A Comparison of Three Protein Sources Used in Medium-Sized Litopenaeus vannamei: Effects on Growth, Immunity, Intestinal Digestive Enzyme Activity, and Microbiota Structure
Abstract
:1. Introduction
2. Materials and Methods
2.1. Diets Preparation
2.2. Feeding Trial
2.3. Sample Collection
2.4. Growth Performance Analysis
2.5. Analysis of Non-Specific Immunometric Indices in the Gill and Intestinal Digestive Enzymes Activities
2.6. Intestinal Microbial Analysis
2.7. Statistical Analysis
3. Results
3.1. Growth Performance and Feed Utilization
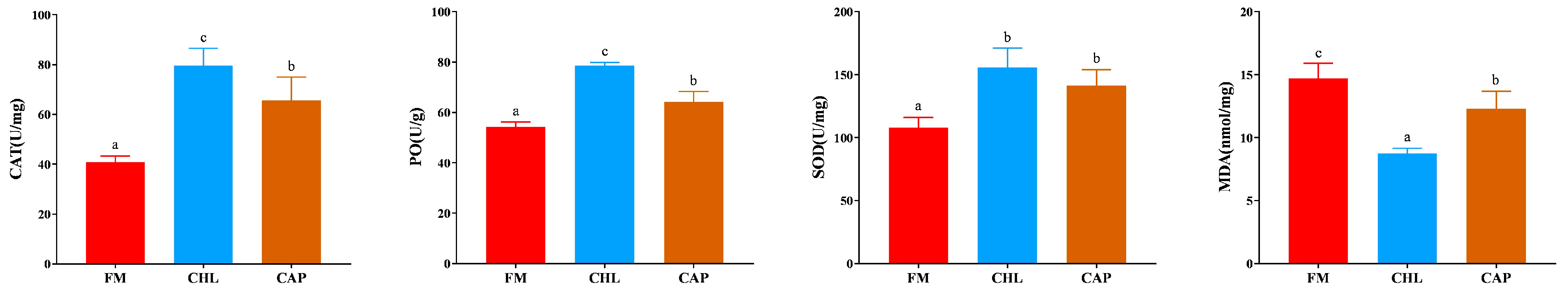
3.2. Non-Specific Immune Indices in the Gills
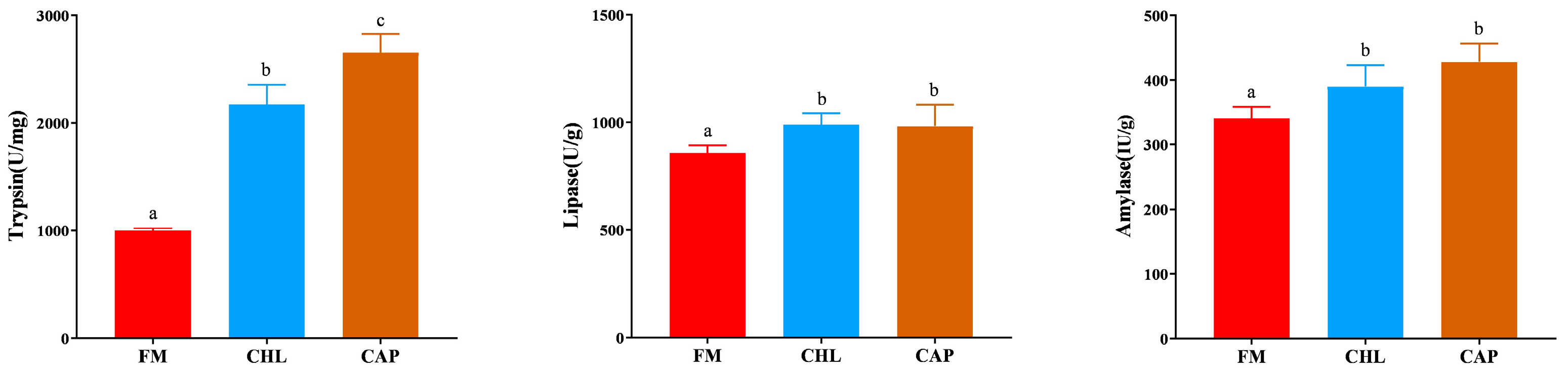
3.3. Digestive Enzyme Activities in the Intestine
3.4. Intestinal Microbiota Analysis
3.4.1. Richness and Diversity Analysis
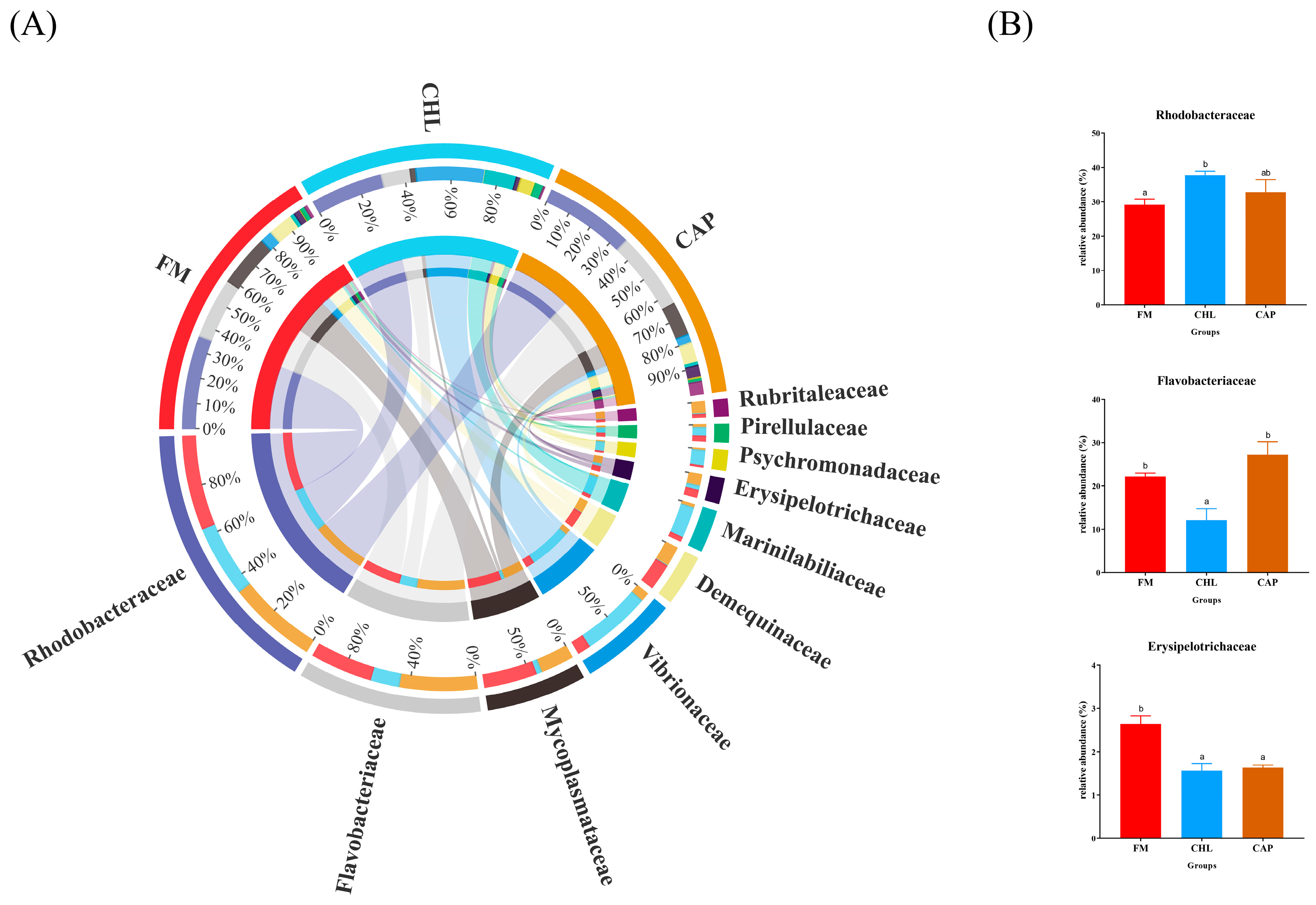
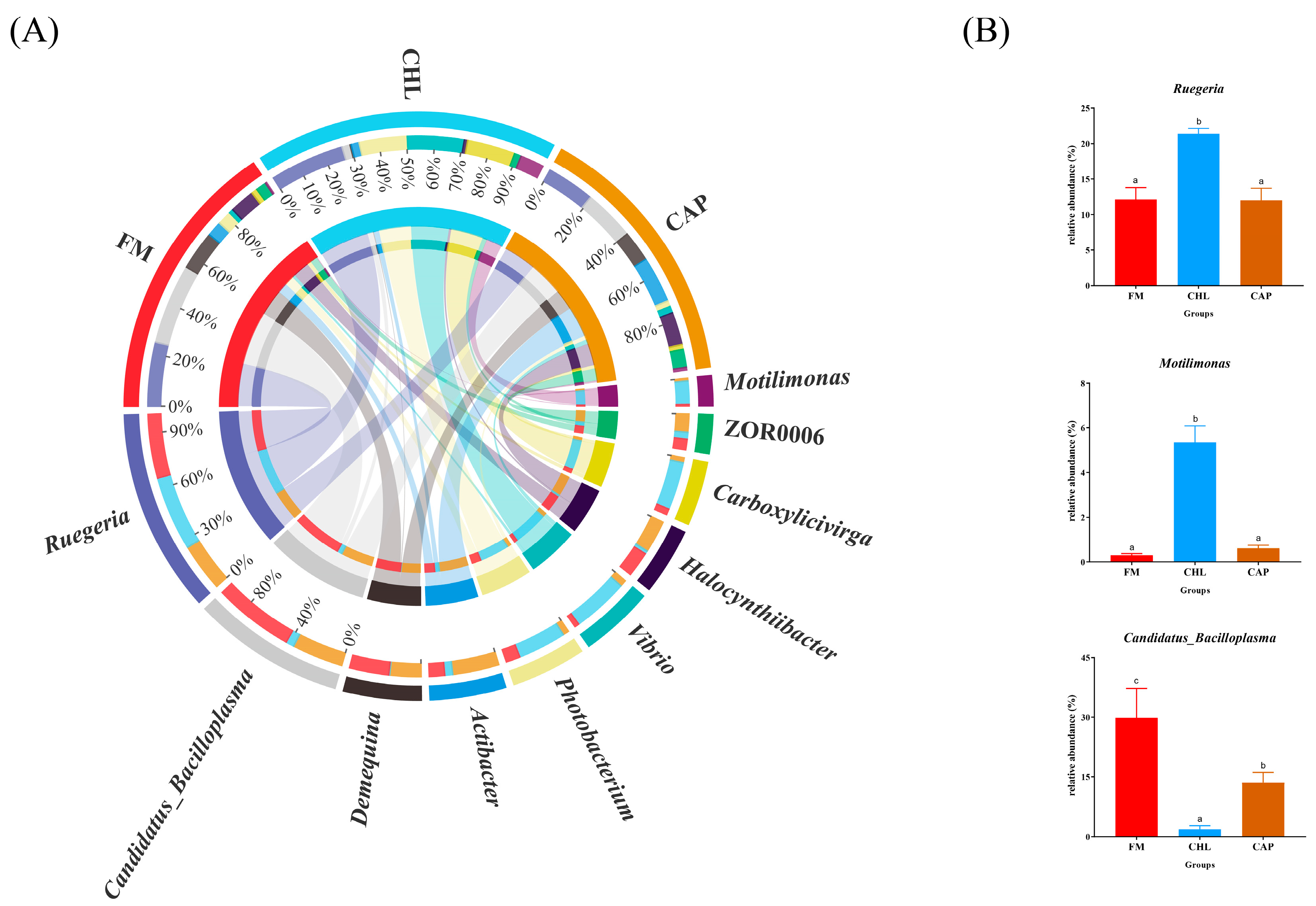
3.4.2. Comparison of the Intestinal Microbiota Composition
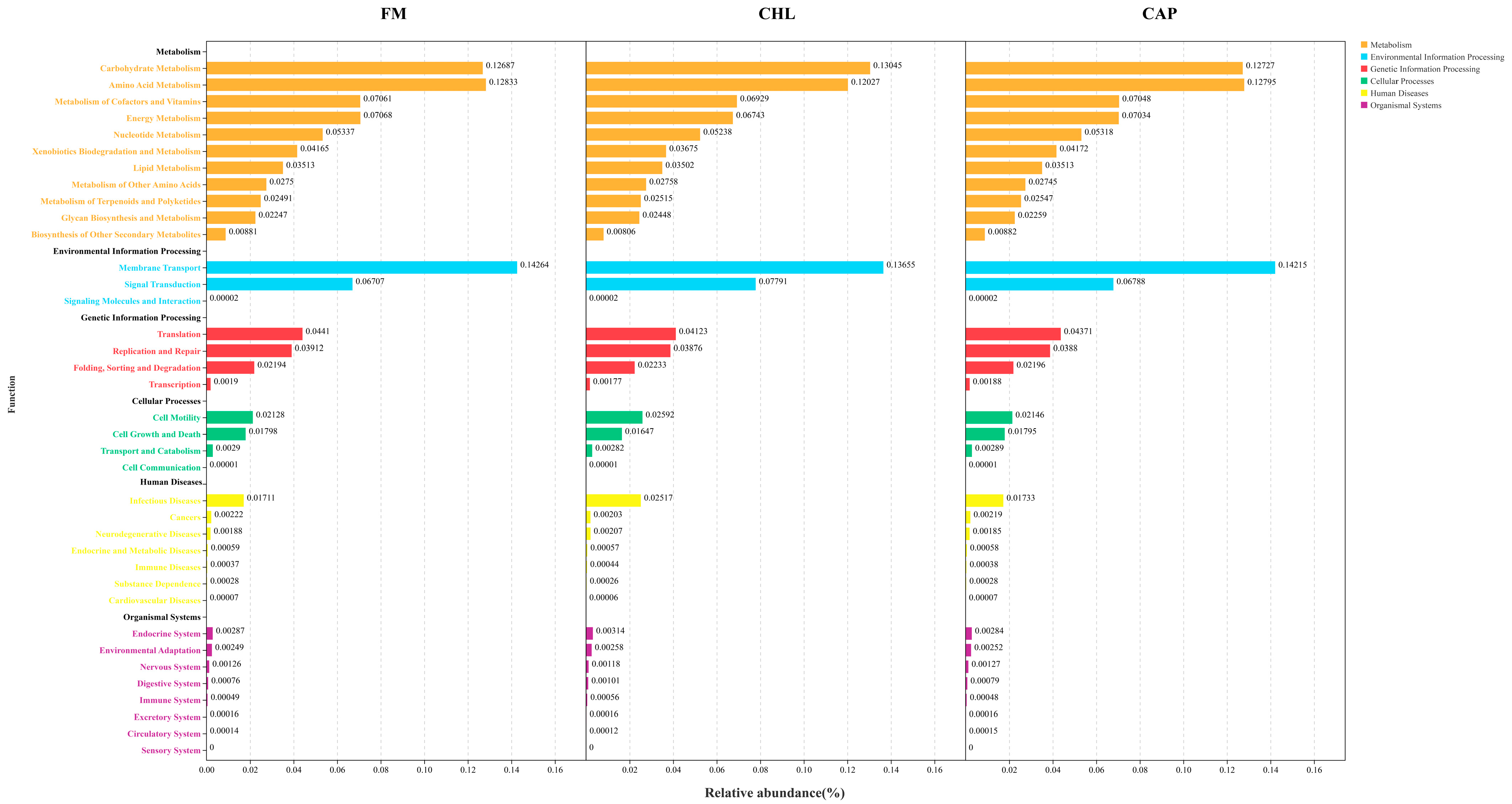
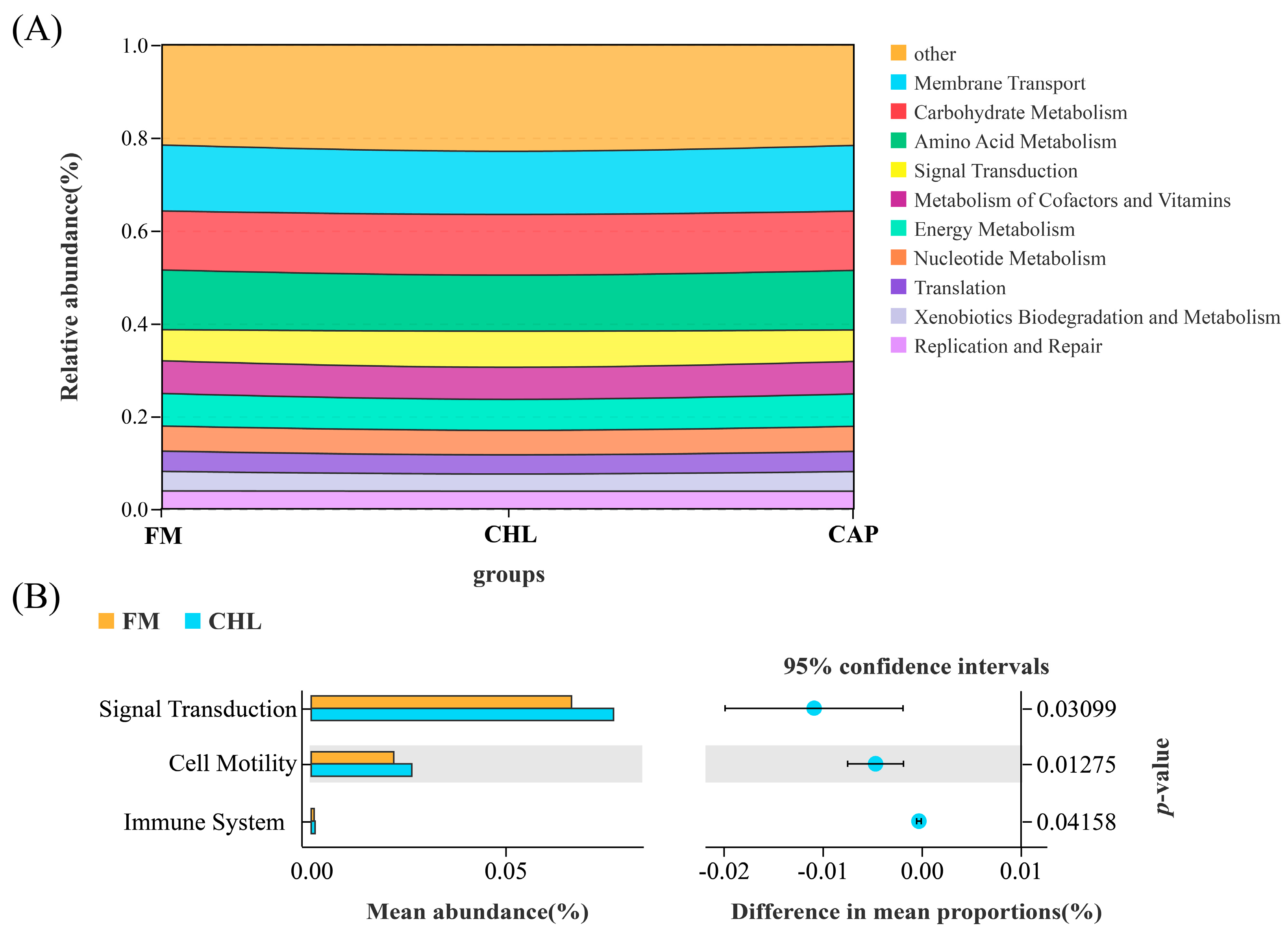
3.4.3. Functional Prediction of the Intestinal Microbial Community
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, S.W.; Tian, L.X.; Jin, Y.; Yang, H.J.; Liang, G.Y.; Liu, Y.J. Effect of Glycine Supplementation on Growth Performance, Body Composition and Salinity Stress of Juvenile Pacific White Shrimp, Litopenaeus vannamei Fed Low Fishmeal Diet. Aquaculture 2014, 418–419, 159–164. [Google Scholar] [CrossRef]
- Olsen, R.L.; Hasan, M.R. A Limited Supply of Fishmeal: Impact on Future Increases in Global Aquaculture Production. Trends Food Sci. Technol. 2014, 27, 120–128. [Google Scholar] [CrossRef]
- Jobling, M.; Jobling, M. National Research Council (NRC): Nutrient Requirements of Fish and Shrimp. Aquac. Int. 2011, 20, 601–602. [Google Scholar] [CrossRef]
- Hardy, R.W. Utilization of Plant Proteins in Fish Diets: Effects of Global Demand and Supplies of Fishmeal. Aquac. Res. 2010, 41, 770–776. [Google Scholar] [CrossRef]
- Li, X.; Zhang, X.; Yao, W.; Leng, X. Dietary Effects of Cottonseed Protein Concentrate Substituting Fishmeal on the Growth and Flesh Quality of Pacific White Shrimp (Litopenaeus vannamei). Aquac. Rep. 2023, 29, 101482. [Google Scholar] [CrossRef]
- Bauer, W.; Prentice-Hernandez, C.; Tesser, M.B.; Wasielesky, W.; Poersch, L.H.S. Substitution of Fishmeal with Microbial Floc Meal and Soy Protein Concentrate in Diets for the Pacific White Shrimp Litopenaeus vannamei. Aquaculture 2012, 342–343, 112–116. [Google Scholar] [CrossRef]
- Forster, I.P.; Dominy, W.; Obaldo, L.; Tacon, A.G.J. Rendered Meat and Bone Meals as Ingredients of Diets for Shrimp Litopenaeus vannamei (Boone, 1931). Aquaculture 2003, 219, 655–670. [Google Scholar] [CrossRef]
- Zheng, Y.; Hou, C.; Chen, J.; Wang, H.; Yuan, H.; Hu, N.; Shi, L.; Zhang, S. Integrating Microbiome and Transcriptome Analyses to Understand the Effect of Replacing Fishmeal with Tenebrio molitor Meal in Pacific White Shrimp (Litopenaeus vannamei) Diets. Aquaculture 2023, 575, 739818. [Google Scholar] [CrossRef]
