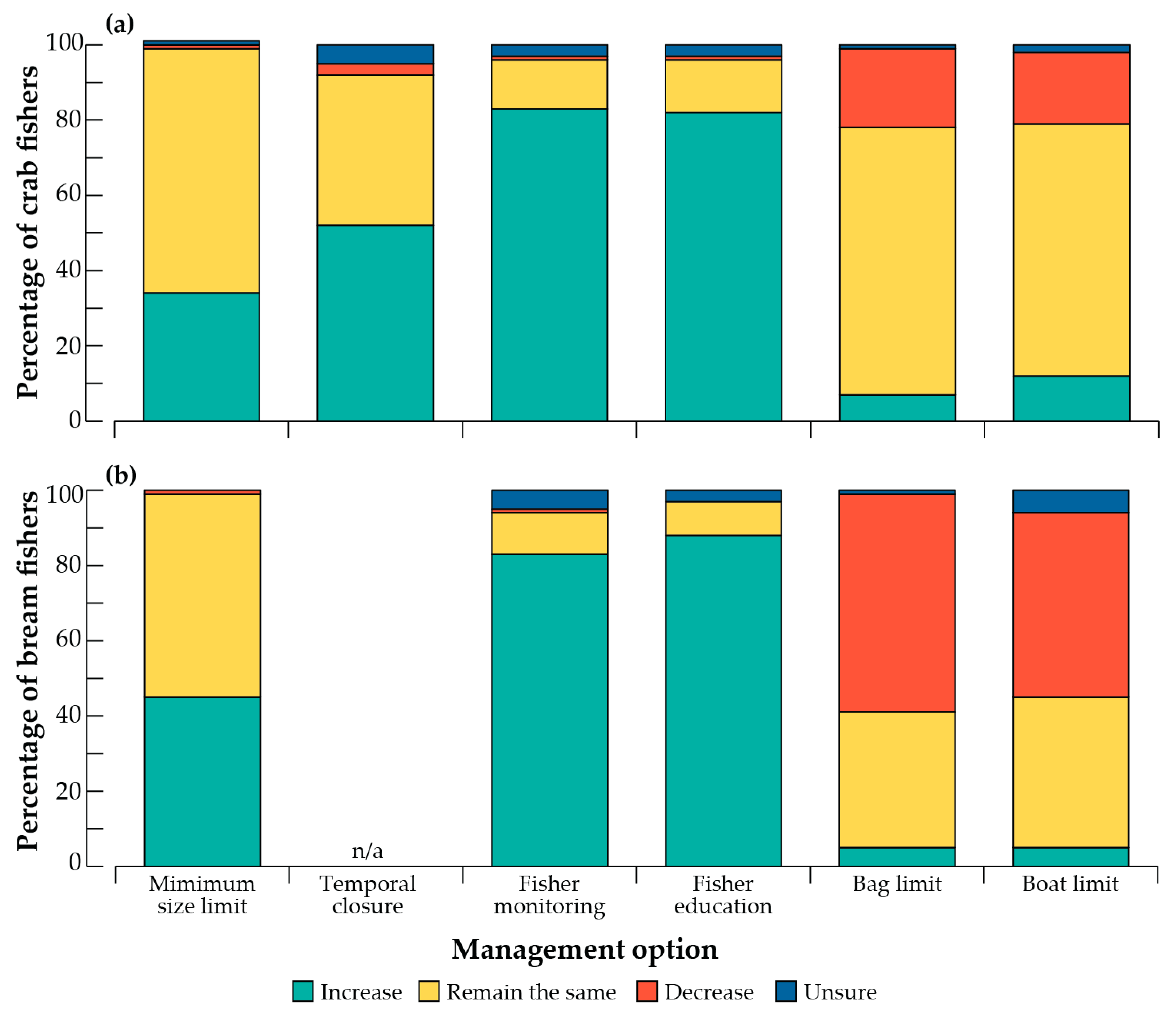
3.3. Views of Current Management and Management Tools
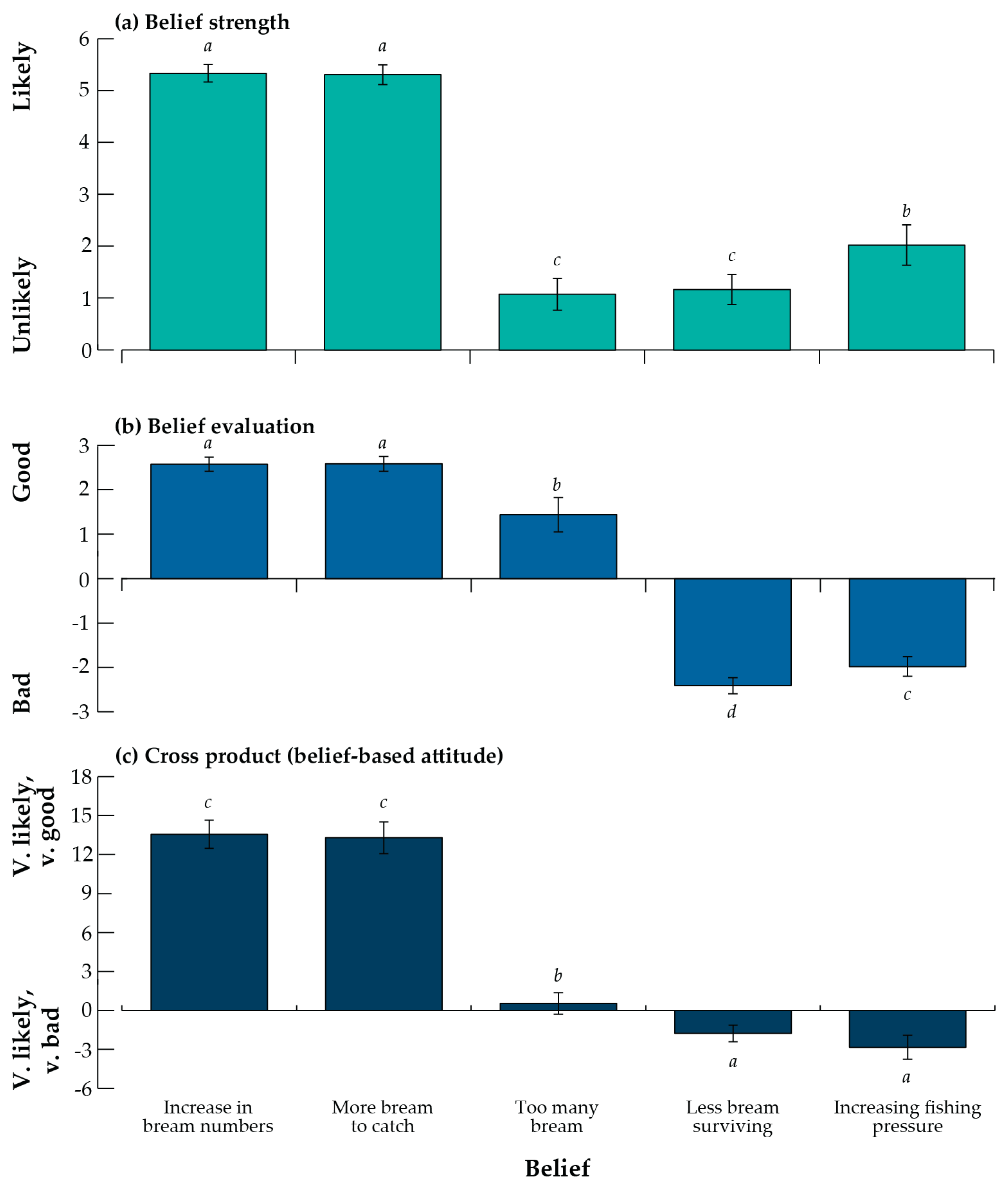
3.4. Beliefs and Attitudes to Stock Enhancement
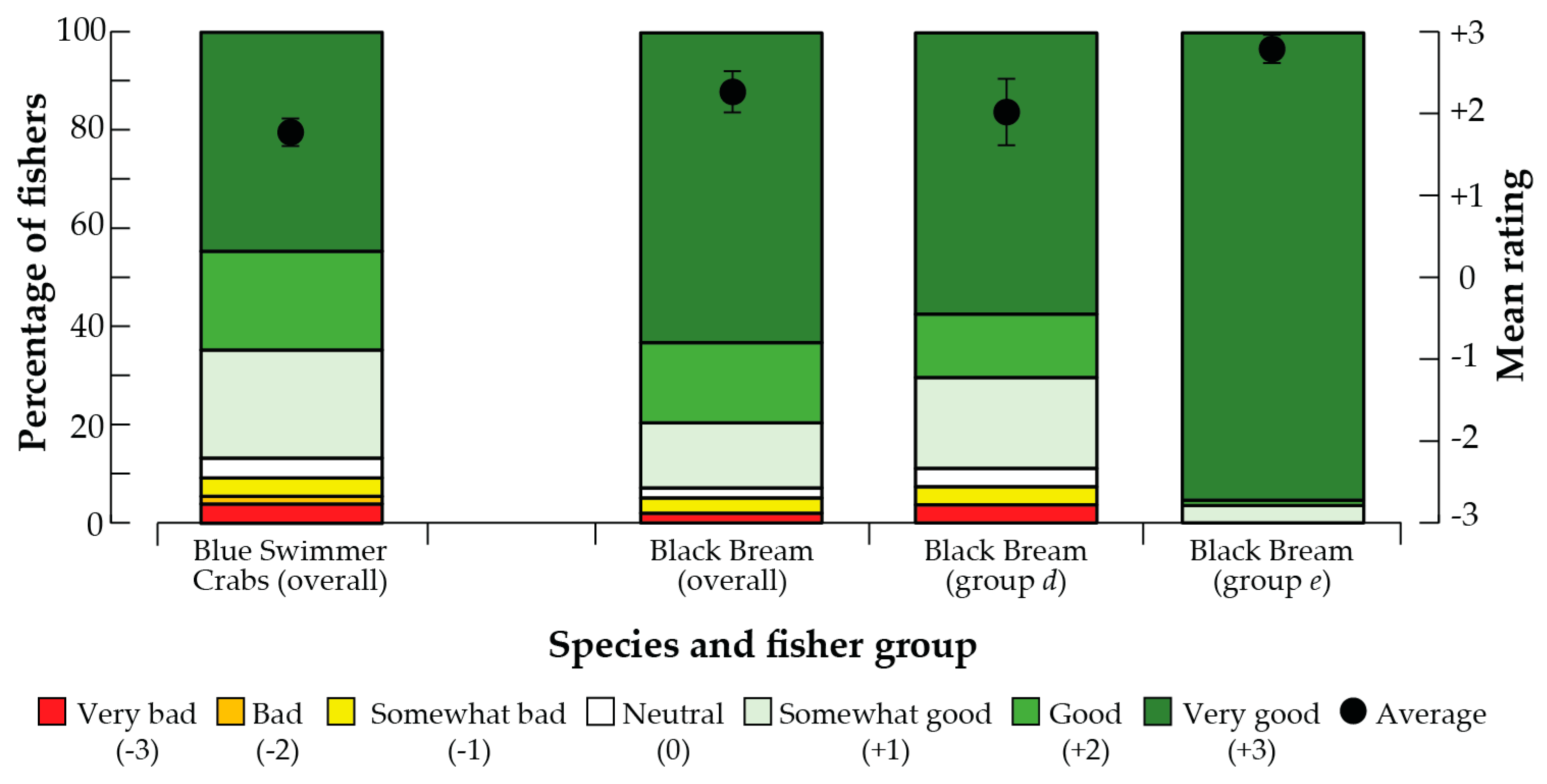
3.5. Implications for Stock Enhancement
4. Discussion
4.1. Perceived Fishery Issues
4.2. Views of Current Catches and Management
4.3. Opinions on Management Tools
4.4. Stocking as a Management Option
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Allison, E.H.; Ellis, F. The livelihoods approach and management of small-scale fisheries. Mar. Policy 2001, 25, 377–388. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture: Sustainability in Action; Food and Agriculture Organisation: Rome, Italy, 2020. [Google Scholar]
- Sutinen, J.G.; Soboil, M. The performance of fisheries management systems and the ecosystem challenge. In Responsible Fisheries in the Marine Ecosystem; CABI: Wallingford, UK, 2003; p. 291. [Google Scholar]
- Selig, E.R.; Kleisner, K.M.; Ahoobim, O.; Arocha, F.; Cruz-Trinidad, A.; Fujita, R.; Hara, M.; Katz, L.; McConney, P.; Ratner, B.D.; et al. A typology of fisheries management tools: Using experience to catalyse greater success. Fish Fish. 2017, 18, 543–570. [Google Scholar] [CrossRef]
- Mascia, M.B.; Claus, C.A.; Naidoo, R. Impacts of marine protected areas on fishing communities. Conserv. Biol. 2010, 24, 1424–1429. [Google Scholar] [CrossRef] [PubMed]
- Magee, C.; Voyer, M.; McIlgorm, A.; Li, O. Chasing the thrill or just passing the time? Trialing a new mixed methods approach to understanding heterogeneity amongst recreational fishers based on motivations. Fish. Res. 2018, 199, 107–118. [Google Scholar] [CrossRef]
