Some Like It Hot: Investigating Thermoregulatory Behavior of Carcharhinid Sharks in a Natural Environment with Artificially Elevated Temperatures
Abstract
:1. Introduction
2. Methods
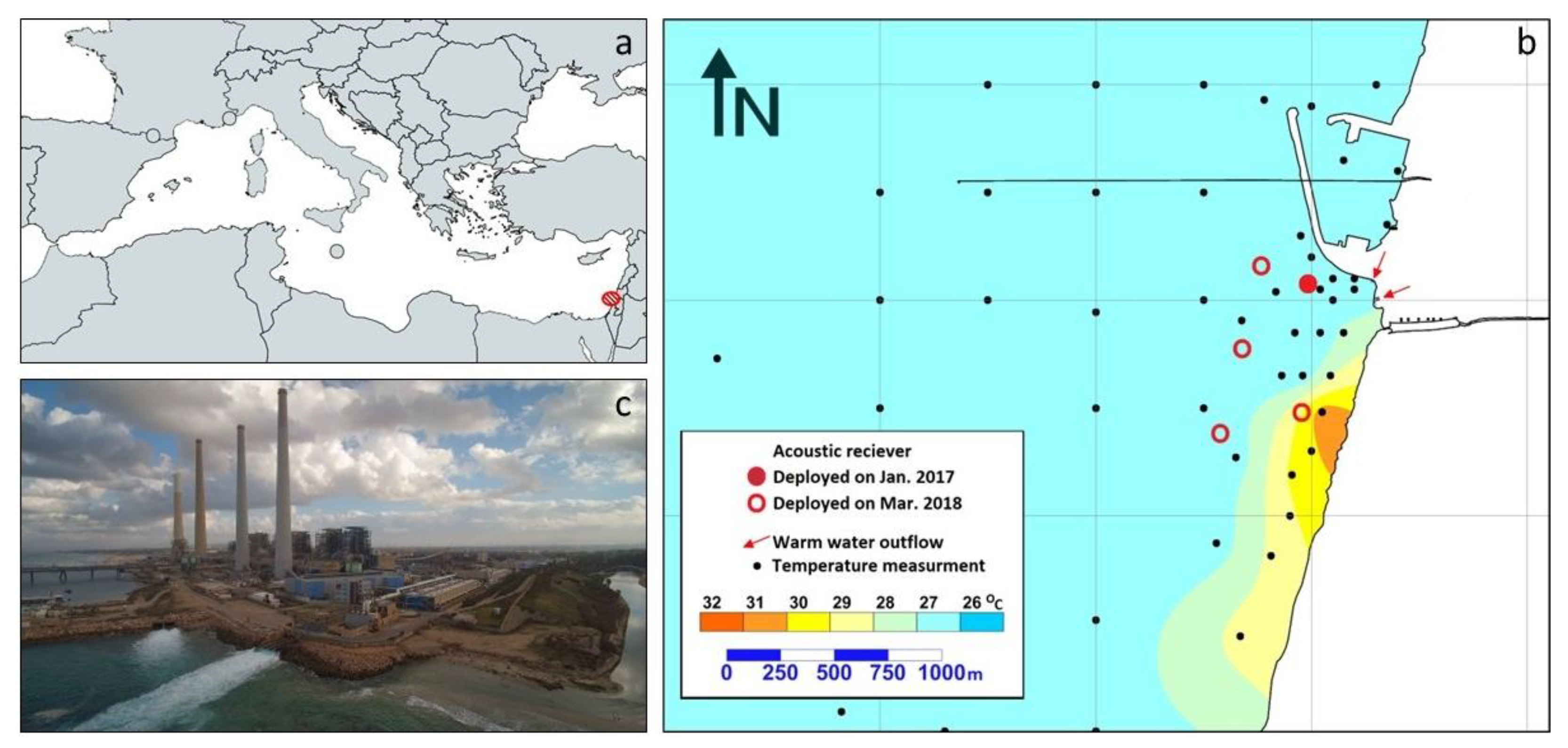
2.1. Study Site
2.2. Shark Tagging
2.3. Water Temperature Measurements
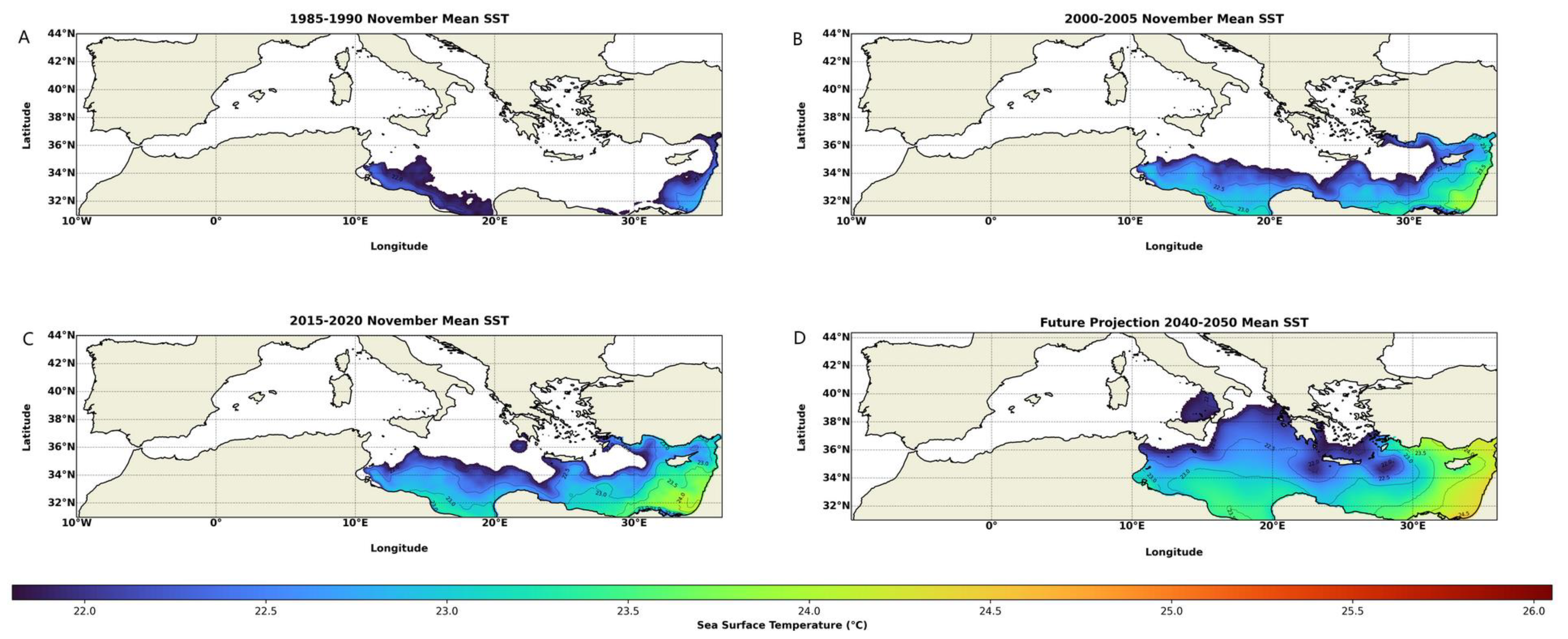
2.4. Mediterranean Water Temperature Measurements and Predictions
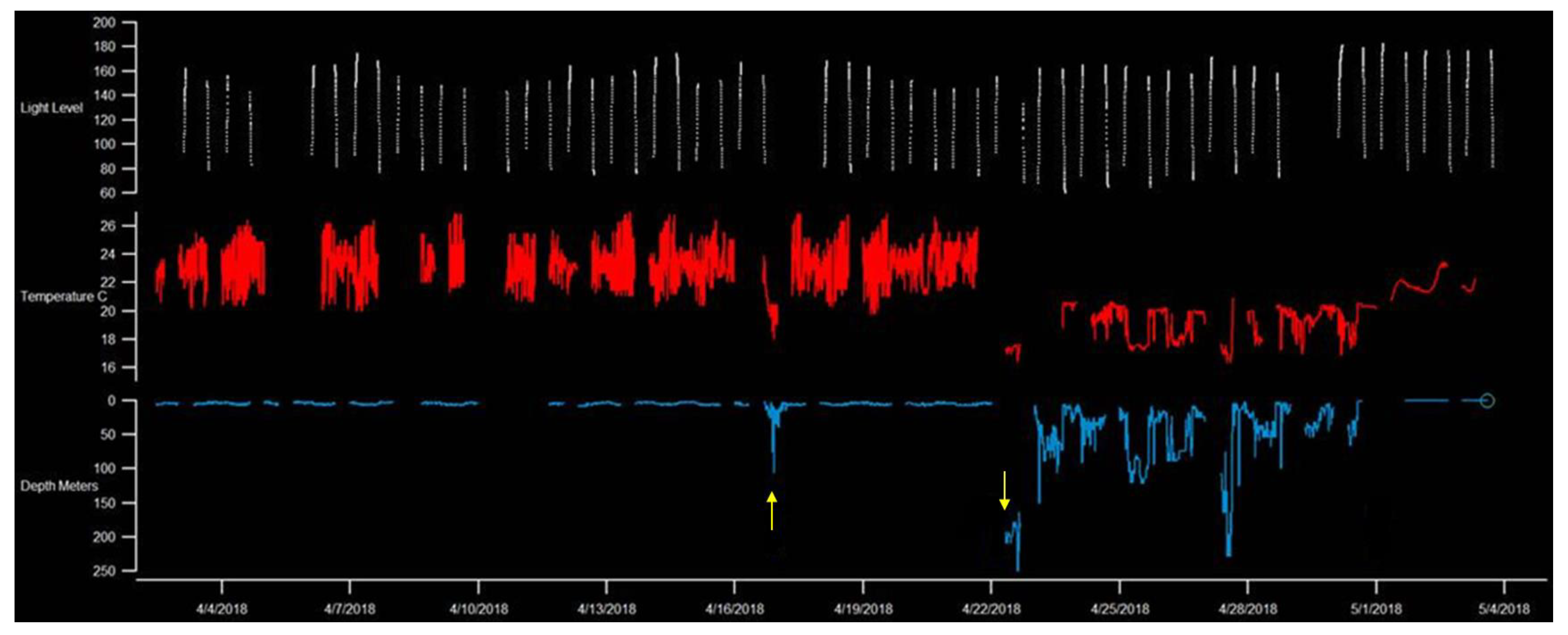
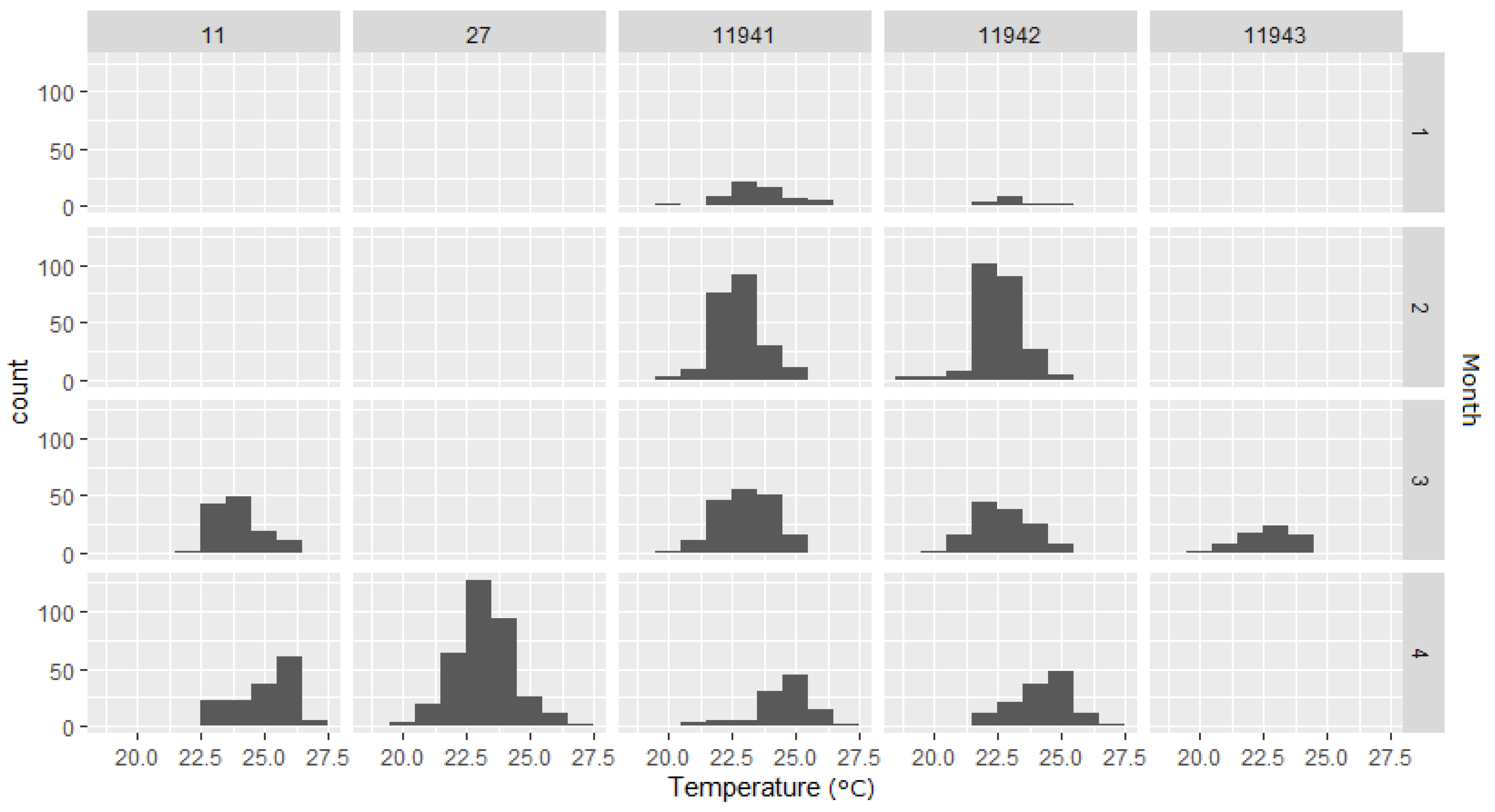
3. Results
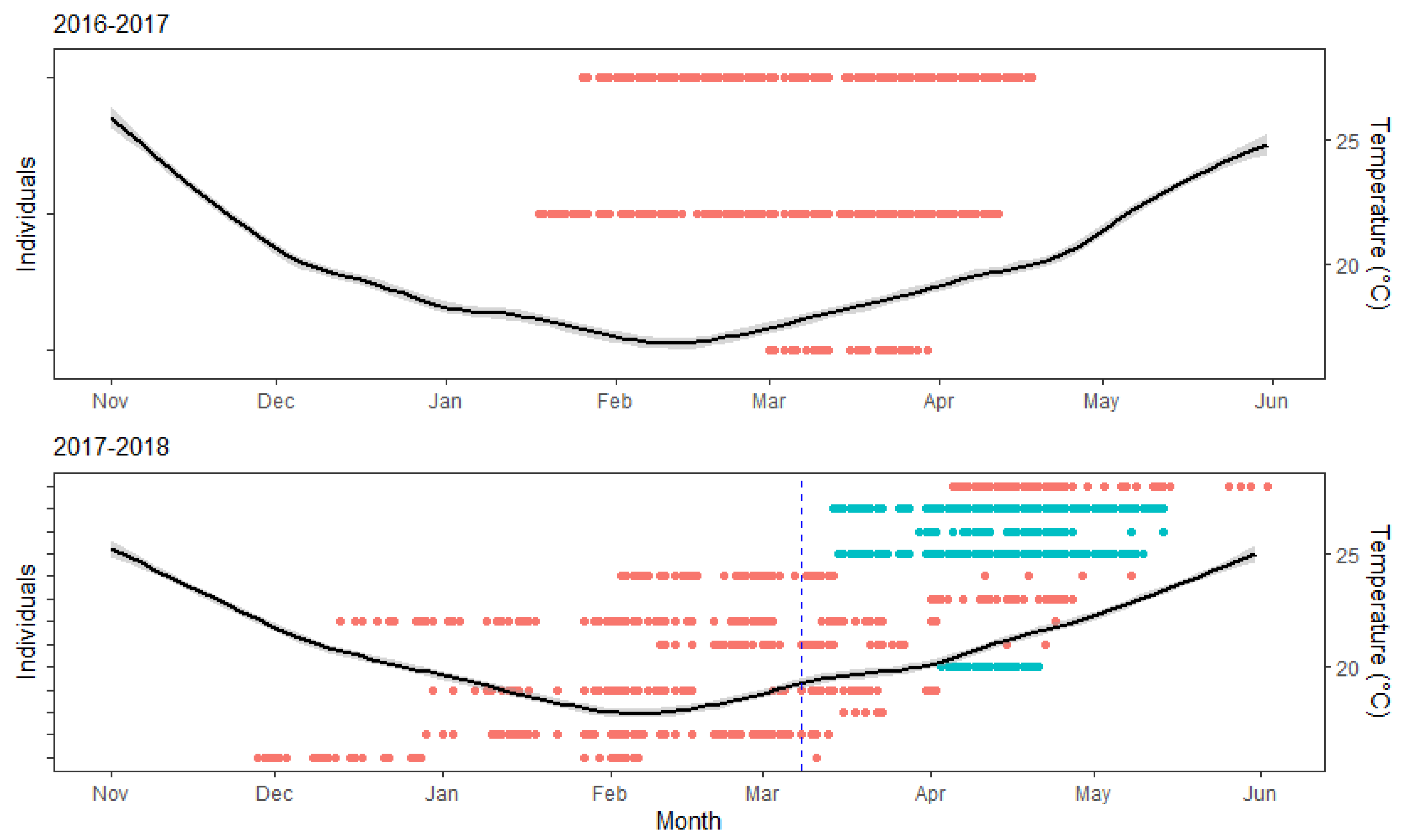
3.1. Residency and Date of Departure
3.2. Temperature
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Humphries, N.E.; Queiroz, N.; Dyer, J.R.M.; Pade, N.G.; Musyl, M.K.; Schaefer, K.M.; Fuller, D.W.; Brunnschweiler, J.M.; Doyle, T.K.; Houghton, J.D.R. Environmental Context Explains Lévy and Brownian Movement Patterns of Marine Predators. Nature 2010, 465, 1066–1069. [Google Scholar] [CrossRef]
