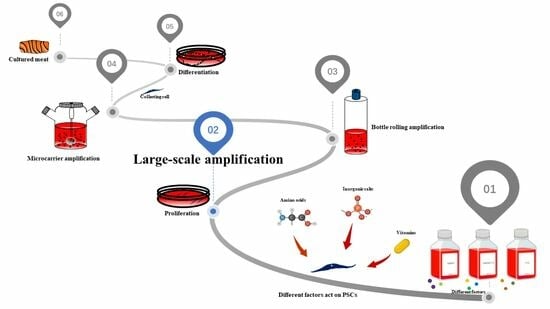
Characterization of Proliferation Medium and Its Effect on Differentiation of Muscle Satellite Cells from Larimichthys crocea in Cultured Fish Meat Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Isolation and Culture of PSCs
2.3. CCK8 Assay
2.4. Cell Cycle Analysis
2.5. Cell Proliferation and Differentiation
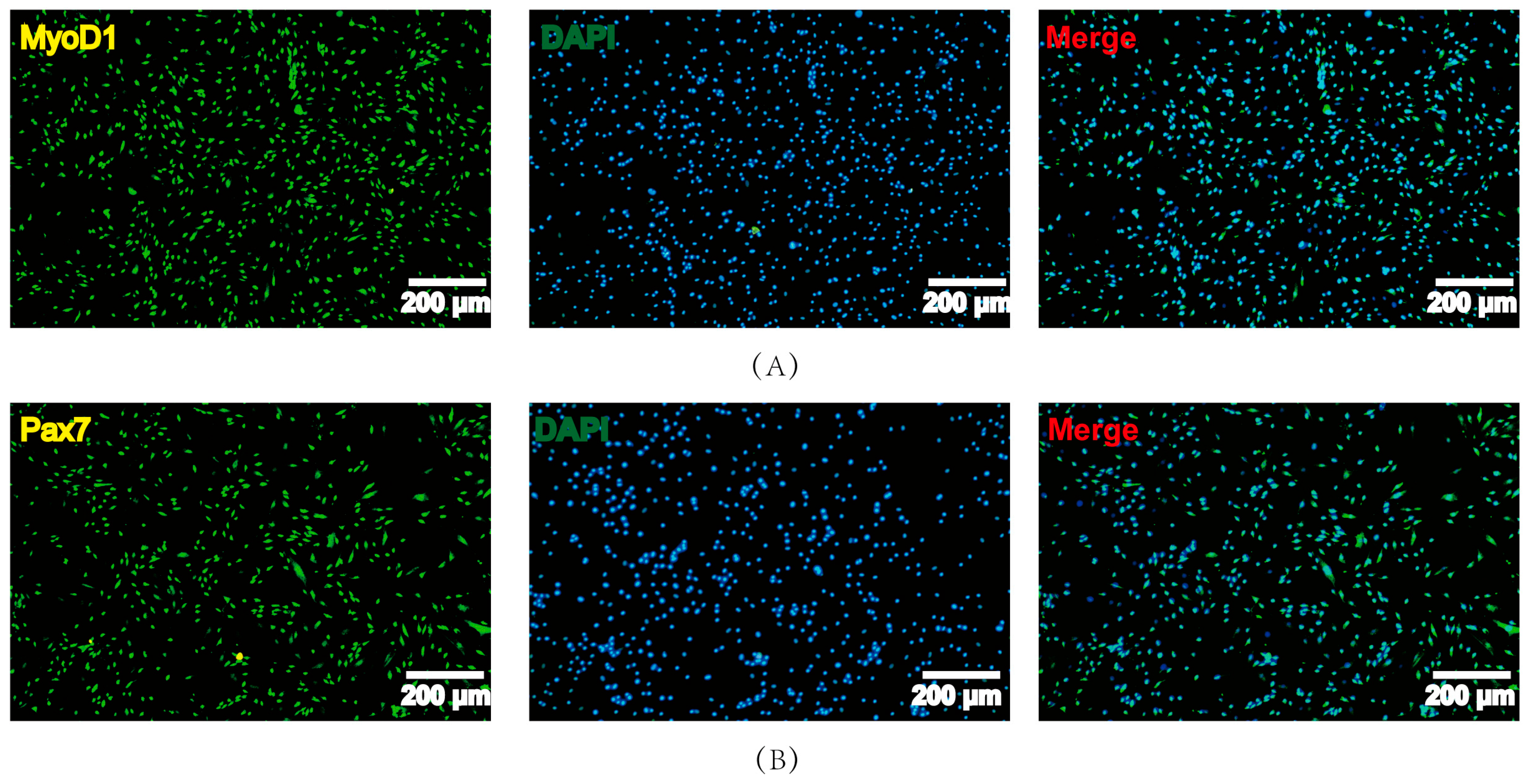
2.6. Immunofluorescence
2.7. RNA Extraction and Quantitative RT-PCR
2.8. Statistical Analysis
3. Results
3.1. Isolation and Identification of PSCs
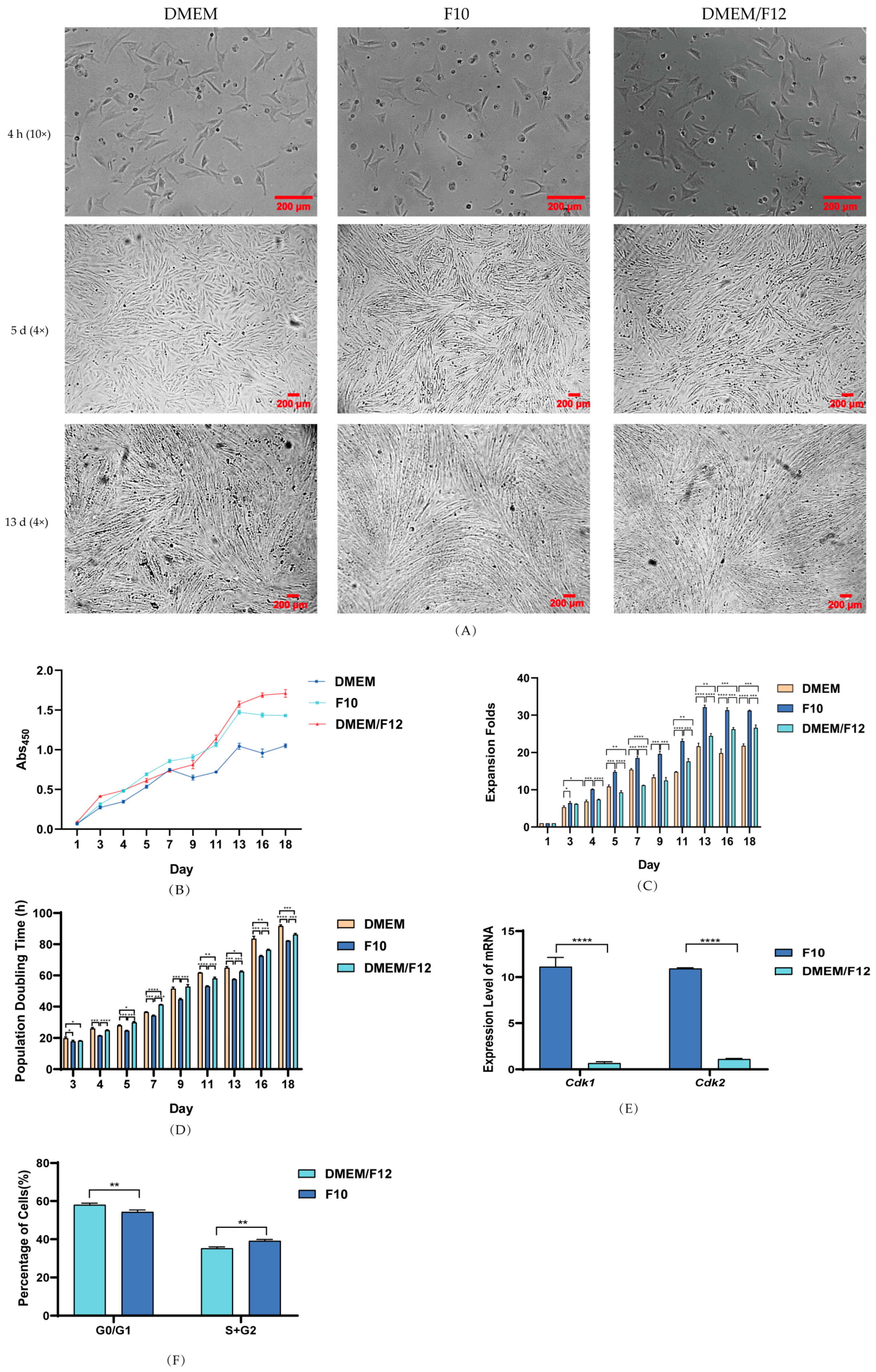
3.2. Effect of Culture Media on PSC Proliferation
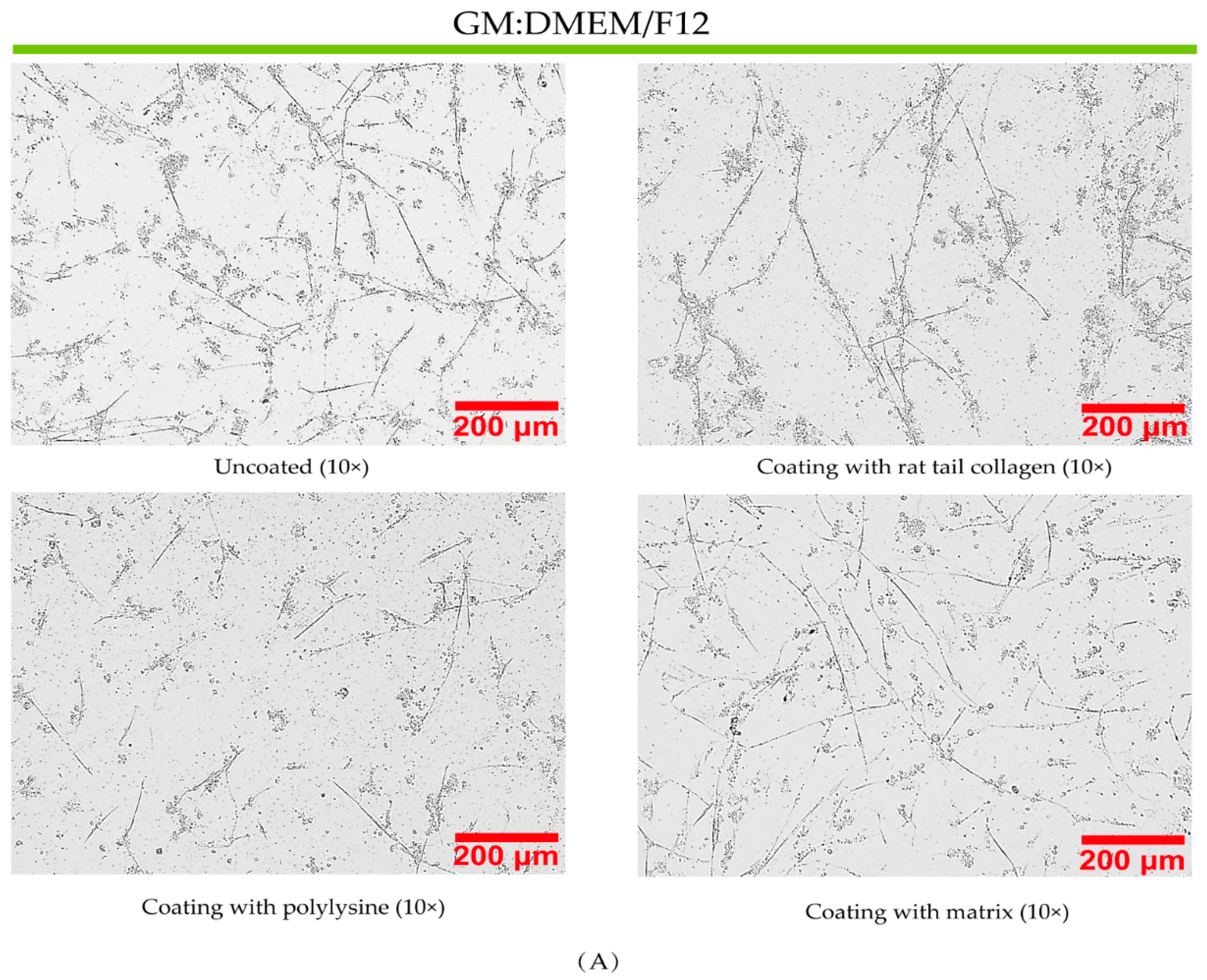
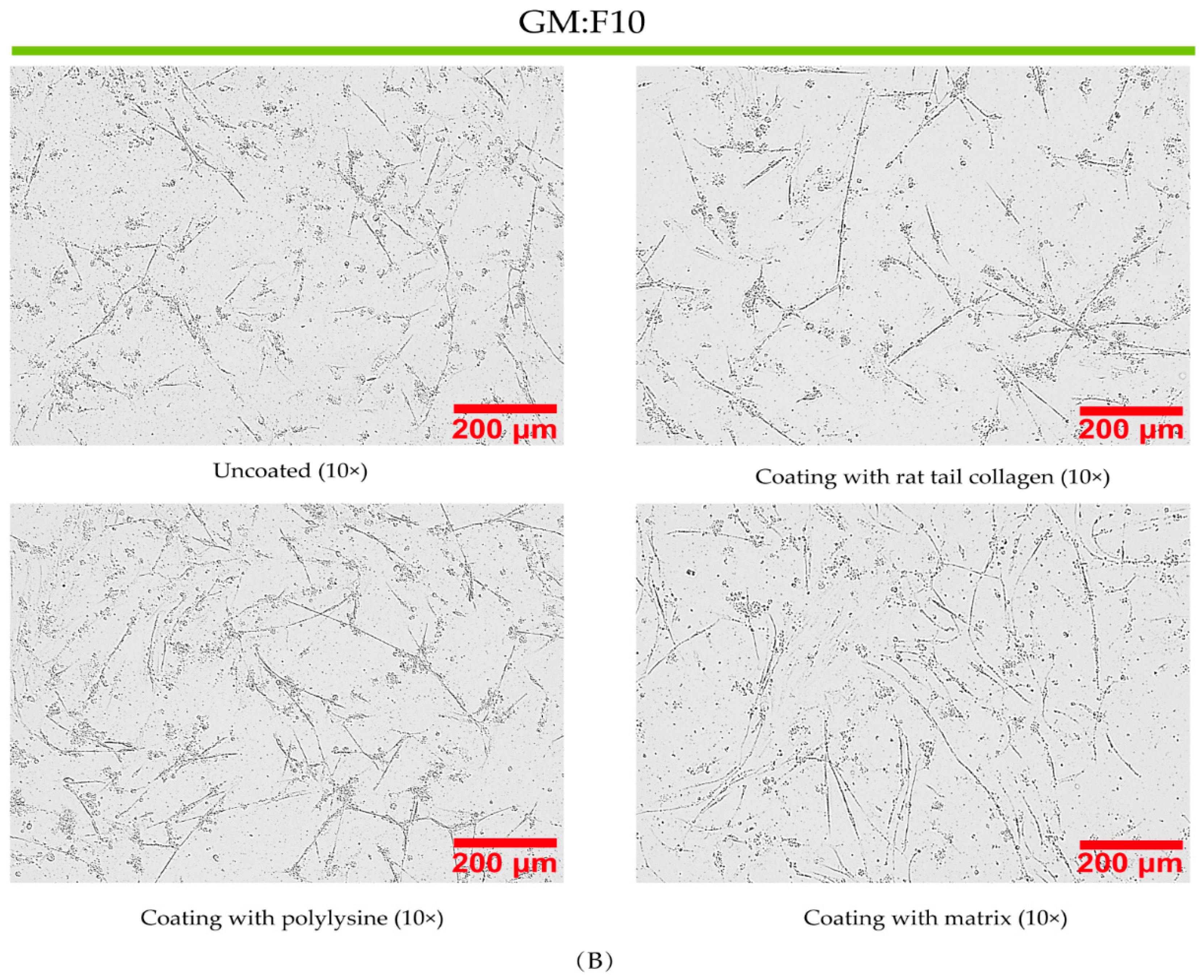
3.3. Effect of Coated and Uncoated Materials on PSC Proliferation
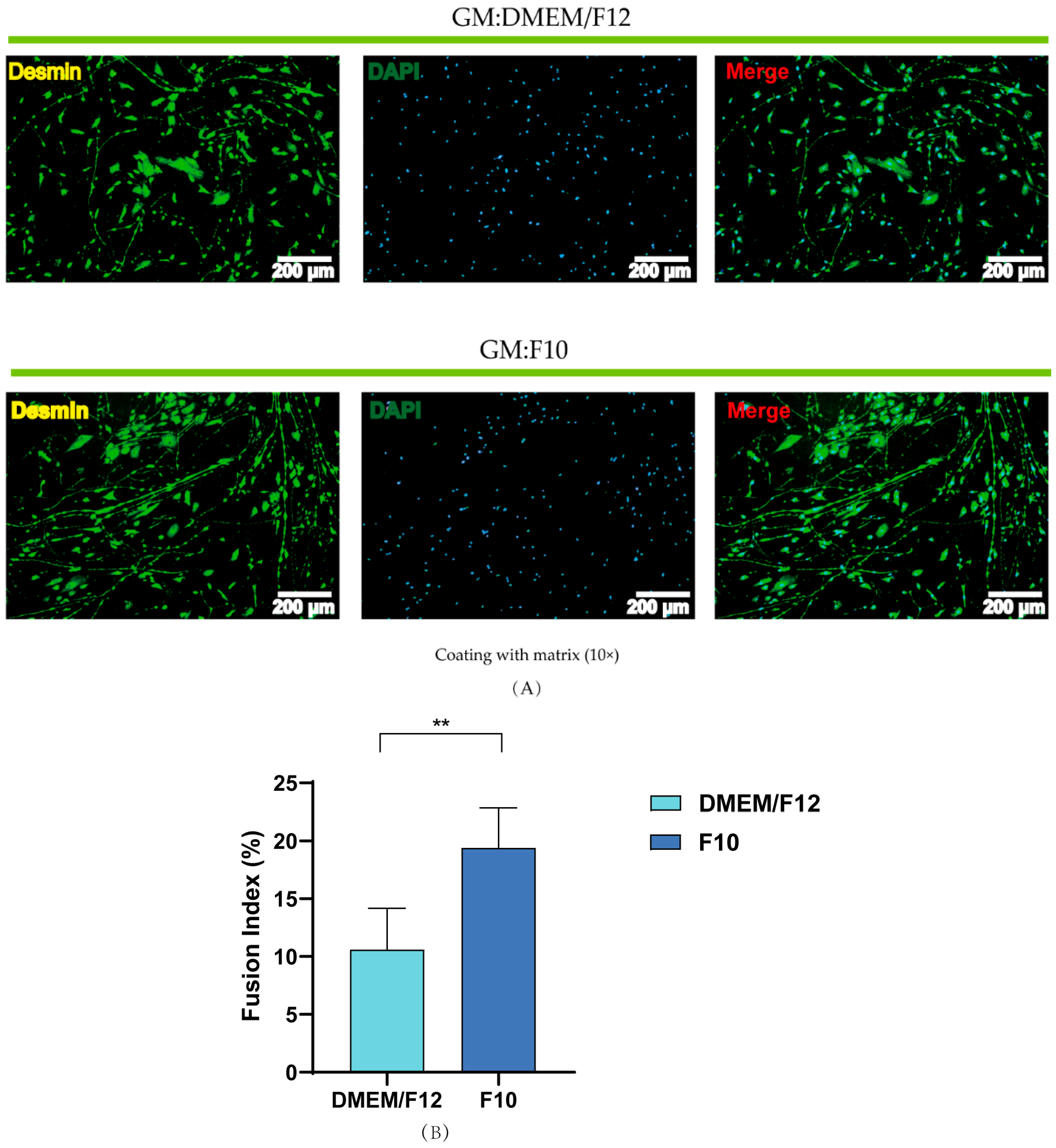
3.4. Effects of Basal Media and Coated Materials on PSC Proliferation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, L.; Shi, X.; Su, Y.; Meng, Z.; Lin, H. Loss of genetic diversity in the cultured stocks of the large yellow croaker, Larimichthys crocea, revealed by microsatellites. Int. J. Mol. Sci. 2012, 13, 5584–5597. