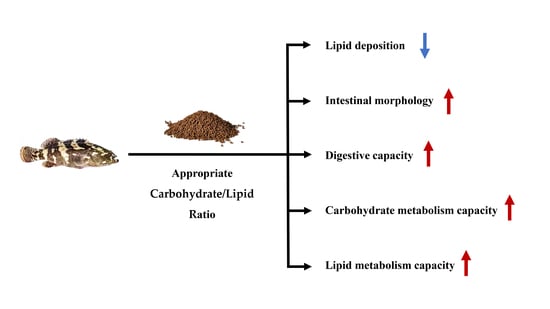
How Do Different Dietary Carbohydrate/Lipid Ratios Influence Intestinal Morphology and Glycolipid Metabolism Capacity in Hybrid Grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Diets
2.2. Experimental Fish and Experimental Conditions
2.3. Sample Collection and Analysis
2.4. Calculations and Statistical Analysis
3. Results
3.1. Growth Performance and Body Morphologic
3.2. Whole Body Composition
3.3. Ether Extract and Glycogen Contents in Liver and Muscle
3.4. Liver and Intestinal Digestive Enzyme Activities
3.5. Intestinal Morphology
3.6. Serum Biochemical Index
3.7. Hepatic Enzyme Activities of Carbohydrate Metabolism
3.8. Hepatic Enzyme Activities of Lipid Metabolism
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, J.T.; Jiang, Y.D.; Li, X.Y.; Han, T.; Yang, Y.X.; Hu, S.X.; Yang, M. Dietary protein requirement of juvenile red spotted grouper (Epinephelus akaara). Aquaculture 2016, 450, 289–294. [Google Scholar] [CrossRef]
- Shpigel, M.; Guttman, L.; Shauli, L.; Odintsov, V.; Ben-Ezra, D.; Harpaz, S. Ulva lactuca from an Integrated Multi-Trophic Aquaculture (IMTA) biofilter system as a protein supplement in gilthead seabream (Sparus aurata) diet. Aquaculture 2017, 481, 112–118. [Google Scholar] [CrossRef]
- Shiau, S.Y.; Huang, S.L. Influence of varying energy levels with 2 protein concentrations in diets for hybrid tilapia (Oreochromis niloticus × Oreochromis aureus) reared in seawater. Aquaculture 1990, 91, 143–152. [Google Scholar] [CrossRef]
- Hemre, G.I.; Mommsen, T.P.; Krogdahl, A. Carbohydrates in fish nutrition: Effects on growth, glucose metabolism and hepatic enzymes. Aquac. Nutr. 2002, 8, 175–194. [Google Scholar] [CrossRef]
- Zhou, C.P.; Ge, X.P.; Niu, J.; Lin, H.Z.; Huang, Z.; Tan, X.H. Effect of dietary carbohydrate levels on growth performance, body composition, intestinal and hepatic enzyme activities, and growth hormone gene expression of juvenile golden pompano, Trachinotus ovatus. Aquaculture 2015, 437, 390–397. [Google Scholar] [CrossRef]
- Moon, T.W. Glucose intolerance in teleost fish: Face or fiction? Comp. Biochem. Phys. B 2001, 129, 243–249. [Google Scholar] [CrossRef]
- Polakof, S.; Panserat, S.; Soengas, J.L.; Moon, T.W. Glucose metabolism in fish: A review. J. Comp. Physiol. B 2012, 182, 1015–1045. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.P.; Wang, M.Q.; Xie, F.J.; Deng, D.F.; Zhou, Q.C. Effects of dietary carbohydrate to lipid ratios on growth performance, digestive enzyme and hepatic carbohydrate metabolic enzyme activities of large yellow croaker (Larmichthys crocea). Aquaculture 2016, 452, 45–51. [Google Scholar] [CrossRef]
- Fernandez, F.; Miquel, A.G.; Cordoba, M.; Varas, M.; Meton, I.; Caseras, A.; Baanante, I.V. Effects of diets with distinct protein-to-carbohydrate ratios on nutrient digestibility, growth performance, body composition and liver intermediary enzyme activities in gilthead sea bream (Sparus aurata, L.) fingerlings. J. Exp. Mar. Biol. Ecol. 2007, 343, 1–10. [Google Scholar] [CrossRef]
- Leung, L.Y.; Woo, N.Y.S. Influence of dietary carbohydrate level on endocrine status and hepatic carbohydrate metabolism in the marine fish Sparus sarba. Fish Physiol. Biochem. 2012, 38, 543–554. [Google Scholar] [CrossRef]
- Ren, M.C.; Ai, Q.H.; Mai, K.S.; Ma, H.M.; Wang, X.J. Effect of dietary carbohydrate level on growth performance, body composition, apparent digestibility coefficient and digestive enzyme activities of juvenile cobia, Rachycentron canadum L. Aquac. Res. 2011, 42, 1467–1475. [Google Scholar] [CrossRef]
- Li, X.Y.; Wang, J.T.; Han, T.; Hu, S.X.; Jiang, Y.D. Effects of dietary carbohydrate level on growth and body composition of juvenile giant croaker Nibea japonica. Aquac. Res. 2015, 46, 2851–2858. [Google Scholar] [CrossRef]