- Lara-Lizardi, F.; Hoyos-Padilla, E.M.; Klimley, A.P.; Grau, M.; Ketchum, J.T. Movement Patterns and Residency of Bull Sharks, Carcharhinus Leucas, in a Marine Protected Area of the Gulf of California. Environ. Biol. Fishes 2022, 105, 1765–1779. [Google Scholar] [CrossRef]
- Braccini, M.; Taylor, S. The Spatial Segregation Patterns of Sharks from Western Australia. R. Soc. Open Sci. 2016, 3, 160306. [Google Scholar] [CrossRef] [PubMed]
- Kock, A.; O’Riain, M.J.; Mauff, K.; Meÿer, M.; Kotze, D.; Griffiths, C. Residency, Habitat Use and Sexual Segregation of White Sharks, Carcharodon Carcharias in False Bay, South Africa. PLoS ONE 2013, 8, e55048. [Google Scholar] [CrossRef] [PubMed]
- Mucientes, G.R.; Queiroz, N.; Sousa, L.L.; Tarroso, P.; Sims, D.W. Sexual Segregation of Pelagic Sharks and the Potential Threat from Fisheries. Biol. Lett. 2009, 5, 156–159. [Google Scholar] [CrossRef]
- Afonso, A.S.; Hazin, F.H.V. Vertical Movement Patterns and Ontogenetic Niche Expansion in the Tiger Shark, Galeocerdo Cuvier. PLoS ONE 2015, 10, e0116720. [Google Scholar] [CrossRef]
- Porter, Z.C. Thermal Niche Requirements of the White-Spotted Bamboo Shark Chiloscyllium Plagiosum; The University of West Florida: Pensacola, FL, USA, 2020; ISBN 9798664798227. [Google Scholar]
- Serena, F. Field Identification Guide to the Sharks and Rays of the Mediterranean and Black Sea; Food & Agriculture Org.: Rome, Italy, 2005; ISBN 9251052913. [Google Scholar]
- Serena, F.; Abella, A.J.; Bargnesi, F.; Barone, M.; Colloca, F.; Ferretti, F.; Fiorentino, F.; Jenrette, J.; Moro, S. Species Diversity, Taxonomy and Distribution of Chondrichthyes in the Mediterranean and Black Sea. Eur. Zool. J. 2020, 87, 497–536. [Google Scholar] [CrossRef]
- Ebert, D.A.; Dando, M. Field Guide to Sharks, Rays & Chimaeras of Europe and the Mediterranean; Princeton University Press: Princeton, NJ, USA, 2020; ISBN 0691205981. [Google Scholar]
- IUCN Carcharhinus Obscurus (Dusky Shark). Available online: https://www.iucnredlist.org/species/3852/2872747#assessment-information (accessed on 15 March 2023).