- Motte, C.; Rios, A.; Lefebvre, T.; Do, H.; Henry, M.; Jintasataporn, O. Replacing Fish Meal with Defatted Insect Meal (Yellow Mealworm Tenebrio molitor) Improves the Growth and Immunity of Pacific White Shrimp (Litopenaeus vannamei). Animals 2019, 9, 258. [Google Scholar] [CrossRef]
- Mendoza, R.; De Dios, A.; Vazquez, C.; Cruz, E.; Ricque, D.; Aguilera, C.; Montemayor, J. Fishmeal Replacement with Feather-Enzymatic Hydrolyzates Co-Extruded with Soya-Bean Meal in Practical Diets for the Pacific White Shrimp (Litopenaeus vannamei). Aquac. Nutr. 2001, 7, 143–151. [Google Scholar] [CrossRef]
- Han, F.; Qian, J.; Qu, Y.; Li, Z.; Chen, H.; Xu, C.; Zhang, H.; Qin, J.G.; Chen, L.; Li, E. Partial Replacement of Soybean Meal with Fermented Cottonseed Meal in a Low Fishmeal Diet Improves the Growth, Digestion and Intestinal Microbiota of Juvenile White Shrimp Litopenaeus vannamei. Aquac. Rep. 2022, 27, 101339. [Google Scholar] [CrossRef]
- Yao, W.; Zhang, C.; Li, X.; He, M.; Wang, J.; Leng, X. The Replacement of Fish Meal with Fermented Soya Bean Meal or Soya Bean Meal in the Diet of Pacific White Shrimp (Litopenaeus vannamei). Aquac. Res. 2020, 51, 2400–2409. [Google Scholar] [CrossRef]
- Van Nguyen, N.; Hoang, L.; Van Khanh, T.; Duy Hai, P.; Hung, L.T. Utilization of Fermented Soybean Meal for Fishmeal Substitution in Diets of Pacific White Shrimp (Litopenaeus vannamei). Aquac. Nutr. 2018, 24, 1092–1100. [Google Scholar] [CrossRef]
- Enyidi, U.; Pirhonen, J.; Vielma, J. Effects of Sesame Seed Meal and Bambaranut Meal on Growth, Feed Utilization and Body Composition of Juvenile African Catfish Clarias gariepinus. Iran. J. Fish. Sci. 2014, 13, 998–1013. [Google Scholar]
- Li, P.; Mai, K.; Trushenski, J.; Wu, G. New Developments in Fish Amino Acid Nutrition: Towards Functional and Environmentally Oriented Aquafeeds. Amino Acids 2009, 37, 43–53. [Google Scholar] [CrossRef]
- Bureau, D.P.; Harris, A.M.; Bevan, D.J.; Simmons, L.A.; Azevedo, P.A.; Cho, C.Y. Feather Meals and Meat and Bone Meals from Different Origins as Protein Sources in Rainbow Trout (Oncorhynchus mykiss) Diets. Aquaculture 2000, 181, 281–291. [Google Scholar] [CrossRef]
- Carneiro, W.F.; Castro, T.F.D.; Orlando, T.M.; Meurer, F.; de Jesus Paula, D.A.; Virote, B.D.C.R.; Murgas, L.D.S. Replacing Fish Meal by Chlorella sp. Meal: Effects on Zebrafish Growth, Reproductive Performance, Biochemical Parameters and Digestive Enzymes. Aquaculture 2020, 528, 735612. [Google Scholar] [CrossRef]
- Xu, W.; Gao, Z.; Qi, Z.; Qiu, M.; Peng, J.Q.; Shao, R. Effect of Dietary Chlorella on the Growth Performance and Physiological Parameters of Gibel Carp, Carassius auratus gibelio. Turk. J. Fish. Aquat. Sci. 2014, 234, 234–253. [Google Scholar] [CrossRef]
- Dineshbabu, G.; Goswami, G.; Kumar, R.; Sinha, A.; Das, D. Microalgae–Nutritious, Sustainable Aqua- and Animal Feed Source. J. Funct. Foods 2019, 62, 103545. [Google Scholar] [CrossRef]
- Vuppaladadiyam, A.K.; Prinsen, P.; Raheem, A.; Luque, R.; Zhao, M. Microalgae Cultivation and Metabolites Production: A Comprehensive Review. Biofuels Bioprod. Biorefining 2018, 12, 304–324. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, X.; Wang, Y.; Xu, H.; Wang, S. Establishment and Characterization of a Outdoor Open Culture System for Microalgae Chlorella sorokiniana. J. Guangdong Ocean Univ. 2021, 41, 33–40. [Google Scholar] [CrossRef]