- Xia, B.; Gao, Q.F.; Wang, J.Y.; Li, P.Y.; Zhang, L.M.; Zhang, Z.D. Effects of dietary carbohydrate level on growth, biochemical composition and glucose metabolism of juvenile sea cucumber Apostichopus japonicus (Selenka). Aquaculture 2015, 448, 63–70. [Google Scholar] [CrossRef]
- Zhou, C.; Liu, B.; Ge, X.; Xie, J.; Xu, P. Effect of dietary carbohydrate on the growth performance, immune response, hepatic antioxidant abilities and heat shock protein 70 expression of Wuchang bream, Megalobrama amblycephala. J. Appl. Ichthyol. 2013, 29, 1348–1356. [Google Scholar] [CrossRef]
- Zhou, C.P.; Ge, X.P.; Lin, H.Z.; Niu, J. Effect of dietary carbohydrate on non-specific immune response, hepatic antioxidative abilities and disease resistance of juvenile golden pompano (Trachinotus ovatus). Fish Shellfish Immunol. 2014, 41, 183–190. [Google Scholar] [CrossRef]
- Lin, S.M.; Shi, C.M.; Mu, M.M.; Chen, Y.J.; Luo, L. Effect of high dietary starch levels on growth, hepatic glucose metabolism, oxidative status and immune response of juvenile largemouth bass, Micropterus salmoides. Fish Shellfish Immunol. 2018, 78, 121–126. [Google Scholar] [CrossRef]
- Watanabe, T. Lipid Nutrition in Fish. Comp. Biochem. Phys. B 1982, 73, 3–15. [Google Scholar] [CrossRef]
- Chou, R.L.; Su, M.S.; Chen, H.Y. Optimal dietary protein and lipid levels for juvenile cobia (Rachycentron canadum). Aquaculture 2001, 193, 81–89. [Google Scholar] [CrossRef]
- Wang, J.T.; Liu, Y.J.; Tian, L.X.; Mai, K.S.; Du, Z.Y.; Wang, Y.; Yang, H.J. Effect of dietary lipid level on growth performance, lipid deposition, hepatic lipogenesis in juvenile cobia (Rachycentron canadum). Aquaculture 2005, 249, 439–447. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, Y.J.; Mai, K.S.; Tian, L.X.; Liu, D.H.; Tan, X.Y.; Lin, H.Z. Effect of dietary lipid level on growth performance, feed utilization and body composition of grouper Epinephelus coioides juveniles fed isonitrogenous diets in floating netcages. Aquac. Int. 2005, 13, 257–269. [Google Scholar] [CrossRef]
- Jiang, Y.D.; Wang, J.T.; Han, T.; Li, X.Y.; Hu, S.X. Effect of dietary lipid level on growth performance, feed utilization and body composition by juvenile red spotted grouper (Epinephelus akaara). Aquac. Int. 2015, 23, 99–110. [Google Scholar] [CrossRef]
- Goncalves, R.; Lund, I.; Gesto, M.; Skov, P.V. The effect of dietary protein, lipid, and carbohydrate levels on the performance, metabolic rate and nitrogen retention in juvenile European lobster (Homarus gammarus, L.). Aquaculture 2020, 525, 735334. [Google Scholar] [CrossRef]
- Lopez, L.M.; Soto, J.O.; Escamilla, I.T.; Ibarra, M.F.; Ochoa, L.; Drawbridge, M.; Peres, H. Evaluation of carbohydrate-to-lipid ratio in diets supplemented with Bacillus subtilis probiotic strain on growth performance, body composition and digestibility in juvenile white seabass (Atractoscion nobilis, Ayres 1860). Aquac. Res. 2016, 47, 1864–1873. [Google Scholar] [CrossRef]
- Dong, L.F.; Tong, T.; Zhang, Q.; Wang, Q.C.; Xu, M.Z.; Yu, H.R.; Wang, J. Effects of dietary carbohydrate to lipid ratio on growth, feed utilization, body composition and digestive enzyme activities of golden pompano (Trachinotus ovatus). Aquac. Nutr. 2018, 24, 341–347. [Google Scholar] [CrossRef]
- Xing, S.J.; Sun, R.J.; Pan, X.Y.; Ma, J.; Zhang, W.B.; Mai, K.S. Effects of dietary carbohydrate-to-lipid ratio on growth performance, body composition, digestive enzyme activities, and hepatic enzyme activities in juvenile large yellow croaker, Larimichthys crocea. J. World Aquac. Soc 2016, 47, 297–307. [Google Scholar] [CrossRef]