- IUCN Carcharhinus Plumbeus (Sandbar Shark). Available online: https://www.iucnredlist.org/species/3853/2874370#assessment-information (accessed on 15 March 2023).
- Ferretti, F.; Walls, R.H.L.; Musick, J.; Stevens, J.; Baum, J.K.; Bradai, M.N.; Fergusson, I.; Grubbs, D.; Soldo, A.; Vacchi, M.; et al. Carcharhinus Plumbeus. The IUCN Red List of Threatened Species 2016: E.T3853A16527809. 2016. Available online: https://www.iucnredlist.org/species/3853/16527809 (accessed on 19 August 2022).
- Barash, A.; Pickholtz, R.; Pickholtz, E.; Blaustein, L.; Rilov, G. Seasonal Aggregations of Sharks near Coastal Power Plants in Israel: An Emerging Phenomenon. Mar. Ecol. Prog. Ser. 2018, 590, 145–154. [Google Scholar] [CrossRef]
- Barash, A.; Scheinin, A.; Bigal, E.; Zemah Shamir, Z.; Martinez, S.; Tchernov, D. Depth Partitioning and Diel Movement of Two Large Carcharhinid Sharks in Extremely Shallow Waters. Fishes 2023, 8, 85. [Google Scholar] [CrossRef]
- Mancusi, C.; Baino, R.; Fortuna, C.; de Sola, L.G.; Morey, G.; Bradai, M.N.; Kallianotis, A.; Soldo, A.; Hemida, F.; Saad, A.A. MEDLEM Database, a Data Collection on Large Elasmobranchs in the Mediterranean and Black Seas. Mediterr. Mar. Sci. 2020, 21, 276–288. [Google Scholar] [CrossRef]
- Kabasakal, H.; Karhan, S.Ü.; Sakınan, S. Review of the Distribution of Large Sharks in the Seas of Turkey (Eastern Mediterranean). Cah. Biol. Mar. 2017, 58, 219–228. [Google Scholar]
- Musick, J.; Grubbs, D.; Baum, J.K.; Cortés, E. Carcharhinus obscurus (Mediterranean Assessment). The IUCN Red List of Threatened Species 2016: E.T3852A16527849. 2016. Available online: https://www.iucnredlist.org/species/3852/16527849 (accessed on 21 March 2021).
- Filiz, H.; Gulsahin, A. First 12 Months of Sandbar Shark Monitoring in Turkey. In Proceedings of the 11th Panhellenic Symposium on Oceanography and Fisheries, Mytilene, Lesvos Island, Greece, 13–17 May 2015. [Google Scholar]
- Merchant, C.J.; Embury, O.; Bulgin, C.E.; Block, T.; Corlett, G.K.; Fiedler, E.; Good, S.A.; Mittaz, J.; Rayner, N.A.; Berry, D. Satellite-Based Time-Series of Sea-Surface Temperature since 1981 for Climate Applications. Sci. Data 2019, 6, 223. [Google Scholar] [CrossRef] [PubMed]
- Tyberghein, L.; Verbruggen, H.; Pauly, K.; Troupin, C.; Mineur, F.; de Clerck, O. Bio-ORACLE: A Global Environmental Dataset for Marine Species Distribution Modelling. Glob. Ecol. Biogeogr. 2012, 21, 272–281. [Google Scholar] [CrossRef]
- Schwalm, C.R.; Glendon, S.; Duffy, P.B. RCP8.5 Tracks Cumulative CO2 Emissions. Proc. Natl. Acad. Sci. USA 2020, 117, 19656–19657. [Google Scholar] [CrossRef]
- Van Rossum, G.; Fred, L.D. Python Reference Manual. Vol. 111. Amsterdam: Centrum voor Wiskunde en Informatica. 1995. Available online: http://www.cs.cmu.edu/afs/cs.cmu.edu/project/gwydion-1/OldFiles/OldFiles/python/Doc/ref.ps (accessed on 21 March 2021).