- Hartill, B.W.; Taylor, S.M.; Keller, K.; Weltersbach, M.S. Digital camera monitoring of recreational fishing effort: Applications and challenges. Fish Fish. 2020, 21, 204–215. [Google Scholar] [CrossRef]
- Provost, E.J.; Butcher, P.A.; Coleman, M.A.; Kelaher, B.P. Assessing the viability of small aerial drones to quantify recreational fishers. Fish. Manag. Ecol. 2020, 27, 615–621. [Google Scholar] [CrossRef]
- Fedler, A.J.; Ditton, R.B. Understanding angler motivations in fisheries management. Fisheries 1994, 19, 6–13. [Google Scholar] [CrossRef]
- Dimech, M.; Darmanin, M.; Philip Smith, I.; Kaiser, M.J.; Schembri, P.J. Fishers’ perception of a 35-year old exclusive fisheries management zone. Biol. Conserv. 2009, 142, 2691–2702. [Google Scholar] [CrossRef]
- Taylor, M.D.; Chick, R.C.; Lorenzen, K.; Agnalt, A.-L.; Leber, K.M.; Blankenship, H.L.; Haegen, G.V.; Loneragan, N.R. Fisheries enhancement and restoration in a changing world. Fish. Res. 2017, 186, 407–412. [Google Scholar] [CrossRef]
- Ingram, B.A.; Hayes, B.; Rourke, M.L. Impacts of stock enhancement strategies on the effective population size of Murray cod, Maccullochella peelii, a threatened Australian fish. Fish. Manag. Ecol. 2011, 18, 467–481. [Google Scholar] [CrossRef]
- Anderson, J.D.; Cason, P.D. Density-dependent impacts on growth and body condition of Red Drum in stock enhancement rearing ponds. N. Am. J. Aquac. 2015, 77, 491–496. [Google Scholar] [CrossRef]
- Arlinghaus, R.; Mehner, T. Management preferences of urban anglers. Fisheries 2003, 28, 10–17. [Google Scholar] [CrossRef]
- Garlock, T.M.; Lorenzen, K. Marine angler characteristics and attitudes toward stock enhancement in Florida. Fish. Res. 2017, 186, 439–445. [Google Scholar] [CrossRef]