- Wang, J.T.; Jiang, Y.D.; Han, T.; Li, X.Y.; Wang, Y.; Liu, Y.J. Effects of dietary carbohydrate-to-lipid ratios on growth and body composition of orange-spotted grouper Epinephelus coioides. N. Am. J. Aquac. 2017, 79, 1–7. [Google Scholar] [CrossRef]
- Lee, K.W.; Kim, H.S.; Jeong, H.S.; Kim, J.; Yun, A.Y.; Cho, S.H. Effect of dietary carbohydrate-to-lipid ratio on growth and carcass composition of juvenile abalone, Haliotis discus, Reeve 1846. J. World Aquac. Soc 2019, 50, 604–613. [Google Scholar] [CrossRef]
- Mozanzadeh, M.T.; Yavari, V.; Marammazi, J.G.; Agh, N.; Gisbert, E. Optimal dietary carbohydrate-to-lipid ratios for silvery-black porgy (Sparidentex hasta) juveniles. Aquac. Nutr. 2017, 23, 470–483. [Google Scholar] [CrossRef]
- Li, S.L.; Yin, J.; Zhang, H.T.; Liu, Z.K.; Chen, N.S. Effects of dietary carbohydrate and lipid levels on growth performance, feed utilization, body composition and nonspecific immunity of large yellow croaker (Larimichthys crocea). Aquac. Nutr. 2019, 25, 995–1005. [Google Scholar] [CrossRef]
- Zhang, C.X.; Huang, K.K.; Wang, L.; Song, K.; Lu, K.L.; Zhang, L.; Li, P. Optimal dietary carbohydrate to lipid ratio for bullfrog Rana (Lithobates) catesbeiana. Aquac. Res. 2016, 47, 3332–3340. [Google Scholar] [CrossRef]
- Fisheries and Fishery Administration Bureau of the Ministry of Agriculture and Rural Affairs; National Fishery Technology Promotion Station. China Society of Fisheries China Fishery Statistics Yearbook 2022; Ministry of Agriculture and Rural Affairs: Beijing, China, 2022; pp. 22–23.
- Wu, M.J.; Lu, S.D.; Wu, X.Y.; Jiang, S.T.; Luo, Y.; Yao, W.; Jin, Z.B. Effects of dietary amino acid patterns on growth, feed utilization and hepatic IGF-I, TOR gene expression levels of hybrid grouper (Epinephelus fuscoguttatus female × Epinephelus lanceolatus male) juveniles. Aquaculture 2017, 468, 508–514. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, Z.W.; Ding, N.; Xiong, W.W.; Zheng, G.F.; Lin, Q.; Zhang, G. Effects of temperature on the survival, feeding, and growth of pearl gentian grouper (Female Epinephelus fuscoguttatus × male Epinephelus lanceolatus). Fish. Sci. 2018, 84, 399–404. [Google Scholar] [CrossRef]
- Guo, X.W.; Tan, B.P.; Chi, S.Y.; Dong, X.H.; Yang, Q.H.; Liu, H.Y.; Zhang, S. Correlation analysis of fish growth performance and serum hormone and digestive enzyme activities of juvenile pearl gentian grouper (Epinephelus lanceolatus ♂ × E. fuscoguttatus ♀) fed with different protein levels diets. J. Fish. China 2019, 43, 1808–1820. [Google Scholar] [CrossRef]
- Jiang, S.T.; Wu, X.Y.; Luo, Y.; Wu, M.J.; Lu, S.D.; Jin, Z.B.; Yao, W. Optimal dietary protein level and protein to energy ratio for hybrid grouper (Epinephelus fuscoguttatus female × Epinephelus lanceolatus male) juveniles. Aquaculture 2016, 465, 28–36. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis of Association of Official Analytical Chemists (AOAC) International. Volume I, Agricultural Chemicals, Contaminants, Drugs; Horwitz, W., Ed.; AOAC International: Gaithersburg, MD, USA, 2010. [Google Scholar]
- Yang, X.Y.; Zhao, X.M.; Wang, G.H.; Dong, X.H.; Yang, Q.H.; Liu, H.Y.; Zhang, S.; Tan, B.P.; Chi, S.Y. Improvement of hybrid grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂) by enzyme-digested poultry by-product: Growth performance, amino acid and peptide transport capacity, and intestinal morphology. Front. Nutr. 2022, 9, 955734. [Google Scholar] [CrossRef]
- Gao, W.; Liu, Y.J.; Tian, L.X.; Mai, K.S.; Liang, G.Y.; Yang, H.J.; Huai, M.Y.; Luo, W.J. Effect of dietary carbohydrate-to-lipid ratios on growth performance, body composition, nutrient utilization and hepatic enzymes activities of herbivorous grass carp (Ctenopharyngodon idella). Aquac. Nutr. 2010, 16, 327–333. [Google Scholar] [CrossRef]