- Chen, Y.; Sagada, G.; Xu, B.; Chao, W.; Zou, F.; Ng, W.K.; Sun, Y.; Wang, L.; Zhong, Z.; Shao, Q. Partial Replacement of Fishmeal with Clostridium autoethanogenum Single-Cell Protein in the Diet for Juvenile Black Sea Bream (Acanthopagrus schlegelii). Aquac. Res. 2020, 51, 1000–1011. [Google Scholar] [CrossRef]
- Wei, H.; Yu, H.; Chen, X.; Chao, W.; Zou, F.; Chen, P.; Zhang, Y.; Wu, X.; Liang, X.; Xue, M. Effects of Soybean Meal Replaced by Clostridium autoethanogenum on Growth Performance, Plasma Biochemical Indexes and Hepatopancreas and Intestinal Histopathology of Grass Carp (Ctenopharyngodon idllus). Chin. J. Anim. Nutr. 2018, 30, 4190–4201. [Google Scholar] [CrossRef]
- Bulynina, S.S.; Ziganshina, E.E.; Ziganshin, A.M. Growth Efficiency of Chlorella sorokiniana in Synthetic Media and Unsterilized Domestic Wastewater. BioTech 2023, 12, 53. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Lu, Q.; Fan, L.; Zhou, W. A Review on the Use of Microalgae for Sustainable Aquaculture. Appl. Sci. 2019, 9, 2377. [Google Scholar] [CrossRef]
- Lai, Y.C.; Chang, C.H.; Chen, C.Y.; Chang, J.S.; Ng, I.S. Towards Protein Production and Application by Using Chlorella species as Circular Economy. Bioresour. Technol. 2019, 289, 121625. [Google Scholar] [CrossRef]
- Ma, S.; Wang, H.; Yang, J.; Liang, X.; Xue, M.; Cheng, H. Effect of Process Parameters on the Physical Quality of Low-Starch Extruded Feed Containing Clostridium autoethanogenum Protein. Anim. Feed Sci. Technol. 2023, 304, 115745. [Google Scholar] [CrossRef]
- Maulu, S.; Hualiang, L.; Ke, J.; Ren, M.; Ge, X.; Huang, D.; Yu, H. Dietary Clostridium autoethanogenum Protein Modulates Intestinal Absorption, Antioxidant Status, and Immune Response in GIFT (Oreochromis niloticus) Juveniles. Aquac. Res. 2021, 52, 5787–5799. [Google Scholar] [CrossRef]
- Raji, A.A.; Junaid, O.Q.; Milow, P.; Taufek, N.M.; Fada, A.M.; Kolawole, A.A.; Alias, Z.; Razak, S.A. Partial Replacement of Fishmeal with Spirulina platensis and Chlorella vulgaris and Its Effect on Growth and Body Composition of African Catfish Clarias gariepinus (Burchell 1822). Indian J. Fish. 2019, 66, 100–111. [Google Scholar] [CrossRef]
- Xi, L.; Lu, Q.; Liu, Y.; Su, J.; Chen, W.; Gong, Y.; Han, D.; Yang, Y.; Zhang, Z.; Jin, J.; et al. Effects of Fish Meal Replacement with Chlorella Meal on Growth Performance, Pigmentation, and Liver Health of Largemouth Bass (Micropterus salmoides). Anim. Nutr. 2022, 10, 26–40. [Google Scholar] [CrossRef]
- Maliwat, G.C.; Velasquez, S.; Robil, J.L.; Chan, M.; Traifalgar, R.F.; Tayamen, M.; Ragaza, J.A. Growth and Immune Response of Giant Freshwater Prawn Macrobrachium rosenbergii (De Man) Postlarvae Fed Diets Containing Chlorella vulgaris (Beijerinck). Aquac. Res. 2017, 48, 1666–1676. [Google Scholar] [CrossRef]