- Costanza, R.; Fisher, B.; Mulder, K.; Liu, S.; Christopher, T. Biodiversity and ecosystem services: A multi-scale empirical study of the relationship between species richness and net primary production. Ecol. Econ. 2007, 61, 478–491. [Google Scholar] [CrossRef]
- Tweedley, J.R.; Warwick, R.M.; Potter, I.C. The contrasting ecology of temperate macrotidal and microtidal estuaries. Oceanogr. Mar. Biol. Annu. Rev. 2016, 54, 73–172. [Google Scholar] [CrossRef]
- Potter, I.C.; Warwick, R.M.; Hall, N.G.; Tweedley, J.R. The physico-chemical characteristics, biota and fisheries of estuaries. In Freshwater Fisheries Ecology; Craig, J., Ed.; Wiley-Blackwell: Chichester, West Sussex, UK, 2015; pp. 48–79. [Google Scholar]
- Creighton, C.; Boon, P.I.; Brookes, J.D.; Sheaves, M. Repairing Australia’s estuaries for improved fisheries production—What benefits, at what cost? Mar. Freshw. Res. 2015, 66, 493–507. [Google Scholar] [CrossRef]
- Warwick, R.M.; Tweedley, J.R.; Potter, I.C. Microtidal estuaries warrant special management measures that recognise their critical vulnerability to pollution and climate change. Mar. Pollut. Bull. 2018, 135, 41–46. [Google Scholar] [CrossRef]
- Hallett, C.S.; Valesini, F.J.; Clarke, K.R.; Hoeksema, S.D. Effects of a harmful algal bloom on the community ecology, movements and spatial distributions of fishes in a microtidal estuary. Hydrobiologia 2016, 763, 267–284. [Google Scholar] [CrossRef]
- Tweedley, J.R.; Hallett, C.S.; Warwick, R.M.; Clarke, K.R.; Potter, I.C. The hypoxia that developed in a microtidal estuary following an extreme storm produced dramatic changes in the benthos. Mar. Freshw. Res. 2016, 67, 327–341. [Google Scholar] [CrossRef]