- Bres, M. The Behaviour of Sharks. Rev. Fish Biol. Fish. 1993, 3, 133–159. [Google Scholar] [CrossRef]
- Romine, J.G.; Musick, J.A.; Burgess, G.H. Demographic Analyses of the Dusky Shark, Carcharhinus obscurus, in the Northwest Atlantic Incorporating Hooking Mortality Estimates and Revised Reproductive Parameters. Environ. Biol. Fishes 2009, 84, 277–289. [Google Scholar] [CrossRef]
- Finke, D.L.; Snyder, W.E. Niche Partitioning Increases Resource Exploitation by Diverse Communities. Science 2008, 321, 1488–1490. [Google Scholar] [CrossRef]
- Lear, K.O.; Whitney, N.M.; Morris, J.J.; Gleiss, A.C. Temporal Niche Partitioning as a Novel Mechanism Promoting Co-Existence of Sympatric Predators in Marine Systems. Proc. R. Soc. B 2021, 288, 20210816. [Google Scholar] [CrossRef]
- Dwyer, R.G.; Campbell, H.A.; Cramp, R.L.; Burke, C.L.; Micheli-Campbell, M.A.; Pillans, R.D.; Lyon, B.J.; Franklin, C.E. Niche Partitioning between River Shark Species Is Driven by Seasonal Fluctuations in Environmental Salinity. Funct. Ecol. 2020, 34, 2170–2185. [Google Scholar] [CrossRef]
- Casper, B.M.; Halvorsen, M.B.; Popper, A.N. Are Sharks Even Bothered by a Noisy Environment? Adv. Exp. Med. Biol. 2012, 730, 93–97. [Google Scholar]
- Weilgart, L. The Impact of Ocean Noise Pollution on Fish and Invertebrates; Report for OceanCare; OceanCare: Wadenswil, Switzerland, 2018. [Google Scholar]
- de Sousa Rangel, B.; Hammerschlag, N.; Moreira, R.G. Urban Living Influences the Nutritional Quality of a Juvenile Shark Species. Sci. Total Environ. 2021, 776, 146025. [Google Scholar] [CrossRef]
- Gelsleichter, J.; Manire, C.A.; Szabo, N.J.; Cortés, E.; Carlson, J.; Lombardi-Carlson, L. Organochlorine Concentrations in Bonnethead Sharks (Sphyrna tiburo) from Four Florida Estuaries. Arch. Environ. Contam. Toxicol. 2005, 48, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Zemah-Shamir, Z. The Human–Wildlife Conflict as a Global Challenge: Anthropogenic Factors Affecting Shark Behavior and Physiology. Winter Aggregation of Sharks at Hadera as a Case Study. Ph.D. Thesis, University of Haifa, Haifa, Israel, 2021. [Google Scholar]
- Shamir, Z.Z.; Shamir, S.Z.; Becker, N.; Scheinin, A.; Tchernov, D. Evidence of the Impacts of Emerging Shark Tourism in the Mediterranean. Ocean Coast Manag. 2019, 178, 104847. [Google Scholar] [CrossRef]
- Barash, A.; Salingre, S.; Grosmark, Y.; Rothman, S. The MECO Project (Mediterranean Elasmobranch Citizen Observations): Creating a Large-Scale Database of Elasmobranchs Observations Using Social. In Proceedings of the 22nd Annual European Elasmobranch Association Meeting, Peniche, Portugal, 12–14 October 2018. [Google Scholar]
- Rasmussen, K.; Palacios, D.M.; Calambokidis, J.; Saborío, M.T.; Dalla Rosa, L.; Secchi, E.R.; Steiger, G.H.; Allen, J.M.; Stone, G.S. Southern Hemisphere Humpback Whales Wintering off Central America: Insights from Water Temperature into the Longest Mammalian Migration. Biol. Lett. 2007, 3, 302–305. [Google Scholar] [CrossRef]
- Jachowski, D.S.; Singh, N.J. Toward a Mechanistic Understanding of Animal Migration: Incorporating Physiological Measurements in the Study of Animal Movement. Conserv. Physiol. 2015, 3, cov035. [Google Scholar] [CrossRef]
- Block, B.A.; Jonsen, I.D.; Jorgensen, S.J.; Winship, A.J.; Shaffer, S.A.; Bograd, S.J.; Hazen, E.L.; Foley, D.G.; Breed, G.A.; Harrison, A.L.; et al. Tracking Apex Marine Predator Movements in a Dynamic Ocean. Nature 2011, 475, 86–90. [Google Scholar] [CrossRef]
- Freitas, C.; Villegas-Ríos, D.; Moland, E.; Olsen, E.M. Sea Temperature Effects on Depth Use and Habitat Selection in a Marine Fish Community. J. Anim. Ecol. 2021, 90, 1787–1800. [Google Scholar] [CrossRef]
- Magnuson, J.J.; Crowder, L.B.; Medvick, P.A. Temperature as an Ecological Resource. Am. Zool. 1979, 19, 331–343. [Google Scholar] [CrossRef]
- Magnuson, J.J.; DeStasio, B.T. Thermal Niche of Fishes and Global Warming. In Society of Experimental Biology Seminar Series; Cambridge University Press: Cambridge, UK, 1997; Volume 61, pp. 377–408. Available online: https://books.google.co.il/books?hl=en&lr=&id=p7L0x74mrNEC&oi=fnd&pg=PA377&dq=Thermal+Niche+of+Fishes+and+Global+Warming.+In+Society+of+Experimental+Biology+Seminar+Series&ots=-4ie7i3NVU&sig=jFCalMt-PiUrX-K_jkbamUDLaCY&redir_esc=y#v=onepage&q&f=false (accessed on 20 May 2022).