- Xie, D.Z.; Yang, L.P.; Yu, R.M.; Chen, F.; Lu, R.H.; Qin, C.B.; Nie, G.X. Effects of dietary carbohydrate and lipid levels on growth and hepatic lipid deposition of juvenile tilapia, Oreochromis niloticus. Aquaculture 2017, 479, 696–703. [Google Scholar] [CrossRef]
- Sterzelecki, F.C.; Sugai, J.K.; Baloi, M.; Passini, G.; de Carvalho, C.V.A.; Fracalossi, D.M.; Cerqueira, V.R. Effect of dietary carbohydrate to lipid ratios on growth, digestive enzyme and blood metabolites of juvenile Brazilian sardines, Sardinella brasiliensis (Steindachner, 1879). Aquac. Res. 2017, 48, 5111–5121. [Google Scholar] [CrossRef]
- Hu, Y.H.; Liu, Y.J.; Tian, L.X.; Yang, H.J.; Liang, G.Y.; Gao, W. Optimal dietary carbohydrate to lipid ratio for juvenile yellowfin seabream (Sparus latus). Aquac. Nutr. 2007, 13, 291–297. [Google Scholar] [CrossRef]
- Li, W.F.; Wu, X.Y. Effects of different dietary carbohydrate/lipid ratios on growth, feed utilization and body composition of early giant grouper Epinephelus lanceolatus. J. Aquac. Res. Dev. 2016, 7, 1000415. [Google Scholar] [CrossRef]
- Erfanullah; Jafri, A.K. Effect of dietary carbohydrate-to-lipid ratio on growth and body composition of walking catfish (Clarias batrachus). Aquaculture 1998, 161, 159–168. [Google Scholar] [CrossRef]
- Wang, L.N.; Liu, W.B.; Lu, K.L.; Xu, W.N.; Cai, D.S.; Zhang, C.N.; Qian, Y. Effects of dietary carbohydrate/lipid ratios on non-specific immune responses, oxidative status and liver histology of juvenile yellow catfish Pelteobagrus fulvidraco. Aquaculture 2014, 426, 41–48. [Google Scholar] [CrossRef]
- Mokoginta, I.; Takeuchi, T.; Hadadi, A.; Dedi, J. Different capabilities in utilizing dietary carbohydrate by fingerling and subadult giant gouramy Osphronemus gouramy. Fish. Sci. 2004, 70, 996–1002. [Google Scholar] [CrossRef]
- Li, S.L.; Li, Z.Q.; Chen, N.S.; Jin, P.F.; Zhang, J.C. Dietary lipid and carbohydrate interactions: Implications on growth performance, feed utilization and non-specific immunity in hybrid grouper (Epinephelus fuscoguttatus female × E-lanceolatus male). Aquaculture 2019, 498, 568–577. [Google Scholar] [CrossRef]
- Moro, G.V.; Camilo, R.; Moraes, G.; Fracalossi, D.M. Dietary non-protein energy sources: Growth, digestive enzyme activities and nutrient utilization by the catfish jundia, Rhamdia quelen. Aquac. Res. 2010, 41, 394–400. [Google Scholar] [CrossRef]
- Tan, Q.; Xie, S.; Zhu, X.; Lei, W.; Yang, Y. Effect of dietary carbohydrate-to-lipid ratios on growth and feed utilization in Chinese longsnout catfish (Leiocassis longirostris Gunther). J. Appl. Ichthyol. 2007, 23, 605–610. [Google Scholar] [CrossRef]
- Guerrero-Zarate, R.; Alvarez-Gonzalez, C.A.; Jesus-Contreras, R.; Pena-Marin, E.S.; Martinez-Garcia, R.; Galaviz, M.A.; Lopez, L.M.; Llera-Herrera, R. Evaluation of carbohydrate/lipid ratios on growth and metabolic response in tropical gar (Atractosteus tropicus) juvenile. Aquac. Res. 2019, 50, 1812–1823. [Google Scholar] [CrossRef]
- Li, X.F.; Wang, Y.; Liu, W.B.; Jiang, G.Z.; Zhu, J. Effects of dietary carbohydrate/lipid ratios on growth performance, body composition and glucose metabolism of fingerling blunt snout bream Megalobrama amblycephala. Aquac. Nutr. 2013, 19, 701–708. [Google Scholar] [CrossRef]
- Wang, J.T.; Li, X.Y.; Han, T.; Yang, Y.X.; Jiang, Y.D.; Yang, M.; Xu, Y.J.; Harpaz, S. Effects of different dietary carbohydrate levels on growth, feed utilization and body composition of juvenile grouper Epinephelus akaara. Aquaculture 2016, 459, 143–147. [Google Scholar] [CrossRef]
- Martino, R.C.; Cyrino, J.E.P.; Portz, L.; Trugo, L.C. Effect of dietary lipid level on nutritional performance of the surubim, Pseudoplatystoma coruscans. Aquaculture 2002, 209, 209–218. [Google Scholar] [CrossRef]