- Tweedley, J.R.; Dittmann, S.R.; Whitfield, A.K.; Withers, K.; Hoeksema, S.D.; Potter, I.C. Hypersalinity: Global distribution, causes, and present and future effects on the biota of estuaries and lagoons. In Coasts and Estuaries; Wolanski, E., Day, J.W., Elliott, M., Ramachandran, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 523–546. [Google Scholar]
- Krispyn, K.N.; Loneragan, N.R.; Whitfield, A.K.; Tweedley, J.R. Salted mullet: Protracted occurrence of Mugil cephalus under extreme hypersaline conditions. Estuar. Coast. Shelf Sci. 2021, 261, 107533. [Google Scholar] [CrossRef]
- Cottingham, A.; Hesp, S.A.; Hall, N.G.; Hipsey, M.R.; Potter, I.C. Marked deleterious changes in the condition, growth and maturity schedules of Acanthopagrus butcheri (Sparidae) in an estuary reflect environmental degradation. Estuar. Coast. Shelf Sci. 2014, 149, 109–119. [Google Scholar] [CrossRef]
- Cottingham, A.; Huang, P.; Hipsey, M.R.; Hall, N.G.; Ashworth, E.; Williams, J.; Potter, I.C. Growth, condition, and maturity schedules of an estuarine fish species change in estuaries following increased hypoxia due to climate change. Ecol. Evol. 2018, 8, 7111–7130. [Google Scholar] [CrossRef] [PubMed]
- DPIRD. Annual Report 2018; Department of Primary Industries and Regional Development: Perth, Australia, 2019; p. 248.
- Henry, G.W.; Lyle, J.M. The National Recreational and Indigenous Fishing Survey; Fisheries Research and Development Corporation: Cronulla, Australia, 2003. [Google Scholar]
- Arlinghaus, R.; Tillner, R.; Bork, M. Explaining participation rates in recreational fishing across industrialised countries. Fish. Manag. Ecol. 2015, 22, 45–55. [Google Scholar] [CrossRef]
- Ryan, K.L.; Hall, N.G.; Lai, E.K.; Smallwood, C.B.; Tate, A.; Taylor, S.M.; Wise, B.S. Statewide Survey of Boat-Based Recreational Fishing in Western Australia 2017/18; Department of Primary Industries and Regional Development: Perth, Australia, 2019.