- Asch, R.G.; Erisman, B. Spawning Aggregations Act as a Bottleneck Influencing Climate Change Impacts on a Critically Endangered Reef Fish. Divers. Distrib. 2018, 24, 1712–1728. [Google Scholar] [CrossRef]
- Collin, R.; Rebolledo, A.P.; Smith, E.; Chan, K.Y.K. Thermal Tolerance of Early Development Predicts the Realized Thermal Niche in Marine Ectotherms. Funct. Ecol. 2021, 35, 1679–1692. [Google Scholar] [CrossRef]
- Wearmouth, V.J.; Sims, D.W. Chapter 2 Sexual Segregation in Marine Fish, Reptiles, Birds and Mammals. Behaviour Patterns, Mechanisms and Conservation Implications. Adv. Mar. Biol. 2008, 54, 107–170. [Google Scholar] [PubMed]
- Bass, N.C.; Mourier, J.; Knott, N.A.; Day, J.; Guttridge, T.; Brown, C. Long-Term Migration Patterns and Bisexual Philopatry in a Benthic Shark Species. Mar. Freshw. Res. 2017, 68, 1414. [Google Scholar] [CrossRef]
- Collatos, C.; Abel, D.C.; Martin, K.L. Seasonal Occurrence, Relative Abundance, and Migratory Movements of Juvenile Sandbar Sharks, Carcharhinus Plumbeus, in Winyah Bay, South Carolina. Environ. Biol. Fishes 2020, 103, 859–873. [Google Scholar] [CrossRef]
- Barnes, C.J.; Butcher, P.A.; Macbeth, W.G.; Mandelman, J.W.; Smith, S.D.A.; Peddemors, V.M. Movements and Mortality of Two Commercially Exploited Carcharhinid Sharks Following Longline Capture and Release off Eastern Australia. Endanger. Species Res. 2016, 30, 193–208. [Google Scholar] [CrossRef]
- Papastamatiou, Y.P.; Itano, D.G.; Dale, J.J.; Meyer, C.G.; Holland, K.N. Site Fidelity and Movements of Sharks Associated with Ocean-Farming Cages in Hawaii. Mar. Freshw. Res. 2010, 61, 1366–1375. [Google Scholar] [CrossRef]
- Conrath, C.L.; Musick, J.A. Investigations into Depth and Temperature Habitat Utilization and Overwintering Grounds of Juvenile Sandbar Sharks, Carcharhinus Plumbeus: The Importance of near Shore North Carolina Waters. Environ. Biol. Fishes 2008, 82, 123–131. [Google Scholar] [CrossRef]
- Jacoby, D.M.P.; Croft, D.P.; Sims, D.W. Social Behaviour in Sharks and Rays: Analysis, Patterns and Implications for Conservation. Fish Fish. 2012, 13, 399–417. [Google Scholar] [CrossRef]
- Visser, M.E.; Perdeck, A.C.; van Balen, J.H.; Both, C. Climate Change Leads to Decreasing Bird Migration Distances. Glob. Chang. Biol. 2009, 15, 1859–1865. [Google Scholar] [CrossRef]
- Miller-Rushing, A.J.; Lloyd-Evans, T.L.; Primack, R.B.; Satzinger, P. Bird Migration Times, Climate Change, and Changing Population Sizes. Glob. Chang. Biol. 2008, 14, 1959–1972. [Google Scholar] [CrossRef]
- Bussière, E.M.S.; Underhill, L.G.; Altwegg, R. Patterns of Bird Migration Phenology in South Africa Suggest Northern Hemisphere Climate as the Most Consistent Driver of Change. Glob. Chang. Biol. 2015, 21, 2179–2190. [Google Scholar] [CrossRef]
- Zaifman, J.; Shan, D.; Ay, A.; Jimenez, A.G. Shifts in Bird Migration Timing in North American Long-Distance and Short-Distance Migrants Are Associated with Climate Change. Int. J. Zool. 2017, 2017, 6025646. [Google Scholar] [CrossRef]
- Parmesan, C.; Yohe, G. A Globally Coherent Fingerprint of Climate Change Impacts across Natural Systems. Nature 2003, 421, 37–42. [Google Scholar] [CrossRef]
- Johnson, G.C.; Lyman, J.M. Warming Trends Increasingly Dominate Global Ocean. Nat. Clim. Chang. 2020, 10, 757–761. [Google Scholar] [CrossRef]