- Lee, S.M.; Kim, K.D. Effects of dietary carbohydrate to lipid ratios on growth and body composition of juvenile and grower rockfish, Sebastes schlegeli. Aquac. Res. 2009, 40, 1830–1837. [Google Scholar] [CrossRef]
- Li, X.F.; Jiang, Y.Y.; Liu, W.B.; Ge, X.P. Protein-sparing effect of dietary lipid in practical diets for blunt snout bream (Megalobrama amblycephala) fingerlings: Effects on digestive and metabolic responses. Fish Physiol. Biochem. 2012, 38, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Giri, S.S.; Sahoo, S.K.; Sahu, A.K.; Meher, P.K. Effect of dietary protein level on growth, survival, feed utilisation and body composition of hybrid Clarias catfish (Clarias batrachus × Clarias gariepinus). Anim. Feed Sci. Tech. 2003, 104, 169–178. [Google Scholar] [CrossRef]
- Lundstedt, L.M.; Melo, J.F.B.; Moraes, G. Digestive enzymes and metabolic profile of Pseudoplatystoma corruscans (Teleostei: Siluriformes) in response to diet composition. Comp. Biochem. Phys. B 2004, 137, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Cahu, C.L.; Infante, J.L.Z. Early weaning of sea bass (Dicentrarchus-Labrax) larvae with a compound diet effect on digestive enzymes. Comp. Biochem. Phys. A 1994, 109, 213–222. [Google Scholar] [CrossRef]
- Tappy, L.; Minehira, K. New data and new concepts on the role of the liver in glucose homeostasis. Curr. Opin. Clin. Nutr. 2001, 4, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.P. Utilization of dietary carbohydrate by fish. Aquaculture 1994, 124, 67–80. [Google Scholar] [CrossRef]
- Moreira, I.S.; Peres, H.; Couto, A.; Enes, P.; Oliva-Teles, A. Temperature and dietary carbohydrate level effects on performance and metabolic utilisation of diets in European sea bass (Dicentrarchus labrax) juveniles. Aquaculture 2008, 274, 153–160. [Google Scholar] [CrossRef]
- Enes, P.; Panserat, S.; Kaushik, S.; Oliva-Teles, A. Nutritional regulation of hepatic glucose metabolism in fish. Fish Physiol. Biochem. 2009, 35, 519–539. [Google Scholar] [CrossRef]
- Liu, H.; Yang, J.J.; Dong, X.H.; Tan, B.P.; Zhang, S.; Chi, S.Y.; Yang, Q.H.; Liu, H.Y.; Yang, Y.Z. Effects of different dietary carbohydrate-to-lipid ratios on growth, plasma biochemical indexes, digestive, and immune enzymes activities of sub-adult orange-spotted grouper Epinephelus coioides. Fish Physiol. Biochem. 2020, 46, 1409–1420. [Google Scholar] [CrossRef]
- Conde-Sieira, M.; Soengas, J.L.; Valente, L.M.P. Potential capacity of Senegalese sole (Solea senegalensis) to use carbohydrates: Metabolic responses to hypo-and hyper-glycaemia. Aquaculture 2015, 438, 59–67. [Google Scholar] [CrossRef]
- Kamalam, B.S.; Medale, F.; Panserat, S. Utilisation of dietary carbohydrates in farmed fishes: New insights on influencing factors, biological limitations and future strategies. Aquaculture 2017, 467, 3–27. [Google Scholar] [CrossRef]
- Geurden, I.; Mennigen, J.; Plagnes-Juan, E.; Veron, V.; Cerezo, T.; Mazurais, D.; Zambonino-Infante, J.; Gatesoupe, J.; Skiba-Cassy, S.; Panserat, S. High or low dietary carbohydrate: Protein ratios during first-feeding affect glucose metabolism and intestinal microbiota in juvenile rainbow trout. J. Exp. Biol. 2014, 217, 3396–3406. [Google Scholar] [CrossRef]
- Aiderus, A.; Black, M.A.; Dunbier, A.K. Fatty acid oxidation is associated with proliferation and prognosis in breast and other cancers. Bmc Cancer 2018, 18, 805–819. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Wu, F.; Yang, C.G.; Jiang, M.; Liu, W.; Wen, H. Dietary lipid levels impact lipoprotein lipase, hormone-sensitive lipase, and fatty acid synthetase gene expression in three tissues of adult GIFT strain of Nile tilapia, Oreochromis niloticus. Fish Physiol. Biochem. 2015, 41, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, E.; Goldberg, E.B.; Makarova, K.S.; Lin, L.; Brown, W.J.; Jackson, C.L. ATGL has a key role in lipid droplet/adiposome degradation in mammalian cells. EMBO Rep. 2006, 7, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Yuan, X.; Liang, L.M.; Liu, K.; Ye, H.Y.; Liu, Z.Y.; Liu, Y.F.; Huang, L.Q.; He, W.J.; Chen, Y.Q.; et al. Overexpression of acetyl-CoA carboxylase increases fatty acid production in the green alga Chlamydomonas reinhardtii. Biotechnol. Lett. 2019, 41, 1133–1145. [Google Scholar] [CrossRef]