- Shi, L.; Chan, S.; Li, C.; Zhang, S. Identification and Characterization of a Laccase from Litopenaeus vannamei Involved in Anti-Bacterial Host Defense. Fish Shellfish Immunol. 2017, 66, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liang, H.; Xie, J.; Chao, W.; Zou, F.; Ge, X.; Ren, M. Diet Supplemented with a Novel Clostridium autoethanogenum Protein Have a Positive Effect on the Growth Performance, Antioxidant Status and Immunity in Juvenile Jian Carp (Cyprinus carpio var. Jian). Aquac. Rep. 2021, 19, 100572. [Google Scholar] [CrossRef]
- Dai, J.; Chen, T.; Guo, X.; Dai, Z.; He, Z.; Hu, Y. Evaluation of Fish Meal Replacement by Clostridium autoethanogenum Protein in Diets for Juvenile Red Swamp Crayfish (Procambarus clrkii). Aquaculture 2023, 570, 739379. [Google Scholar] [CrossRef]
- Xu, Z.; Li, M.; Wang, Y.; Feng, M.; Gan, Z.; Leng, X.; Li, X. Affecting Mechanism of Chlorella sorokiniana Meal Replacing Fish Meal on Growth and Immunity of Litopenaeus vannamei Based on Transcriptome Analysis. Aquac. Rep. 2023, 31, 101645. [Google Scholar] [CrossRef]
- Yao, W.; Yang, P.; Zhang, X.; Xu, X.; Zhang, C.; Li, X.; Leng, X. Effects of Replacing Dietary Fish Meal with Clostridium autoethanogenum Protein on Growth and Flesh Quality of Pacific White Shrimp (Litopenaeus vannamei). Aquaculture 2022, 549, 737770. [Google Scholar] [CrossRef]
- Jiang, X.; Yao, W.; Yang, H.; Tan, S.; Leng, X.; Li, X. Dietary Effects of Clostridium autoethanogenum Protein Substituting Fish Meal on Growth, Intestinal Histology and Immunity of Pacific White Shrimp (Litopenaeus vannamei) Based on Transcriptome Analysis. Fish Shellfish Immunol. 2021, 119, 635–644. [Google Scholar] [CrossRef]
- Yadav, G.; Meena, D.K.; Sahoo, A.K.; Das, B.K.; Sen, R. Effective Valorization of Microalgal Biomass for the Production of Nutritional Fish-Feed Supplements. J. Clean. Prod. 2020, 243, 118697. [Google Scholar] [CrossRef]
- Jayant, M.; Hassan, M.A.; Srivastava, P.P.; Meena, D.K.; Kumar, P.; Kumar, A.; Wagde, M.S. Brewer’s Spent Grains (BSGs) as Feedstuff for Striped Catfish, Pangasianodon hypophthalmus fingerlings: An Approach to Transform Waste into Wealth. J. Clean. Prod. 2018, 199, 716–722. [Google Scholar] [CrossRef]
- Macias-Sancho, J.; Poersch, L.H.; Bauer, W.; Romano, L.A.; Wasielesky, W.; Tesser, M.B. Fishmeal Substitution with Arthrospira (Spirulina platensis) in a Practical Diet for Litopenaeus vannamei: Effects on Growth and Immunological Parameters. Aquaculture 2014, 426–427, 120–125. [Google Scholar] [CrossRef]
- Ju, Z.Y.; Deng, D.F.; Dominy, W.A. Defatted Microalgae (Haematococcus pluvialis) Meal as a Protein Ingredient to Partially Replace Fishmeal in Diets of Pacific White Shrimp (Litopenaeus vannamei, Boone, 1931). Aquaculture 2012, 354–355, 50–55. [Google Scholar] [CrossRef]
- Liao, Z.; Gong, Y.; Zhao, W.; He, X.; Wei, D.; Niu, J. Comparison Effect of Rhodobacter sphaeroides Protein Replace Fishmeal on Growth Performance, Intestinal Morphology, Hepatic Antioxidant Capacity and Immune Gene Expression of Litopenaeus vannamei under Low Salt Stress. Aquaculture 2022, 547, 737488. [Google Scholar] [CrossRef]
- Aas, T.S.; Hatlen, B.; Grisdale-Helland, B.; Terjesen, B.F.; Bakke-McKellep, A.M.; Helland, S.J. Effects of Diets Containing a Bacterial Protein Meal on Growth and Feed Utilisation in Rainbow Trout (Oncorhynchus mykiss). Aquaculture 2006, 261, 357–368. [Google Scholar] [CrossRef]