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Lee, S.Y.; Jung, J.W.; Kim, J.H.; Oh, D.H.; Kim, H.W.; Kang, J.H.; Choi, J.S.; Kim, G.D.; Joo, S.T.; et al. Review of technology and materials for the development of cultured meat. Crit. Rev. Food Sci. Nutr. 2022, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Xu, E.; Niu, R.; Lao, J.; Zhang, S.; Li, J.; Zhu, Y.; Shi, H.; Zhu, Q.; Chen, Y.; Jiang, Y.; et al. Tissue-like cultured fish fillets through a synthetic food pipeline. NPJ Sci. Food 2023, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Bols, N.C.; Lee, L.E.J.; Dowd, G.C. Distinguishing between ante factum and post factum properties of animal cell lines and demonstrating their use in grouping ray-finned fish cell lines into invitromes. In Vitro Cell Dev. Biol. Anim. 2023, 59, 41–62. [Google Scholar] [CrossRef]
- Goswami, M.; Pinto, N.; Yashwanth, B.S.; Sathiyanarayanan, A.; Ovissipour, R. Development of a cell line from skeletal trunk muscle of the fish Labeo rohita. Cytotechnology 2023, 75, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Kadim, I.T.; Mahgoub, O.; Baqir, S.; Faye, B.; Purchas, R. Cultured meat from muscle stem cells: A review of challenges and prospects. J. Integr. Agric. 2015, 14, 222–233. [Google Scholar] [CrossRef]
- Manneken, J.D.; Dauer, M.V.P.; Currie, P.D. Dynamics of muscle growth and regeneration: Lessons from the teleost. Exp. Cell Res. 2022, 411, 112991. [Google Scholar] [CrossRef]