- Regost, C.; Arzel, J.; Cardinal, M.; Robin, J.; Laroche, M.; Kaushik, S.J. Dietary lipid level, hepatic lipogenesis and flesh quality in turbot (Psetta maxima). Aquaculture 2001, 193, 291–309. [Google Scholar] [CrossRef]
- Oku, H.; Ogata, H.Y.; Liang, X.F. Organization of the lipoprotein lipase gene of red sea bream Pagrus major. Comp. Biochem. Phys. B 2002, 131, 775–785. [Google Scholar] [CrossRef]
- Nilssonehle, P.; Garfinkel, A.S.; Schotz, M.C. Lipolytic enzymes and plasma-lipoprotein metabolism. Annu. Rev. Biochem. 1980, 49, 667–693. [Google Scholar] [CrossRef]
- Han, H.; Dai, D.; Wang, W.; Zhu, J.; Zhu, Z.; Lu, L.; Zhang, R. Impact of serum levels of lipoprotein lipase, hepatic lipase, and endothelial lipase on the progression of coronary artery disease. J. Interv. Med. 2019, 2, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Albalat, A.; Saera-Vila, A.; Capilla, E.; Gutierrez, J.; Perez-Sanchez, J.; Navarro, I. Insulin regulation of lipoprotein lipase (LPL) activity and expression in gilthead sea bream (Sparus aurata). Comp. Biochem. Phys. B 2007, 148, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.K.; Liu, W.B.; Xu, C.; Cao, X.F.; Zhong, X.Q.; Shi, H.J.; Li, X.F. Dietary carbohydrate levels and lipid sources modulate the growth performance, fatty acid profiles and intermediary metabolism of blunt snout bream Megalobrama amblycephala in an interactive pattern. Aquaculture 2017, 481, 140–153. [Google Scholar] [CrossRef]



| Ingredients | Level of CHO:L Ratios in Diet | |||||
|---|---|---|---|---|---|---|
| 0.82 | 1.03 | 1.28 | 1.58 | 1.94 | 2.27 | |
| Fish meal | 380.0 | 380.0 | 380.0 | 380.0 | 380.0 | 380.0 |
| Dehulled soybean meal | 80.0 | 80.0 | 80.0 | 80.0 | 80.0 | 80.0 |
| Wheat gluten flour | 140.0 | 140.0 | 140.0 | 140.0 | 140.0 | 140.0 |
| Chicken powder | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 |
| Fish oil | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 |
| Soybean oil | 58.2 | 47.4 | 36.6 | 25.8 | 15.0 | 4.2 |
| Soybean lecithin | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 |
| Corn starch | 105.0 | 130.0 | 155.0 | 180.0 | 205.0 | 230.0 |
| Calcium dihydrogen phosphate | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 | 15.0 |
| Vitamin premix 1 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Mineral premix 1 | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 | 7.0 |
| Vitamin C | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 | 0.5 |
| Choline chloride | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Ethoxyquin | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Attractant | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Carboxymethyl Cellulose | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 | 1.0 |
| Cellulose | 71.0 | 56.8 | 42.6 | 28.4 | 14.2 | 0.0 |
| Total | 1000.0 | 1000.0 | 1000.0 | 1000.0 | 1000.0 | 1000.0 |
| Proximate composition 2 | ||||||
| Crude protein | 504.9 | 506.8 | 502.5 | 503.8 | 505.6 | 505.7 |
| Crude lipid | 188.0 | 172.6 | 159.4 | 144.8 | 130.7 | 122.4 |
| Nitrogen-free extract 3 | 153.4 | 178.4 | 203.4 | 222.7 | 253.4 | 278.4 |
| Gross energy (MJ/Kg) | 200.3 | 196.5 | 192.8 | 188.1 | 185.7 | 182.4 |