- Ryan, K.L.; Hall, N.G.; Lai, E.K.; Smallwood, C.B.; Taylor, S.M.; Wise, B.S. State-Wide Survey of Boat-Based Recreational Fishing in Western Australia 2013/14; Department of Fisheries, Western Australia: Perth, Australia, 2015; p. 208.
- Tweedley, J.R.; Obregón, C.; Beukes, S.J.; Loneragan, N.R.; Hughes, M. Differences in recreational fishers’ motivations for utilising two estuarine fisheries. Fishes 2023, 8, 292. [Google Scholar] [CrossRef]
- Johnston, D.; Yeoh, D.; Harris, D.; Denham, A.; Fisher, E. Blue Swimmer Crab (Portunus armatus) Resource in the West Coast Bioregion, Western Australia. Part 1: Peel-Harvey Estuary, Cockburn Sound and Swan-Canning Estuary; Department of Primary Indistrues and Regional Development: Perth, Australia, 2020.
- Newman, S.J.; Wise, B.S.; Santoro, K.G.; Gaughan, D.J. Status Reports of the Fisheries and Aquatic Resources of Western Australia 2020/21: The State of the Fisheries; Department of Primary Industries and Regional Development: Perth, Australia, 2021.
- Obregón, C.; Christensen, J.; Zeller, D.; Hughes, M.; Tweedley, J.R.; Gaynor, A.; Loneragan, N.R. Local fisher knowledge reveals changes in size of blue swimmer crabs in small-scale fisheries. Mar. Policy 2022, 143, 105144. [Google Scholar] [CrossRef]
- Johnston, D.; Harris, D.; Caputi, N.; Thomson, A. Decline of a Blue Swimmer Crab (Portunus pelagicus) fishery in Western Australia—History, contributing factors and future management strategy. Fish. Res. 2011, 109, 119–130. [Google Scholar] [CrossRef]
- Jenkins, G.; Michael, R.; Tweedley, J.R. Identifying Future Stock Enhancement Options for Blue Swimmer Crabs (Portunus armatus); Project 2015/09; Recreational Fishing Initiatives Fund: Perth, Australia, 2017. [Google Scholar]
- Malseed, B.E.; Sumner, N.R. A 12-Month Survey of Recreational Fishing in the Swan-Canning Estuary Basin of Western Australia during 1998-99; Department of Fisheries, Western Australia: Perth, Australia, 2001; p. 44.
- Morison, A.; Daume, S.; Gardner, C.; Lack, M. Western Australia Peel Harvey Estuarine Fishery MSC Full Assessment Public Certification Report; SCS Global Services, Sustainable Seafood Program—Australasia: Victoria, Australia, 2016. [Google Scholar]
- DPIRD. Protecting Breeding Stock Levels of the Blue Swimmer Crab Resource in the South West; Department of Primary Industries and Regional Development: Perth, Australia, 2018; p. 39.
- Christensen, J.; Jones, R. World Heritage and local change: Conflict, transformation and scale at Shark Bay, Western Australia. J. Rural Stud. 2020, 74, 235–243. [Google Scholar] [CrossRef]
- Chandrapavan, A.; Caputi, N.; Kangas, M.I. The decline and recovery of a crab population from an extreme marine heatwave and a changing climate. Front. Mar. Sci. 2019, 6, 510. [Google Scholar] [CrossRef]
- Lindley, J.; Quinn, L. Perceptions of compliance in recreational fisheries: Case study of the Peel-Harvey blue swimmer crab fishery. Front. Conserv. Sci. 2022, 3, 968518. [Google Scholar] [CrossRef]
- Hoeksema, S.D.; Potter, I.C. Diel, seasonal, regional and annual variations in the characteristics of the ichthyofauna of the upper reaches of a large Australian microtidal estuary. Estuar. Coast. Shelf Sci. 2006, 67, 503–520. [Google Scholar] [CrossRef]
- Smallwood, C.B.; Sumner, N.R. Twelve-Month Survey of Recreational Estuarine Fishing in the South Coast Bioregion of Western Australia during 2002/03; Department of Fisheries, Western Australia: Perth, Australia, 2007; p. 56.
- Cottingham, A.; Hall, N.G.; Potter, I.C. Performance and contribution to commercial catches and egg production by restocked Acanthopagrus butcheri (Sparidae) in an estuary. Estuar. Coast. Shelf Sci. 2015, 164, 194–203. [Google Scholar] [CrossRef]
- Cottingham, A.; Hall, N.G.; Loneragan, N.R.; Jenkins, G.I.; Potter, I.C. Efficacy of restocking an estuarine-resident species demonstrated by long-term monitoring of cultured fish with alizarin complexone-stained otoliths. A case study. Fish. Res. 2020, 227, 105556. [Google Scholar] [CrossRef]
- Malseed, B.E.; Sumner, N.R. A 12-Month Survey of Recreational Fishing in the Peel-Harvey Estuary of Western Australia during 1998-99; Department of Fisheries, Western Australia: Perth, Australia, 2001; p. 52.