- Song, W.J.; Liu, P.P.; Meng, Z.Q.; Jie Ding, S.; Xia Li, H. N-acetylcysteine promotes the proliferation of porcine adipose-derived stem cells during in vitro long-term expansion for cultured meat production. Food Res. Int. 2023, 166, 112606. [Google Scholar] [CrossRef]
- Post, M.J. Cultured beef: Medical technology to produce food. J. Sci. Food Agric. 2014, 94, 1039–1041. [Google Scholar] [CrossRef]
- Hong, T.K.; Shin, D.M.; Choi, J.; Do, J.T.; Han, S.G. Current Issues and Technical Advances in Cultured Meat Production: A Review. Food Sci. Anim. Resour. 2021, 41, 355–372. [Google Scholar] [CrossRef]
- Kang, D.H.; Louis, F.; Liu, H.; Shimoda, H.; Nishiyama, Y.; Nozawa, H.; Kakitani, M.; Takagi, D.; Kasa, D.; Nagamori, E.; et al. Engineered whole cut meat-like tissue by the assembly of cell fibers using tendon-gel integrated bioprinting. Nat. Commun. 2021, 12, 5059. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Wu, Z.; Ding, X.; Post, M.J.; Guo, R.; Wang, J.; Wu, J.; Tang, W.; Ding, S.; Zhou, G. Production of cultured meat from pig muscle stem cells. Biomaterials 2022, 287, 121650. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Suyama, Y.; Notsu, T.; Fukuoka, K.; Ikuta, K.; Kanayama, H.; Umeda, R.; Teraoka, S.; Minato, H.; Ninomiya, H.; et al. Effects of Conditioned Medium of Adipose-Derived Stem Cells Exposed to Platelet-Rich Plasma on the Expression of Endothelial