- Murray, A.P.; Marchant, R. Nitrogen Utilization in Rainbow Trout Fingerlings (Salmo gairdneri richardson) Fed Mixed Microbial Biomass. Aquaculture 1986, 54, 263–275. [Google Scholar] [CrossRef]
- Rumsey, G.L.; Hughes, S.G.; Smith, R.R.; Kinsella, J.E.; Shetty, K.J. Digestibility and Energy Values of Intact, Disrupted and Extracts from Brewer’s Dried Yeast Fed to Rainbow Trout (Oncorhynchus mykiss). Anim. Feed Sci. Technol. 1991, 33, 185–193. [Google Scholar] [CrossRef]
- Yuan, H.; Xie, M.; Hu, N.; Zheng, Y.; Hou, C.; Tan, B.; Shi, L.; Zhang, S. Growth, Immunity and Transcriptome Response to Different Stocking Densities in Litopenaeus vannamei. Fish Shellfish Immunol. 2023, 139, 108924. [Google Scholar] [CrossRef]
- Xu, S.L.; Wang, D.L.; Jia, C.Y.; Jin, S.; Wang, C.L.; Zou, X. Effects of Vibrio alginolyticus Infection on Immune-Related Enzyme Activities and Ultrastructure of Charybdis japonica Gills. Aquaculture 2013, 396–399, 82–88. [Google Scholar] [CrossRef]
- Sahin, K.; Yazlak, H.; Orhan, C.; Tuzcu, M.; Akdemir, F.; Sahin, N. The Effect of Lycopene on Antioxidant Status in Rainbow Trout (Oncorhynchus mykiss) Reared under High Stocking Density. Aquaculture 2014, 418–419, 132–138. [Google Scholar] [CrossRef]
- Krupesha Sharma, S.R.; Rathore, G.; Verma, D.K.; Sadhu, N.; Philipose, K.K. Vibrio alginolyticus Infection in Asian Seabass (Lates calcarifer, Bloch) Reared in Open Sea Floating Cages in India. Aquac. Res. 2012, 44, 86–92. [Google Scholar] [CrossRef]
- Xu, Z.; Li, T.; Li, E.; Chen, K.; Ding, Z.; Qin, J.G.; Chen, L.; Ye, J. Comparative Transcriptome Analysis Reveals Molecular Strategies of Oriental River Prawn Macrobrachium nipponense in Response to Acute and Chronic Nitrite Stress. Fish Shellfish Immunol. 2015, 134, 121312. [Google Scholar] [CrossRef] [PubMed]
- Raji, A.A.; Alaba, P.A.; Yusuf, H.; Abu Bakar, N.H.; Mohd Taufek, N.; Muin, H.; Alias, Z.; Milow, P.; Abdul Razak, S. Fishmeal Replacement with Spirulina platensis and Chlorella vulgaris in African Catfish (Clarias gariepinus) Diet: Effect on Antioxidant Enzyme Activities and Haematological Parameters. Res. Vet. Sci. 2018, 119, 67–75. [Google Scholar] [CrossRef]
- Bito, T.; Okumura, E.; Fujishima, M.; Watanabe, F. Potential of Chlorella as a Dietary Supplement to Promote Human Health. Nutrients 2020, 12, 2524. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.T.; Shariff, M.; Yusoff, F.M.; Goh, Y.M.; Banerjee, S. Applications of Microalga Chlorella vulgaris in Aquaculture. Rev. Aquac. 2020, 12, 328–346. [Google Scholar] [CrossRef]
- Kumar, V. Enzymes in Human and Animal Nutrition Principles and Perspectives; Academic Press: Cambridge, MA, USA, 2018; ISBN 9780128054192. [Google Scholar]
- Sánchez-Paz, A.; García-Carreño, F.; Hernández-López, J.; Muhlia-Almazán, A.; Yepiz-Plascencia, G. Effect of Short-Term Starvation on Hepatopancreas and Plasma Energy Reserves of the Pacific White Shrimp (Litopenaeus vannamei). J. Exp. Mar. Biol. Ecol. 2007, 340, 184–193. [Google Scholar] [CrossRef]