- Prior, S.; Beckley, L.E. Characteristics of recreational anglers in the Blackwood Estuary, a popular tourist destination in southwestern Australia. Tour. Mar. Environ. 2007, 4, 15–28. [Google Scholar] [CrossRef][Green Version]
- DEC. Walpole and Nornalup Inlets Marine Park Management Plan 2009–2019; Department of Environment and Conservation: Perth, Australia, 2009.
- DPIRD. Recreational Fishing Guide 2022; Department of Primary Industries and Regional Development: Perth, Australia, 2022; p. 64.
- Middlestadt, S.E.; Bhattacharyya, K.; Rosenbaum, J.; Fishbein, M.; Shepherd, M. The use of theory based semistructured elicitation questionnaires: Formative research for CDS’s prevention marketing initiative. Public Health Rep. 1996, 111, 18–27. [Google Scholar] [PubMed]
- Francis, J.; Eccles, M.; Johnston, M.; Walker, A.; Grimshaw, J.; Foy, R.; Kaner, E.; Smith, L.; Bonetti, D.; Francis, J.; et al. Constructing Questionnaires Based on the Theory of Planned Behaviour: A Manual for Health Services Researchers; Centre for Health Services Research, University of Newcastle upon Tyne: Newcastle-upon-Tyne, UK, 2004. [Google Scholar]
- Obregón, C.; Admiraal, R.; van Putten, I.; Hughes, M.; Tweedley, J.R.; Loneragan, N.R. Who you speak to matters: Information sharing and the management of a small-scale fishery. Front. Mar. Sci. 2020, 7, 578014. [Google Scholar] [CrossRef]
- Johnston, D.; Harris, D.; Yeoh, D. Blue Swimmer Crab (Portunus armatus) Resource in the West Coast Bioregion, Western Australia Part 2: Warnbro Sound, Comet Bay, Mandurah to Bunbury, Leschenault Estuary, Geographe Bay and Hardy Inlet; Department of Primary Indistrues and Regional Development: Perth, Australia, 2020.
- Kennish, M.J. Environmental threats and environmental future of estuaries. Environ. Conserv. 2002, 29, 78–107. [Google Scholar] [CrossRef]
- Tweedley, J.R.; Warwick, R.M.; Potter, I.C. Can biotic indicators distinguish between natural and anthropogenic environmental stress in estuaries? J. Sea Res. 2015, 102, 10–21. [Google Scholar] [CrossRef]
- Brearley, A. Ernest Hodgkin’s Swanland, 1st ed.; University of Western Australia Press: Perth, Australia, 2005; p. 550. [Google Scholar]
- Potter, I.C.; Rose, T.H.; Huisman, J.M.; Hall, N.G.; Denham, A.; Tweedley, J.R. Large variations in eutrophication among estuaries reflect massive differences in composition and biomass of macroalgal drift. Mar. Pollut. Bull. 2021, 167, 112330. [Google Scholar] [CrossRef]
- Lepesteur, M.; Wegner, A.; Moore, S.A.; McComb, A. Importance of public information and perception for managing recreational activities in the Peel-Harvey estuary, Western Australia. J. Environ. Manag. 2008, 87, 389–395. [Google Scholar] [CrossRef]
- Ryan, K.L.; Shaw, J.; Tracey, S.R.; Lyle, J.M. Recreational fishers’ perceptions of climate change. ICES J. Mar. Sci. 2021, 79, 540–551. [Google Scholar] [CrossRef]
- Leviston, Z.; Greenhill, M.; Walker, I. Australians Attitudes to Climate Change and Adaptation: 2010–2014; CSIRO: Canberra, Australia, 2015.
- Coulson, P.G.; Leary, T.; Chandrapavan, A.; Wakefield, C.B.; Newman, S.J. Going with the flow: The case of three tropical reef fish transported to cool temperate waters following an extreme marine heatwave. Reg. Stud. Mar. Sci. 2023, 61, 102856. [Google Scholar] [CrossRef]
- Pauly, D. The gill-oxygen limitation theory (GOLT) and its critics. Sci. Adv. 2021, 7, eabc6050. [Google Scholar] [CrossRef] [PubMed]
- Asch, R.G.; Stock, C.A.; Sarmiento, J.L. Climate change impacts on mismatches between phytoplankton blooms and fish spawning phenology. Glob. Change Biol. 2019, 25, 2544–2559. [Google Scholar] [CrossRef] [PubMed]
- Valesini, F.J.; Cottingham, A.; Hallett, C.S.; Clarke, K.R. Interdecadal changes in the community, population and individual levels of the fish fauna of an extensively modified estuary. J. Fish Biol. 2017, 90, 1734–1767. [Google Scholar] [CrossRef]
- Elliott, M.; Houde, E.D.; Lamberth, S.J.; Lonsdale, J.-A.; Tweedley, J.R. Management of fishes and fisheries in estuaries. Fish Fish. Estuaries 2022, 2, 706–797. [Google Scholar]
- Rubio, G.; Brinson, A.; Wallmo, K. Attitudes and Preferences of Saltwater Recreational Anglers: Report from the 2013 National Saltwater Angler Survey, Volume II Regional Analysis; National Oceanic and Atmospheric Administration: Washington, DC, USA, 2014; p. 115.