Nitric Oxide Synthase and Angiogenesis by Endothelial Cells. Ann. Plast. Surg. 2023, 90, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.K.; Odena, A.; Karottki, K.J.C.; Kim, C.L.; Hefzi, H.; Lee, G.M.; Faustrup Kildegaard, H.; Nielsen, L.K.; Grav, L.M.; Lewis, N.E. Enhancing CHO cell productivity through a dual selection system using Aspg and Gs in glutamine free medium. Biotechnol. Bioeng. 2022, 120, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Lund, M.; Heaton, R.; Hargreaves, I.P.; Gregersen, N.; Olsen, R.K.J. Odd- and even-numbered medium-chained fatty acids protect against glutathione depletion in very long-chain acyl-CoA dehydrogenase deficiency. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2023, 1868, 159248. [Google Scholar] [CrossRef]
- Farahbakhshian, E.; Verstegen, M.M.; Visser, T.P.; Kheradmandkia, S.; Geerts, D.; Arshad, S.; Riaz, N.; Grosveld, F.; van Til, N.P.; Meijerink, J.P. Angiopoietin-like protein 3 promotes preservation of stemness during ex vivo expansion of murine hematopoietic stem cells. PLoS ONE 2014, 9, e105642. [Google Scholar] [CrossRef]
- Fu, X.; Xiao, J.; Wei, Y.; Li, S.; Liu, Y.; Yin, J.; Sun, K.; Sun, H.; Wang, H.; Zhang, Z.; et al. Combination of inflammation-related cytokines promotes long-term muscle stem cell expansion. Cell Res. 2015, 25, 1082–1083. [Google Scholar] [CrossRef]