- Tang, H.; Zhang, J.; Chen, G.; Huang, J.; Wang, Z.; Tang, B.; Zhuo, H. Combined Effects of Breeding Density, Feeding Frequency and Feeding Level on Specific Growth Rate, Feed Conversion Rate and Pepsin Activity of Juvenile Hybrid Groupers (Epinephelus fuscoguttatus♀×E. lanceolatus♂). J. Guangdong Ocean Univ. 2018, 38, 22–31. [Google Scholar] [CrossRef]
- Cai, Y.; Huang, H.; Yao, W.; Yang, H.; Xue, M.; Li, X.; Leng, X. Effects of Fish Meal Replacement by Three Protein Sources on Physical Pellet Quality and Growth Performance of Pacific White Shrimp (Litopenaeus vannamei). Aquac. Rep. 2022, 25, 101210. [Google Scholar] [CrossRef]
- Felix, N.; Manikandan, K.; Uma, A.; Kaushik, S.J. Evaluation of Single Cell Protein on the Growth Performance, Digestibility and Immune Gene Expression of Pacific White Shrimp, Litopenaeus vannamei. Anim. Feed Sci. Technol. 2023, 296, 115549. [Google Scholar] [CrossRef]
- Radhakrishnan, S.; Belal, I.E.H.; Seenivasan, C.; Muralisankar, T.; Bhavan, P.S. Impact of Fishmeal Replacement with Arthrospira platensis on Growth Performance, Body Composition and Digestive Enzyme Activities of the Freshwater Prawn, Macrobrachium rosenbergii. Aquac. Rep. 2016, 3, 35–44. [Google Scholar] [CrossRef]
- Li, L.; Liu, H.; Li, W.; Wang, Q.; Lin, Z.; Tan, B.; Dong, X.; Xie, R. Clostridium butyricum Improves the Digestive Enzyme Activity, Antioxidant and Immunity Related Genes Expression and Intestinal Microbiota of Litopenaeus vannamei Fed a Replacing Fishmeal with Cottonseed Protein Concentrate (CPC) Diet. Aquac. Rep. 2023, 29, 101517. [Google Scholar] [CrossRef]
- He, Y.; Chi, S.; Tan, B.; Zhang, H.; Dong, X.; Yang, Q.; Liu, H.; Zhang, S. Effect of Yeast Cultures on Intestinal Microbiota of Litopenaeus vannamei. J. Guangdong Ocean Univ. 2017, 37, 21–27. [Google Scholar] [CrossRef]
- Chen, J.; Wei, H.; Yang, Q.; Tan, B.; Chen, Y.; Lin, H. Effects of Galla Chinensis on Growth, Antioxidant Capacity, Non-Specific and Intestinal Structure for Litopenaeus vannamei. J. Guangdong Ocean Univ. 2023, 43, 11–17. [Google Scholar] [CrossRef]
- Binda, C.; Lopetuso, L.R.; Rizzatti, G.; Gibiino, G.; Cennamo, V.; Gasbarrini, A. Actinobacteria: A Relevant Minority for the Maintenance of Gut Homeostasis. Dig. Liver Dis. 2018, 50, 421–428. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Guo, X.; Tan, B.; Dong, X.; Yang, Q.; Liu, H.; Zhang, S.; Chi, S. Partial Fishmeal Protein Replacement with Peptides from Swine Blood Modulates the Nutritional Status, Immune Response, and Intestinal Microbiota of Hybrid Groupers (Female Epinephelus fuscoguttatus× Male E. Lanceolatus). Aquaculture 2021, 533, 736154. [Google Scholar] [CrossRef]
- Yang, C.; Jiang, M.; Lu, X.; Wen, H. Effects of Dietary Protein Level on the Gut Microbiome and Nutrient Metabolism in Tilapia (Oreochromis niloticus). Animals 2021, 11, 1024. [Google Scholar] [CrossRef]
- Ringø, E.; Zhou, Z.; Vecino, J.L.G.; Wadsworth, S.; Romero, J.; Krogdahl, Å.; Olsen, R.E.; Dimitroglou, A.; Foey, A.; Davies, S.; et al. Effect of Dietary Components on the Gut Microbiota of Aquatic Animals. A Never-Ending Story? Aquac. Nutr. 2016, 22, 219–282. [Google Scholar] [CrossRef]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A Common Factor in Human Diseases. BioMed. Res. Int. 2017, 17, 232–246. [Google Scholar] [CrossRef]