- Wilde, G.R.; Ditton, R.B. Diversity among anglers in support for fisheries management tools. In Proceedings of the Warmwater Fisheries Symposium 1, Scottsdale, Arizona, 4–8 June 1991; pp. 329–335. [Google Scholar]
- Renyard, T.S.; Hilborn, R. Sports angler preferences for alternative regulatory methods. Can. J. Fish. Aquat. Sci. 1986, 43, 240–242. [Google Scholar] [CrossRef]
- Helfrich, L.; Chipman, B.; Kauffman, J. Profiles of Shenandoah River anglers fishing under three Black Bass length limit regulations. Proc. Annu. Conf. Southeast. Assoc. Fish Wildl. Agencies 1987, 41, 178–186. [Google Scholar]
- FAO. Recreational Fisheries; Food and Agriculture Organization of the United Nations: Rome, Italy, 2012; p. 176. [Google Scholar]
- Lindley, J.; Quinn, L. Compliance in recreational fisheries: Case study of two blue swimmer crab fisheries. PLoS ONE 2023, 18, e0279600. [Google Scholar] [CrossRef]
- McNeill, A.; Clifton, J.; Harvey, E.S. Specialised recreational fishers reject sanctuary zones and favour fisheries management. Mar. Policy 2019, 107, 103592. [Google Scholar] [CrossRef]
- Cottingham, A.; Hall, N.G.; Hesp, S.A.; Potter, I.C. Differential changes in production measures for an estuarine-resident sparid in deep and shallow waters following increases in hypoxia. Estuar. Coast. Shelf Sci. 2018, 202, 155–163. [Google Scholar] [CrossRef]
- Klefoth, T.; Wegener, N.; Meyerhoff, J.; Arlinghaus, R. Do anglers and managers think similarly about stocking, habitat management and harvest regulations? Implications for the management of community-governed recreational fisheries. Fish. Res. 2023, 260, 106589. [Google Scholar] [CrossRef]
- Muthén, B.; Muthén, L.K. Integrating person-centered and variable-centered analyses: Growth mixture modeling with latent trajectory classes. Alcohol Clin. Exp. Res. 2000, 24, 882–891. [Google Scholar] [CrossRef] [PubMed]
- Obregón, C.; Hughes, M.; Loneragan, N.R.; Poulton, S.J.; Tweedley, J.R. A two-phase approach to elicit and measure beliefs on management strategies: Fishers supportive and aware of trade-offs associated with stock enhancement. Ambio 2020, 49, 640–649. [Google Scholar] [CrossRef]
- Arlinghaus, R. Overcoming human obstacles to conservation of recreational fishery resources, with emphasis on central Europe. Environ. Conserv. 2006, 33, 46–59. [Google Scholar] [CrossRef]
- Hunt, L.M.; Gonder, D.; Haider, W. Hearing voices from the silent majority: A comparison of preferred fish stocking outcomes for Lake Huron by anglers from representative and convenience samples. Hum. Dimens. Wildl. 2010, 15, 27–44. [Google Scholar] [CrossRef]
- Jenkins, G.I.; Michael, R.; Tweedley, J.R.; Oberstein, D.; Loneragan, N.R.; Johnston, D.J. Blue Swimmer Crab (Portunus armatus) Stocking Trial: Reducing Costs and Increasing Survival; Project 2016/03; Recreational Fishing Initiatives Fund: Perth, Australia, 2018. [Google Scholar]
- Jenkins, G.I.; French, D.J.W.; Potter, I.C.; de Lestang, S.; Hall, N.G.; Partridge, G.J.; Hesp, S.A.; Sarre, G.A. Restocking the Blackwood River Estuary with the Black Bream Acanthopagrus butcheri; Fisheries Research and Development: Perth, Australia, 2006. [Google Scholar]
- Arlinghaus, R.; Beardmore, B.; Riepe, C.; Meyerhoff, J.; Pagel, T. Species-specific preferences of German recreational anglers for freshwater fishing experiences, with emphasis on the intrinsic utilities of fish stocking and wild fishes. J. Fish Biol. 2014, 85, 1843–1867. [Google Scholar] [CrossRef] [PubMed]
- Molony, B. Environmental Requirements and Tolerances of Rainbow Trout (Oncorhynchus mykiss) and Brown Trout (Salmo trutta) with Special Reference to Western Australia: A Review; Department of Fisheries, Western Australia: Perth, Australia, 2001; p. 28.