- Ng, C.P.; Sharif, A.R.; Heath, D.E.; Chow, J.W.; Zhang, C.B.; Chan-Park, M.B.; Hammond, P.T.; Chan, J.K.; Griffith, L.G. Enhanced ex vivo expansion of adult mesenchymal stem cells by fetal mesenchymal stem cell ECM. Biomaterials 2014, 35, 4046–4057. [Google Scholar] [CrossRef]
- Yen, F.C.; Glusac, J.; Levi, S.; Zernov, A.; Baruch, L.; Davidovich-Pinhas, M.; Fishman, A.; Machluf, M. Cultured meat platform developed through the structuring of edible microcarrier-derived microtissues with oleogel-based fat substitute. Nat. Commun. 2023, 14, 2942. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, R.; Ding, S.; Deng, L.; Zhang, Y.; Li, J.; Shi, Z.; Wu, Z.; Liang, K.; Yan, X.; et al. Engineered meatballs via scalable skeletal muscle cell expansion and modular micro-tissue assembly using porous gelatin micro-carriers. Biomaterials 2022, 287, 121615. [Google Scholar] [CrossRef]
- Messmer, T.; Klevernic, I.; Furquim, C.; Ovchinnikova, E.; Dogan, A.; Cruz, H.; Post, M.J.; Flack, J.E. A serum-free media formulation for cultured meat production supports bovine satellite cell differentiation in the absence of serum starvation. Nat. Food 2022, 3, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Wagner, W.; Horn, P.; Castoldi, M.; Diehlmann, A.; Bork, S.; Saffrich, R.; Benes, V.; Blake, J.; Pfister, S.; Eckstein, V.; et al. Replicative senescence of mesenchymal stem cells: A continuous and organized process. PLoS ONE 2008, 3, e2213. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.; Li, M.; Du, G.; Zhou, J.; Guan, X. An effective cytokine combination for ex vivo expansion of porcine muscle stem cells. Food Biosci. 2022, 46, 101571. [Google Scholar] [CrossRef]
- Mahadevan, M.M.; Miller, M.M.; Moutos, D.M. Improved human zygote development in a modified Ham’s F1O medium in vitro. J. Assist. Reprod. Genet. 1996, 13, 722–725. [Google Scholar] [CrossRef] [PubMed]
- Fayaz, M.A.; Honaramooz, A. Culture media and supplements affect proliferation, colony-formation, and potency of porcine male germ cells. Theriogenology 2022, 187, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Pahlavanneshan, S.; Behmanesh, M.; Tahamtani, Y.; Hajizadeh-Saffar, E.; Basiri, M.; Baharvand, H. Induction of ss Cell Replication by Small Molecule-Mediated Menin Inhibition and Combined PKC Activation and TGF-ss Inhibition as Revealed by A Refined Primary Culture Screening. Cell J. 2021, 23, 633–639. [Google Scholar] [CrossRef]
- Hua, J.; Wei, Y.; Zhang, Y.; Xu, H.; Ge, J.; Liu, M.; Wang, Y.; Shi, Y.; Hou, L.; Jiang, H. Adaptation process of engineered cell line FCHO/IL-24 stably secreted rhIL-24 in serum-free suspension culture. Protein Expr. Purif. 2022, 199, 106154. [Google Scholar] [CrossRef]