- Wiegand, S.; Jogler, M.; Jogler, C. On the Maverick Planctomycetes. FEMS Microbiol. Rev. 2018, 42, 739–760. [Google Scholar] [CrossRef]
- Van Keulen, G.; Dyson, P.J. Production of Specialized Metabolites by Streptomyces coelicolor A3(2). Adv. Appl. Microbiol. 2014, 89, 217–266. [Google Scholar] [CrossRef]
- Ley, R.E.; Turnbaugh, P.J.; Klein, S.; Gordon, J.I. Microbial Ecology: Human Gut Microbes Associated with Obesity. Nature 2006, 444, 1022–1023. [Google Scholar] [CrossRef]
- Garibay-Valdez, E.; Martínez-Porchas, M.; Calderón, K.; Vargas-Albores, F.; Gollas-Galván, T.; Martínez-Córdova, L. Taxonomic and Functional Changes in the Microbiota of the White Shrimp (Litopenaeus vannamei) Associated with Postlarval Ontogenetic Development. Aquaculture 2020, 518, 734842. [Google Scholar] [CrossRef]
- Liu, J.; Wang, K.; Wang, Y.; Chen, W.; Jin, Z.; Yao, Z.; Zhang, D. Strain-Specific Changes in the Gut Microbiota Profiles of the White Shrimp Litopenaeus vannamei in Response to Cold Stress. Aquaculture 2019, 503, 357–366. [Google Scholar] [CrossRef]
- Mudarris, M.; Austin, B.; Segers, P.; Vancanneyt, M.; Hoste, B.; Bernardet, J.F. Flavobacterium scophthalmum sp. Nov., a Pathogen of Turbot (Scophthalmus maximus L.). Int. J. Syst. Bacteriol. 1994, 44, 447–453. [Google Scholar] [CrossRef] [PubMed]
- Miura, N.; Motone, K.; Takagi, T.; Aburaya, S.; Watanabe, S.; Aoki, W.; Ueda, M. Ruegeria sp. Strains Isolated from the Reef-Building Coral Galaxea fascicularis Inhibit Growth of the Temperature-Dependent Pathogen Vibrio coralliilyticus. Mar. Biotechnol. 2019, 21, 1–8. [Google Scholar] [CrossRef]
- Barreto-Curiel, F.; Ramirez-Puebla, S.T.; Ringø, E.; Escobar-Zepeda, A.; Godoy-Lozano, E.; Vazquez-Duhalt, R.; Sanchez-Flores, A.; Viana, M.T. Effects of Extruded Aquafeed on Growth Performance and Gut Microbiome of Juvenile Totoaba macdonaldi. Anim. Feed Sci. Technol. 2018, 245, 91–103. [Google Scholar] [CrossRef]
- Baum, R.; Bieńkowski, J. Eco-Efficiency in Measuring the Sustainable Production of Agricultural Crops. Sustainability 2020, 12, 1418. [Google Scholar] [CrossRef]


| Ingredients | FM | CHL | CAP |
|---|---|---|---|
| Brown fish meal | 58.90 | 15.00 | 15.00 |
| Chlorella sorokiniana | 0.00 | 49.20 | 0.00 |
| Clostridium autoethanogenum protein | 0.00 | 0.00 | 35.40 |
| Corn starch | 20.00 | 20.00 | 20.00 |
| Fish oil | 0.36 | 2.44 | 2.52 |
| Corn oil | 0.36 | 2.44 | 2.52 |
| Soyabean lecithin | 1.00 | 1.00 | 1.00 |
| Vitamin and mineral premix a | 1.20 | 1.20 | 1.20 |
| Choline chloride | 0.50 | 0.50 | 0.50 |
| Ethoxyquin | 0.05 | 0.05 | 0.05 |
| Attractant | 0.10 | 0.10 | 0.10 |
| Ca(H2PO4)2 | 1.20 | 1.20 | 1.20 |
| Vitamin C | 0.05 | 0.05 | 0.05 |
| Cellulose microcrystalline | 16.28 | 6.82 | 20.46 |
| Total | 100 | 100 | 100 |
| Proximate Composition | |||
| Crude protein | 41.39 | 40.97 | 40.85 |
| Crude lipids | 7.53 | 7.41 | 7.66 |
| Ash | 11.56 | 6.86 | 5.16 |
| Moisture | 7.32 | 6.48 | 7.64 |
| Index | FM | CHL | CAP |
|---|---|---|---|
| IBW (g) | 3.68 ± 0.002 | 3.68 ± 0.002 | 3.68 ± 0.002 |