| Index | Dietary CHO:L Ratios | Pooled SEM | p-Values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.82 | 1.03 | 1.28 | 1.58 | 1.94 | 2.27 | Treatment | Linear | Quadratic | ||
| IBW (g) | 7.74 | 7.80 | 7.69 | 7.74 | 7.71 | 7.92 | 0.12 | 0.772 | 0.465 | 0.464 |
| FBW (g) | 56.57 | 57.44 | 57.32 | 63.56 | 57.33 | 58.08 | 2.35 | 0.364 | 0.626 | 0.425 |
| WGR (%) | 611.72 | 646.02 | 664.07 | 725.45 | 687.86 | 654.22 | 33.21 | 0.317 | 0.363 | 0.061 |
| SGR (%/d) | 3.50 | 3.59 | 3.63 | 3.77 | 3.69 | 3.60 | 0.07 | 0.280 | 0.342 | 0.046 |
| FCR | 0.96 | 1.01 | 1.00 | 0.99 | 1.02 | 1.07 | 0.02 | 0.055 | 0.012 | 0.521 |
| PER | 0.97 | 0.98 | 0.99 | 1.11 | 0.98 | 1.00 | 0.05 | 0.362 | 0.665 | 0.400 |
| SR (%) | 95.84 | 95.00 | 94.17 | 97.34 | 97.34 | 95.84 | 0.40 | 0.162 | 0.184 | 0.071 |
| PDR (%) | 39.45 | 38.30 | 39.51 | 38.31 | 38.93 | 36.95 | 0.41 | 0.547 | 0.200 | 0.507 |
| LDR (%) | 43.39 a | 40.50 b | 39.98 b | 40.92 b | 39.00 b | 39.29 b | 0.38 | <0.001 | <0.001 | 0.026 |
| HSI (%) | 1.79 a | 1.69 ab | 1.64 ab | 1.48 abc | 1.13 bc | 1.00 c | 0.08 | 0.006 | <0.001 | 0.280 |
| VSI (%) | 9.51 a | 8.69 ab | 8.85 ab | 7.84 ab | 8.07 ab | 7.53 b | 0.21 | 0.033 | 0.002 | 0.639 |
| CF (%) | 2.67 | 2.80 | 2.86 | 3.00 | 2.84 | 2.82 | 0.04 | 0.221 | 0.165 | 0.055 |
| Index | Dietary CHO:L Ratios | Pooled SEM | p-Values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.82 | 1.03 | 1.28 | 1.58 | 1.94 | 2.27 | Treatment | Linear | Quadratic | ||
| Moisture (wet) | 691.9 b | 697.7 ab | 700.3 ab | 700.6 ab | 701.6 ab | 707.5 a | 0.15 | 0.038 | 0.002 | 0.827 |
| Crude protein | 565.8 | 570.2 | 574.9 | 571.2 | 572.9 | 581.7 | 0.26 | 0.315 | 0.171 | 0.876 |
| Crude lipid | 346.4 a | 315.4 ab | 312.1 ab | 304.0 b | 292.3 b | 297.2 b | 0.50 | 0.666 | <0.001 | 0.055 |
| Ash | 139.7 | 143.6 | 158.7 | 155.0 | 152.5 | 153.1 | 0.26 | 0.273 | 0.092 | 0.134 |
| Index (g/kg) | Dietary CHO:L Ratios | Pooled SEM | p-Values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.82 | 1.03 | 1.28 | 1.58 | 1.94 | 2.27 | Treatment | Linear | Quadratic | ||
| Liver | ||||||||||
| Crude lipid | 586.4 a | 569.6 ab | 576.0 ab | 536.1 ab | 514.2 ab | 485.4 b | 2.53 | 0.015 | 0.002 | 0.009 |
| Glycogen | 273.3 ab | 377.7 a | 308.5 ab | 301.0 ab | 283.5 ab | 230.1 b | 1.38 | 0.027 | 0.033 | 0.026 |
| Muscle | ||||||||||
| Crude lipid | 390.1 c | 410.0 bc | 461.8 a | 458.3 a | 430.0 b | 387.0 c | 0.68 | 0.001 | 0.869 | <0.001 |
| Glycogen | 13.2 | 13.0 | 13.9 | 13.2 | 13.3 | 12.0 | 0.03 | 0.738 | 0.428 | 0.272 |
| Index (U/mg Protein) | Dietary CHO:L Ratios | Pooled SEM | p-Values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.82 | 1.03 | 1.28 | 1.58 | 1.94 | 2.27 | Treatment | Linear | Quadratic | ||
| Intestine | ||||||||||
| Trypsin | 2467.20 bc | 1443.87 d | 2910.72 b | 3718.29 a | 2405.41 bc | 2248.73 c | 169.45 | 0.001 | 0.712 | 0.219 |
| Lipase | 5.55 d | 9.26 c | 12.90 b | 15.47 a | 8.57 c | 4.52 e | 0.27 | <0.001 | 0.544 | <0.001 |
| Amylase | 0.25 a | 0.21 b | 0.18 c | 0.17 c | 0.15 d | 0.12 e | 0.006 | <0.001 | <0.001 | <0.001 |
| Liver | ||||||||||
| Trypsin | 1593.69 c | 1677.94 c | 1596.91 c | 2733.20 a | 2175.07 b | 830.77 d | 72.96 | <0.001 | 0.548 | 0.008 |
| Lipase | 3.59 a | 2.00 bc | 3.52 a | 2.54 b | 1.57 cd | 1.13 d | 0.21 | <0.001 | 0.005 | 0.019 |
| Amylase | 0.10 b | 0.14 a | 0.15 a | 0.10 b | 0.11 b | 0.11 b | 0.01 | <0.001 | 0.569 | 0.141 |
| Index (μm) | Dietary CHO:L Ratios | Pooled SEM | p-Values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.82 | 1.03 | 1.28 | 1.58 | 1.94 | 2.27 | Treatment | Linear | Quadratic | ||
| VL | 264.14 bc | 284.20 b | 253.60 bc | 309.68 a | 284.33 b | 238.83 c | 13.83 | <0.001 | 0.598 | <0.001 |
| VW | 36.87 a | 37.67 a | 34.21 a | 32.34 a | 31.67 ab | 25.64 b | 1.07 | 0.001 | <0.001 | 0.091 |