- Parmesan, C. Ecological and Evolutionary Responses to Recent Climate Change. Annu. Rev. Ecol. Evol. Syst. 2006, 37, 637–669. [Google Scholar] [CrossRef]
- Philippart, C.J.M.; Anadón, R.; Danovaro, R.; Dippner, J.W.; Drinkwater, K.F.; Hawkins, S.J.; Oguz, T.; O’Sullivan, G.; Reid, P.C. Impacts of Climate Change on European Marine Ecosystems: Observations, Expectations and Indicators. J. Exp. Mar. Biol. Ecol. 2011, 400, 52–69. [Google Scholar] [CrossRef]
- Pinsky, M.L.; Eikeset, A.M.; McCauley, D.J.; Payne, J.L.; Sunday, J.M. Greater Vulnerability to Warming of Marine versus Terrestrial Ectotherms. Nature 2019, 569, 108–111. [Google Scholar] [CrossRef]
- Andrzejaczek, S.; Gleiss, A.C.; Jordan, L.K.B.; Pattiaratchi, C.B.; Howey, L.A.; Brooks, E.J.; Meekan, M.G. Temperature and the Vertical Movements of Oceanic Whitetip Sharks, Carcharhinus Longimanus. Sci. Rep. 2018, 8, 8351. [Google Scholar] [CrossRef]
- Hammerschlag, N.; McDonnell, L.H.; Rider, M.J.; Street, G.M.; Hazen, E.L.; Natanson, L.J.; McCandless, C.T.; Boudreau, M.R.; Gallagher, A.J.; Pinsky, M.L.; et al. Ocean Warming Alters the Distributional Range, Migratory Timing, and Spatial Protections of an Apex Predator, the Tiger Shark (Galeocerdo cuvier). Glob. Chang. Biol. 2022, 28, 1990–2005. [Google Scholar] [CrossRef]
- Kessel, S.; Chapman, D.; Franks, B.; Gedamke, T.; Gruber, S.; Newman, J.; White, E.; Perkins, R. Predictable Temperature-Regulated Residency, Movement and Migration in a Large, Highly Mobile Marine Predator (Negaprion brevirostris). Mar. Ecol. Prog. Ser. 2014, 514, 175–190. [Google Scholar] [CrossRef]
- Rodriguez-Burgos, A.M.; Briceño-Zuluaga, F.J.; Ávila Jiménez, J.L.; Hearn, A.; Peñaherrera-Palma, C.; Espinoza, E.; Ketchum, J.; Klimley, P.; Steiner, T.; Arauz, R.; et al. The Impact of Climate Change on the Distribution of Sphyrna Lewini in the Tropical Eastern Pacific. Mar. Environ. Res. 2022, 180, 105696. [Google Scholar] [CrossRef]
- Pisano, A.; Marullo, S.; Artale, V.; Falcini, F.; Yang, C.; Leonelli, F.E.; Santoleri, R.; Buongiorno Nardelli, B. New Evidence of Mediterranean Climate Change and Variability from Sea Surface Temperature Observations. Remote Sens. 2020, 12, 132. [Google Scholar] [CrossRef]
- Osgood, G.J.; White, E.R.; Baum, J.K. Effects of Climate-change-driven Gradual and Acute Temperature Changes on Shark and Ray Species. J. Anim. Ecol. 2021, 90, 2547–2559. [Google Scholar] [CrossRef] [PubMed]
- Beca-Carretero, P.; Teichberg, M.; Winters, G.; Procaccini, G.; Reuter, H. Projected Rapid Habitat Expansion of Tropical Seagrass Species in the Mediterranean Sea as Climate Change Progresses. Front. Plant Sci. 2020, 11, 1762. [Google Scholar] [CrossRef] [PubMed]
- Assis, J.; Tyberghein, L.; Bosch, S.; Verbruggen, H.; Serrão, E.A.; De Clerck, O. Bio-ORACLE v2.0: Extending Marine Data Layers for Bioclimatic Modelling. Glob. Ecol. Biogeogr. 2018, 27, 277–284. [Google Scholar] [CrossRef]
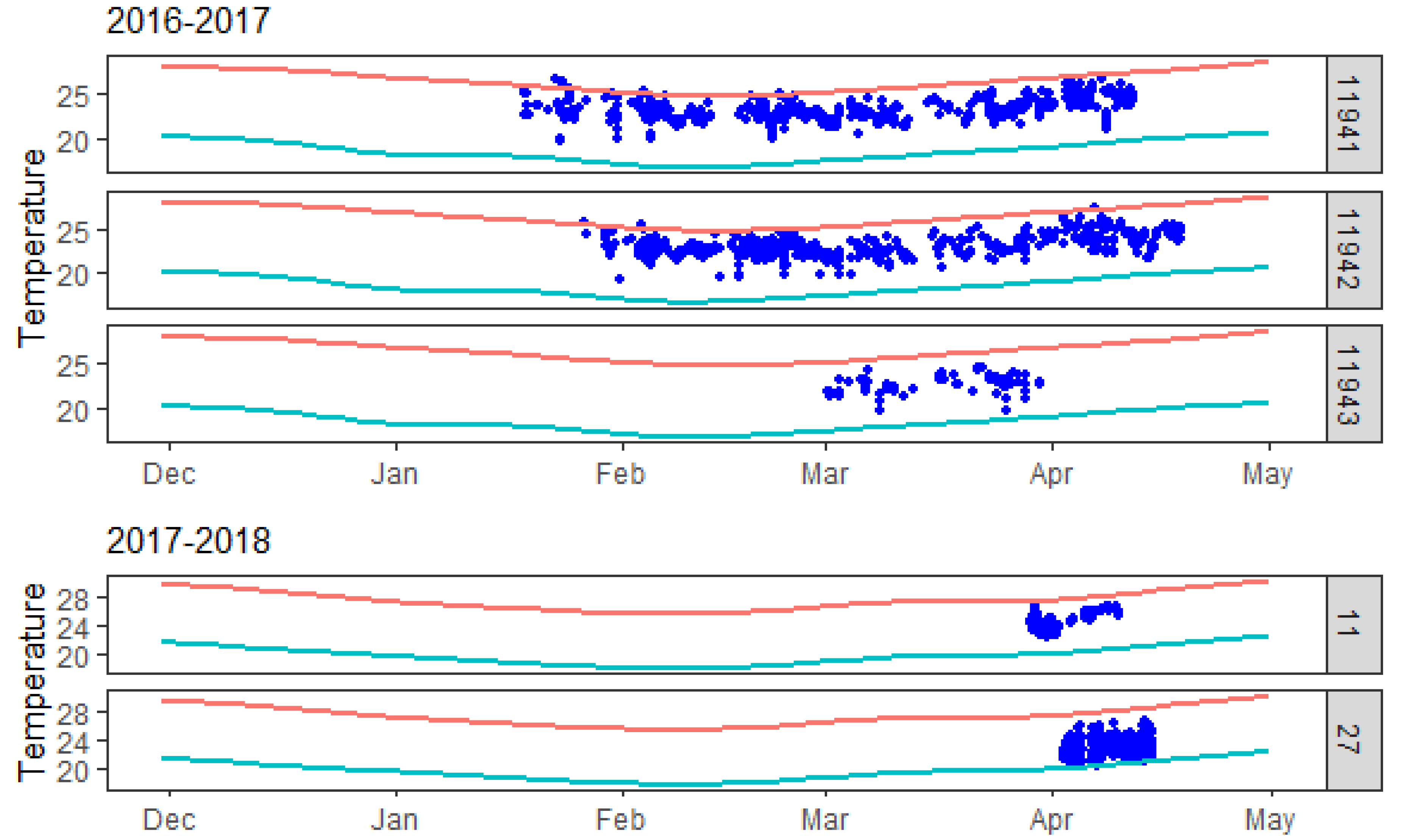
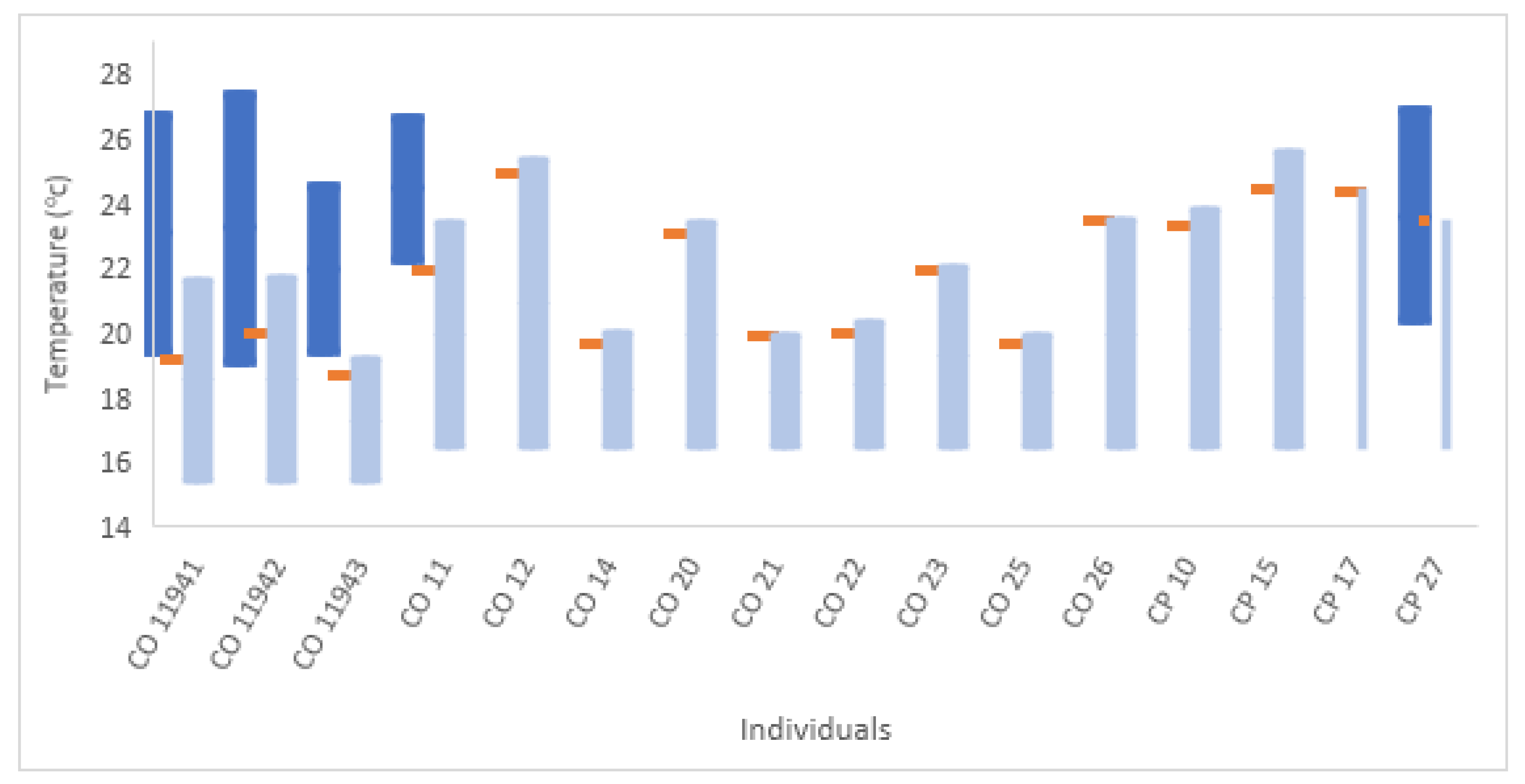
| Shark Serial | Tagging Season | Species | Sex | TL (cm) | Transmitter Type (Sensors) | Detections | Tagging Date | Last Detected | Min Temp (°C) | Max Temp (°C) | Days Tracked | Detec./Day/Rec | Transmitter Model |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 11941 | 2016–2017 | C. obscurus | F | 307 | Acoustic (temperature) | 1532 | 17 January 2017 | 12 April 2017 | 19.5 | 26.7 | 86 | 3.7 | V16T |