- van Poorten, B.T.; Arlinghaus, R.; Daedlow, K.; Haertel-Borer, S.S. Social-ecological interactions, management panaceas, and the future of wild fish populations. Proc. Natl. Acad. Sci. USA 2011, 108, 12554–12559. [Google Scholar] [CrossRef]
- Hilborn, R. The economic performance of marine stock enhancement projects. Bull. Mar. Sci. 1998, 62, 661–674. [Google Scholar]
- Camp, E.V.; Larkin, S.L.; Ahrens, R.N.M.; Lorenzen, K. Trade-offs between socioeconomic and conservation management objectives in stock enhancement of marine recreational fisheries. Fish. Res. 2017, 186, 446–459. [Google Scholar] [CrossRef]
- Lorenzen, K.; Beveridge, M.C.M.; Mangel, M. Cultured fish: Integrative biology and management of domestication and interactions with wild fish. Biol. Rev. 2012, 87, 639–660. [Google Scholar] [CrossRef]
- Tweedley, J.R.; Obregón, C.; Hughes, M.; Loneragan, N.R.; Cottingham, A.; Abagna, D.; Tull, M.; Beukes, S.J.; Garnett, A.M. Golden Fish: Evaluating and Optimising the Biological, Social and Economic Returns of Small-Scale Fisheries; Murdoch University: Perth, Australia, 2023. [Google Scholar]
- Dabrowska, K.; Haider, W.; Hunt, L. Examining the impact of fisheries resources and quality on licence sales. J. Outdoor Recreat. Tour. 2014, 5–6, 58–67. [Google Scholar] [CrossRef]
- Tweedley, J.R.; Loneragan, N.R.; Crisp, J.A.; Poh, B.; Broadley, A.D.; Bennett, A.L.; Hodson, K.P.; Trayler, K.M.; Jenkins, G.I.; Chaplin, J.A. Stock Enhancement of the Western School Prawn (Metapenaeus dalli) in the Swan-Canning Estuary; Evaluating Recruitment Limitation, Environment and Release Strategies; Murdoch University: Perth, Australia, 2017. [Google Scholar]
- Salas, S.; Gaertner, D. The behavioural dynamics of fishers: Management implications. Fish Fish. 2004, 5, 153–167. [Google Scholar] [CrossRef]



| Fisher Group | Name | Description |
|---|---|---|
| (a) Blue Swimmer Crab | ||
| a | Very frequent fishers | Fishers that fish > 150 times per year and of intermediate/expert skill level |
| b | Experienced boat-based fishers | Fishers that have fished for >40 years, primarily from a boat using drop nets |
| c | Inexperienced boat-based fishers | Fishers that have fished for ~10 years, primarily from a boat using drop nets |
| d | Relatively experienced, expert shore-based fishers | Expert fishers that have fished for ~25 years, primarily from the shore, using a range of methods |
| e | Inexperienced shore-based drop net fishers | Fishers that have fished for ~7 years, primarily from a shore using drop nets |
| f | Inexperienced, shore-based novice fishers | Novice fishers that have fished for <10 years, primarily from a shore, using a range of methods |
| g | Bi-monthly, hand fishers | Fishers that fish every two months and catch their crabs by hand |
| (b) Black Bream | ||
| a | Very frequent fishers | Fishers that fish >150 times per year and of intermediate skill level |
| b | Very frequent, expert lure fishers | Fishers that fish >150 times per year are of expert skill level; they use expensive fishing gear and lures and fish in competitions |
| c | Experienced fishers | Fishers that have fished for ~40 years, primarily from a kayak/boat and of intermediate/expert skill, some of whom fish in competitions |
| d | Inexperienced intermediate-skilled fishers | Intermediate skills fishers that have fished for <10 years, primarily from a kayak using relatively cheap gear, and who do not enter competitions |
| e | Inexperienced but keen fishers | Intermediate/expert fishers who have fished for <10 years, use expensive gear, and fish in competitions |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tweedley, J.R.; Obregón, C.; Beukes, S.J.; Loneragan, N.R.; Hughes, M. Selecting from the Fisheries Managers’ Tool-Box: Recreational Fishers’ Views of Stock Enhancement and Other Management Options. Fishes 2023, 8, 460. https://doi.org/10.3390/fishes8090460
Tweedley JR, Obregón C, Beukes SJ, Loneragan NR, Hughes M. Selecting from the Fisheries Managers’ Tool-Box: Recreational Fishers’ Views of Stock Enhancement and Other Management Options. Fishes. 2023; 8(9):460. https://doi.org/10.3390/fishes8090460
Chicago/Turabian StyleTweedley, James R., Clara Obregón, Sarah J. Beukes, Neil R. Loneragan, and Michael Hughes. 2023. "Selecting from the Fisheries Managers’ Tool-Box: Recreational Fishers’ Views of Stock Enhancement and Other Management Options" Fishes 8, no. 9: 460. https://doi.org/10.3390/fishes8090460
APA StyleTweedley, J. R., Obregón, C., Beukes, S. J., Loneragan, N. R., & Hughes, M. (2023). Selecting from the Fisheries Managers’ Tool-Box: Recreational Fishers’ Views of Stock Enhancement and Other Management Options. Fishes, 8(9), 460. https://doi.org/10.3390/fishes8090460