- Wei, N.; Liu, Y.P.; Wang, R.R.; Zhong, Z.J.; Wang, X.L.; Yang, Y.; He, T.; Zhao, S.J.; Wang, H.; Yu, Y.Q. Glutamine Maintains Satellite Glial Cells Growth and Survival in Culture. Neurochem. Res. 2022, 47, 3635–3646. [Google Scholar] [CrossRef]
- Du, H.; Chen, Z.; Gong, X.; Jiang, M.; Chen, G.; Wang, F. Surface grafting of sericin onto thermoplastic polyurethanes to improve cell adhesion and function. J. Biomater. Sci. Polym. Ed. 2023, 34, 1382–1397. [Google Scholar] [CrossRef]
- Dhania, S.; Rani, R.; Kumar, R.; Thakur, R. Fabricated polyhydroxyalkanoates blend scaffolds enhance cell viability and cell proliferation. J. Biotechnol. 2023, 361, 30–40. [Google Scholar] [CrossRef]
- Zhu, L.; Yuhan, J.; Yu, H.; Zhang, B.; Huang, K.; Zhu, L. Decellularized Extracellular Matrix for Remodeling Bioengineering Organoid’s Microenvironment. Small 2023, 19, 2207752. [Google Scholar] [CrossRef] [PubMed]
- Louisthelmy, R.; Burke, B.M.; Cornelison, R.C. Brain Cancer Cell-Derived Matrices and Effects on Astrocyte Migration. Cells Tissues Organs 2023, 212, 21–31. [Google Scholar] [CrossRef] [PubMed]

| Gene | Primer Sequences (5′ > 3′) |
|---|---|
| β-actin | Forward: TGACGCGGTATAAAAGGCGA |
| Reverse: ACCAACCATCACACCCTGAT | |
| Cdk1 | Forward: GCTCTTCAGGATCTTCAGGACTCT |