| WGR (%) | 205.0 ± 3.71 a | 221.2 ± 3.39 a | 251.3 ± 4.95 b |
| SGR (%/day) | 1.96 ± 0.07 a | 2.08 ± 0.03 a | 2.24 ± 0.03 b |
| FCR | 2.07 ± 0.12 | 2.07 ± 0.13 | 1.85 ± 0.04 |
| SR (%) | 93.33 ± 1.44 | 92.50 ± 0.00 | 91.67 ± 0.83 |
| PRE (%) | 1.19 ± 0.02 a | 1.19 ± 0.02 a | 1.33 ± 0.03 b |
| Sample Name | Indexes | ||||||
|---|---|---|---|---|---|---|---|
| Raw Reads | Clean Reads | Raw Tags | Clean Tags | Chimera | Effective Tags | Effective Ratio (%) | |
| FM-1 | 128,181 | 128,058 | 126,554 | 125,613 | 10,876 | 114,737 | 89.51 |
| FM-2 | 127,138 | 127,021 | 125,551 | 123,413 | 12,814 | 110,599 | 86.99 |
| FM-3 | 125,115 | 125,018 | 123,601 | 122,754 | 12,260 | 110,494 | 88.31 |
| FM-4 | 137,013 | 136,879 | 135,244 | 133,840 | 9,649 | 124,191 | 90.64 |
| CAP-1 | 120,946 | 120,817 | 119,133 | 117,788 | 12,882 | 104,906 | 86.74 |
| CAP-2 | 124,558 | 124,432 | 122,772 | 121,582 | 15,632 | 105,950 | 85.06 |
| CAP-3 | 136,435 | 136,308 | 134,547 | 133,180 | 17,186 | 115,994 | 85.02 |
| CAP-4 | 135,872 | 135,737 | 134,114 | 132,707 | 12,863 | 119,844 | 88.20 |
| CHL-1 | 126,594 | 126,455 | 124,853 | 124,059 | 12,665 | 111,394 | 87.99 |
| CHL-2 | 121,213 | 121,071 | 119,074 | 117,909 | 11,455 | 106,454 | 87.82 |
| CHL-3 | 126,517 | 126,380 | 124,693 | 123,766 | 14,000 | 109,766 | 86.76 |
| CHL-4 | 121,977 | 121,827 | 120,041 | 119,371 | 11,172 | 108,199 | 88.70 |
| Index | FM | CHL | CAP |
|---|---|---|---|
| Sobs | 570.0 ± 12.73 a | 772.00 ± 2.83 c | 631.33 ± 22.75 b |
| Shannon | 4.26 ± 0.23 a | 5.19 ± 0.25 c | 4.74 ± 0.05 b |
| Simpson | 0.89 ± 0.03 | 0.90 ± 0.03 | 0.92 ± 0.02 |
| Chao1 | 717.25 ± 9.45 a | 861.72 ± 9.21 c | 762.33 ± 10.53 b |
| ACE | 666.80 ± 38.09 a | 888.60 ± 17.00 b | 834.52 ± 41.92 b |
| Pielou | 0.46 ± 0.02 a | 0.54 ± 0.02 b | 0.51 ± 0.01 b |
| Pd | 82.74 ± 5.53 a | 104.2 ± 6.06 b | 97.17 ± 7.29 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, H.; Hu, N.; Zheng, Y.; Hou, C.; Tan, B.; Shi, L.; Zhang, S. A Comparison of Three Protein Sources Used in Medium-Sized Litopenaeus vannamei: Effects on Growth, Immunity, Intestinal Digestive Enzyme Activity, and Microbiota Structure. Fishes 2023, 8, 449. https://doi.org/10.3390/fishes8090449
Yuan H, Hu N, Zheng Y, Hou C, Tan B, Shi L, Zhang S. A Comparison of Three Protein Sources Used in Medium-Sized Litopenaeus vannamei: Effects on Growth, Immunity, Intestinal Digestive Enzyme Activity, and Microbiota Structure. Fishes. 2023; 8(9):449. https://doi.org/10.3390/fishes8090449
Chicago/Turabian StyleYuan, Hang, Naijie Hu, Yudong Zheng, Cuihong Hou, Beiping Tan, Lili Shi, and Shuang Zhang. 2023. "A Comparison of Three Protein Sources Used in Medium-Sized Litopenaeus vannamei: Effects on Growth, Immunity, Intestinal Digestive Enzyme Activity, and Microbiota Structure" Fishes 8, no. 9: 449. https://doi.org/10.3390/fishes8090449
APA StyleYuan, H., Hu, N., Zheng, Y., Hou, C., Tan, B., Shi, L., & Zhang, S. (2023). A Comparison of Three Protein Sources Used in Medium-Sized Litopenaeus vannamei: Effects on Growth, Immunity, Intestinal Digestive Enzyme Activity, and Microbiota Structure. Fishes, 8(9), 449. https://doi.org/10.3390/fishes8090449