| MT | 37.58 c | 38.57 c | 35.89 c | 49.98 ab | 55.29 a | 49.52 b | 1.84 | <0.001 | <0.001 | 0.869 |
| Index (mmol/L) | Dietary CHO:L Ratios | Pooled SEM | p-Values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.82 | 1.03 | 1.28 | 1.58 | 1.94 | 2.27 | Treatment | Linear | Quadratic | ||
| TP | 49.77 | 51.03 | 50.57 | 50.76 | 51.34 | 49.62 | 0.87 | 0.684 | 0.898 | 0.344 |
| GLU | 4.78 b | 6.87 ab | 8.53 a | 8.01 ab | 8.11 ab | 8.64 a | 1.02 | 0.023 | 0.031 | 0.024 |
| TG | 1.03 a | 0.84 a | 0.80 a | 0.91 a | 0.81 a | 0.53 b | 0.08 | 0.048 | 0.011 | 0.033 |
| CHOL | 4.3 a | 3.73 ab | 4.01 a | 3.01 bc | 2.65 cd | 2.10 d | 0.25 | 0.001 | <0.001 | <0.001 |
| Index | Dietary CHO:L Ratios | Pooled SEM | p-Values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.82 | 1.03 | 1.28 | 1.58 | 1.94 | 2.27 | Treatment | Linear | Quadratic | ||
| HK (U/g protein) | 0.0048 c | 0.0057 b | 0.0060 b | 0.0070 a | 0.0073 a | 0.0059 b | 0.0002 | <0.001 | 0.023 | <0.001 |
| PFK-1 (U/g protein) | 131.95 d | 147.21 c | 160.47 b | 165.73 b | 177.85 a | 128.34 d | 1.73 | <0.001 | 0.193 | <0.001 |
| PK (mU/g protein) | 2.26 d | 2.51 b | 3.16 a | 3.32 a | 2.76 b | 2.38 cd | 0.06 | <0.001 | 0.875 | <0.001 |
| GP (U/g protein) | 0.93 a | 0.84 b | 0.76 c | 0.66 d | 0.86 b | 0.94 a | 0.02 | <0.001 | 0.681 | <0.001 |
| GS (U/g protein) | 2.53 d | 3.62 a | 3.37 b | 3.77 a | 3.63 a | 2.87 c | 0.06 | <0.001 | 0.654 | <0.001 |
| PEPCK (U/g protein) | 0.74 a | 0.71 a | 0.56 b | 0.52 b | 0.55 b | 0.45 c | 0.01 | <0.001 | 0.015 | 0.055 |
| Index (U/g protein) | Dietary CHO:L Ratios | Pooled SEM | p-Values | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.82 | 1.03 | 1.28 | 1.58 | 1.94 | 2.27 | Treatment | Linear | Quadratic | ||
| CPT | 1.98 d | 2.73 c | 3.64 b | 3.96 a | 4.05 a | 3.91 a | 0.05 | <0.001 | <0.001 | <0.001 |
| ATGL | 3.57 c | 3.80 b | 4.01 a | 4.12 a | 3.99 a | 3.40 c | 0.06 | <0.001 | 0.715 | <0.001 |
| HSL | 5.75 d | 6.96 c | 8.34 b | 11.43 a | 11.46 a | 8.67 b | 0.17 | <0.001 | 0.005 | <0.001 |
| LPL | 23.54 a | 20.71 ab | 15.65 ab | 13.60 b | 17.51 ab | 23.18 a | 2.24 | 0.007 | 0.888 | 0.005 |
| HL | 31.40 a | 27.91 ab | 29.18 ab | 16.20 c | 25.79 ab | 24.03 b | 1.96 | 0.006 | 0.140 | 0.056 |
| ACC | 240.01 e | 275.30 d | 302.05 c | 341.42 b | 371.14 a | 248.19 e | 3.54 | <0.001 | 0.273 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, X.; Guo, X.; Dong, X.; Yang, Q.; Liu, H.; Zhang, S.; Tan, B.; Chi, S. How Do Different Dietary Carbohydrate/Lipid Ratios Influence Intestinal Morphology and Glycolipid Metabolism Capacity in Hybrid Grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂). Fishes 2023, 8, 467. https://doi.org/10.3390/fishes8090467
Yang X, Guo X, Dong X, Yang Q, Liu H, Zhang S, Tan B, Chi S. How Do Different Dietary Carbohydrate/Lipid Ratios Influence Intestinal Morphology and Glycolipid Metabolism Capacity in Hybrid Grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂). Fishes. 2023; 8(9):467. https://doi.org/10.3390/fishes8090467
Chicago/Turabian StyleYang, Xuanyi, Xinwei Guo, Xiaohui Dong, Qihui Yang, Hongyu Liu, Shuang Zhang, Beiping Tan, and Shuyan Chi. 2023. "How Do Different Dietary Carbohydrate/Lipid Ratios Influence Intestinal Morphology and Glycolipid Metabolism Capacity in Hybrid Grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂)" Fishes 8, no. 9: 467. https://doi.org/10.3390/fishes8090467
APA StyleYang, X., Guo, X., Dong, X., Yang, Q., Liu, H., Zhang, S., Tan, B., & Chi, S. (2023). How Do Different Dietary Carbohydrate/Lipid Ratios Influence Intestinal Morphology and Glycolipid Metabolism Capacity in Hybrid Grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂). Fishes, 8(9), 467. https://doi.org/10.3390/fishes8090467