| Reverse: ATTGATGACAGGTTGCCAGACTTC | |
| Cdk2 | Forward: CACGGCACCAGTATCCAGTATGA |
| Reverse: CGTCTTGAGGTCTTGGTCCACAT |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, S.; Lou, H.; Lu, H.; Xu, E.; Liu, D.; Chen, Q. Characterization of Proliferation Medium and Its Effect on Differentiation of Muscle Satellite Cells from Larimichthys crocea in Cultured Fish Meat Production. Fishes 2023, 8, 429. https://doi.org/10.3390/fishes8090429
Zhang S, Lou H, Lu H, Xu E, Liu D, Chen Q. Characterization of Proliferation Medium and Its Effect on Differentiation of Muscle Satellite Cells from Larimichthys crocea in Cultured Fish Meat Production. Fishes. 2023; 8(9):429. https://doi.org/10.3390/fishes8090429
Chicago/Turabian StyleZhang, Shengliang, Hanghang Lou, Hongyun Lu, Enbo Xu, Donghong Liu, and Qihe Chen. 2023. "Characterization of Proliferation Medium and Its Effect on Differentiation of Muscle Satellite Cells from Larimichthys crocea in Cultured Fish Meat Production" Fishes 8, no. 9: 429. https://doi.org/10.3390/fishes8090429
APA StyleZhang, S., Lou, H., Lu, H., Xu, E., Liu, D., & Chen, Q. (2023). Characterization of Proliferation Medium and Its Effect on Differentiation of Muscle Satellite Cells from Larimichthys crocea in Cultured Fish Meat Production. Fishes, 8(9), 429. https://doi.org/10.3390/fishes8090429