| 11942 | 2016–2017 | C. obscurus | F | 285 | Acoustic (temperature) | 1709 | 24 January 2017 | 18 April 2017 | 19.2 | 27.3 | 85 | 4.2 | V16T |
| 11943 | 2016–2017 | C. obscurus | F | 289 | Acoustic (temperature) | 242 | 28 February 2017 | 30 March 2017 | 19.5 | 24.5 | 31 | 0.6 | V16T |
| CO 21 | 2017–2018 | C. obscurus | F | 289 | acoustic | 318 | 27 November 2017 | 11 March 2018 | NA | NA | 105 | 3.2 | HP16 |
| CO 23 | 2017–2018 | C. obscurus | F | 276 | acoustic | 737 | 12 December 2017 | 24 April 2018 | NA | NA | 134 | 6.3 | HP16 |
| CO 22 | 2017–2018 | C. obscurus | F | 315 | acoustic | 482 | 27 December 2017 | 2 April 2018 | NA | NA | 97 | 6.1 | HP16 |
| CO 14 | 2017–2018 | C. obscurus | F | 355 | acoustic | 424 | 27 December 2017 | 13 March 2018 | NA | NA | 77 | 7.4 | HP16 |
| CO 20 | 2017–2018 | C. obscurus | F | 300 | acoustic | 267 | 2 January 2018 | 8 May 2018 | NA | NA | 127 | 3.1 | HP16 |
| CO 26 | 2017–2018 | C. obscurus | F | 275 | acoustic | 1051 | 5 February 2018 | 22 April 2018 | NA | NA | 77 | 8.2 | HP16 |
| CP 15 | 2017–2018 | C. plumbeus | M | 169 | acoustic | 17117 | 12 March 2018 | 14 May 2018 | NA | NA | 64 | 53.5 | HP16 |
| CO 25 | 2017–2018 | C. obscurus | F | 280 | acoustic | 63 | 12 March 2018 | 23 March 2018 | NA | NA | 12 | 1.1 | HP16 |
| CP 10 | 2017–2018 | C. plumbeus | M | 191 | acoustic | 17231 | 14 March 2018 | 10 May 2018 | NA | NA | 58 | 59.4 | HP16 |
| CO 11 * | 2017–2018 | C. obscurus | F | 294 | Acoustic, PSAT | 969 | 28 March 2018 | 27 April 2018 | 22.3 * | 26.6 * | 31 | 6.3 | HP16 |
| CP 17 | 2017–2018 | C. plumbeus | M | 180 | acoustic | 4706 | 28 March 2018 | 14 May 2018 | NA | NA | 48 | 19.6 | HP16 |
| CO 12 | 2017–2018 | C. obscurus | F | 300 | acoustic | 1895 | 2 April 2018 | 2 June 2018 | NA | NA | 62 | 6.1 | HP16 |
| CP 27 * | 2017–2018 | C. plumbeus | M | 180 | Acoustic, PSAT | 4348 | 2 April 2018 | 21 April 2018 | 20.4 * | 26.8 * | 20 | 43.5 | HP16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barash, A.; Scheinin, A.; Bigal, E.; Zemah Shamir, Z.; Martinez, S.; Davidi, A.; Fadida, Y.; Pickholtz, R.; Tchernov, D. Some Like It Hot: Investigating Thermoregulatory Behavior of Carcharhinid Sharks in a Natural Environment with Artificially Elevated Temperatures. Fishes 2023, 8, 428. https://doi.org/10.3390/fishes8090428
Barash A, Scheinin A, Bigal E, Zemah Shamir Z, Martinez S, Davidi A, Fadida Y, Pickholtz R, Tchernov D. Some Like It Hot: Investigating Thermoregulatory Behavior of Carcharhinid Sharks in a Natural Environment with Artificially Elevated Temperatures. Fishes. 2023; 8(9):428. https://doi.org/10.3390/fishes8090428
Chicago/Turabian StyleBarash, Adi, Aviad Scheinin, Eyal Bigal, Ziv Zemah Shamir, Stephane Martinez, Aileen Davidi, Yotam Fadida, Renanel Pickholtz, and Dan Tchernov. 2023. "Some Like It Hot: Investigating Thermoregulatory Behavior of Carcharhinid Sharks in a Natural Environment with Artificially Elevated Temperatures" Fishes 8, no. 9: 428. https://doi.org/10.3390/fishes8090428
APA StyleBarash, A., Scheinin, A., Bigal, E., Zemah Shamir, Z., Martinez, S., Davidi, A., Fadida, Y., Pickholtz, R., & Tchernov, D. (2023). Some Like It Hot: Investigating Thermoregulatory Behavior of Carcharhinid Sharks in a Natural Environment with Artificially Elevated Temperatures. Fishes, 8(9), 428. https://doi.org/10.3390/fishes8090428







