Abstract
Two species of carcharhinid sharks aggregate every winter at the warm water effluent of a coastal power plant on the Israeli Mediterranean coast. The two species (Carcharhinus obscurus and Carcharhinus plumbeus) cooccur in a highly confined area for several months every year and are highly associated with the area in and around the hot water effluent. Niche partitioning has recently been suggested as a mechanism that enables the coexistence of similar shark species by resource partitioning, spatial partitioning, and temporal partitioning. In this study, we used acoustic telemetry to study the individual diel movement and activity patterns within this enclosed area and examined the differences between the two species sharing it. Although this location only reaches a maximum depth of 7.5 m, we found both species perform a diel vertical movement, rising closer to the surface at night and moving deeper during daytime. Furthermore, the two shark species swam at different depths both day and night, with C. obscurus swimming in the upper column, about 2 m shallower than C. plumbeus. The very small scale of movement, which nearly equals the sharks’ body length, suggests movement patterns might be conserved at the species level. Moreover, spatiotemporal differences between the two species may reflect a mean of interspecific partitioning that occurs even in a highly confined and shallow habitat.
1. Introduction
In the Eastern Mediterranean, carcharhinid sharks aggregate near the coast of Israel at warm water effluents of coastal power stations [1]. Every year for the last two decades, dozens of sharks of two species, the dusky shark Carcharhinus obscurus (Lesueur, 1818) and the sandbar shark C. plumbeus (Nardo, 1827), aggregate in this relatively small area between November and May, most likely due to elevated temperatures and their thermoregulatory advantages [1]. Both species are large coastal sharks (C. obscurus up to 4.2 m and C. plumbeus up to 2.5 m [2]), with similar food preferences and trophic levels [3], and seem to coexist in large numbers in a small and extremely shallow area.
Niche partitioning has been found to be a significant mechanism allowing multiple species to share common space or resources [4]. Studies have shown that in areas where different species of large sharks coexist, differences were found in the use of space among species. For example, in Queensland, Australia, two shark species inhabit close but separated areas along the same river [5]. Around a small, elongated island near Mexico, four species of sharks have been documented with high affinity to only one site on the island, suggesting spatial partitioning for some of the species [6]. Six shark species in the Gulf of Mexico showed a diel temporal partition when each species utilized the same space at a different time of the day, with minimal overlap between the activity hours [7].
Little is known about niche partitioning in terms of depth distribution. Based on isotope analysis of mercury accumulation, reference [8] suggested that foraging depth can explain resource allocation between species, and reference [6] described the varied use of depth among individuals of different species on the same site.
In this study, we used acoustic telemetry to examine how two large coastal shark species coexist within a small area of a few kilometres, limited by extremely shallow water. We also examine the hypothesis that niche partitioning facilitates their coexistence.
2. Materials and Methods
2.1. Study Site
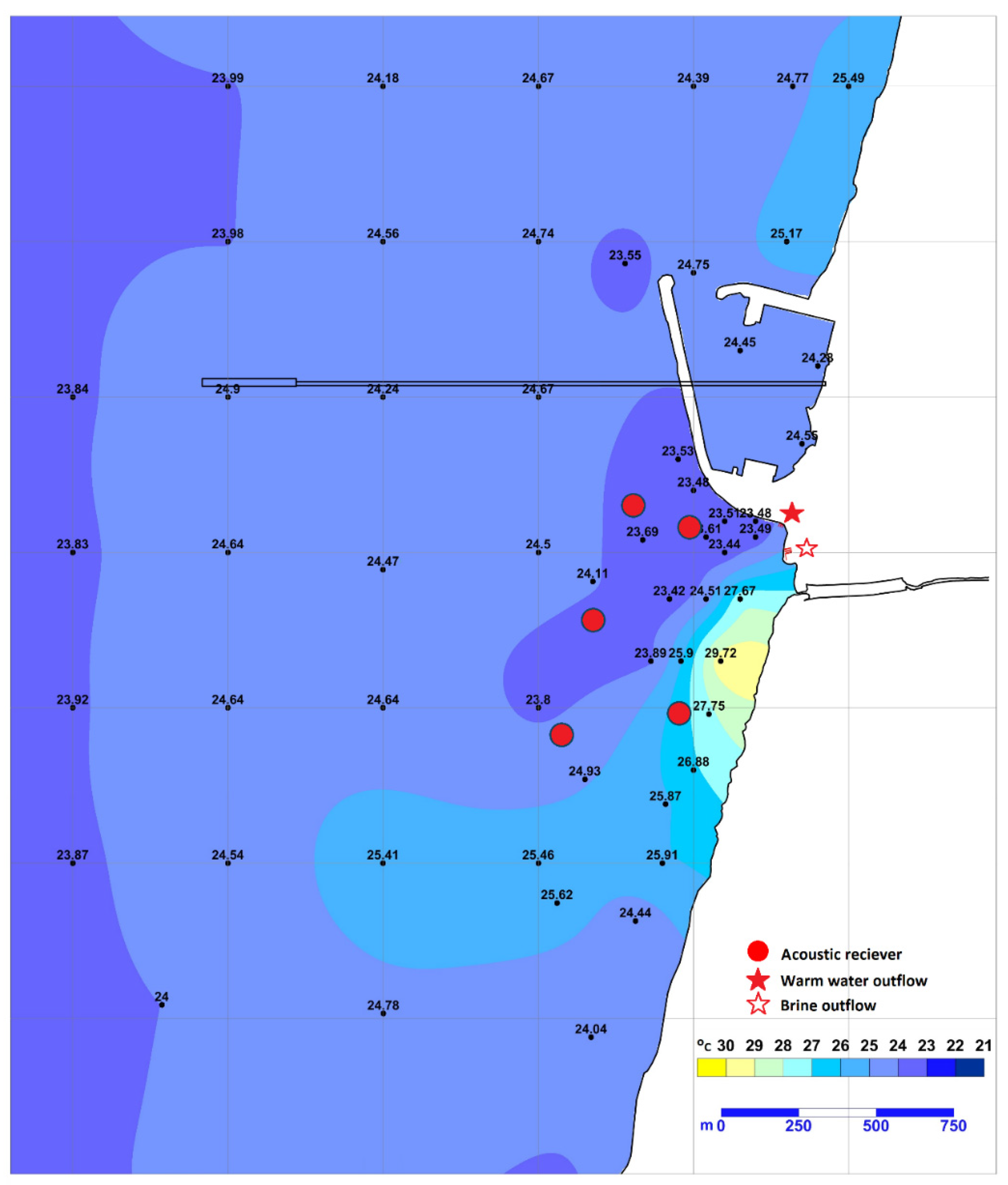
Orot Rabin (OR) power station (32.466814 N, 34.880232 E), located near the city of Hadera, Israel, on the easternmost Mediterranean Sea coast, is one of three coastal power stations found to attract sharks to their warm water effluent [1]. OR pumps seawater to cool its turbines and discharges the water back into the sea at approximately 8 °C above ambient temperature. The discharge creates a heated plume expanding a few kilometres along the coast, with a strong temperature gradient between the point of release and the ambient sea temperature (Figure 1).
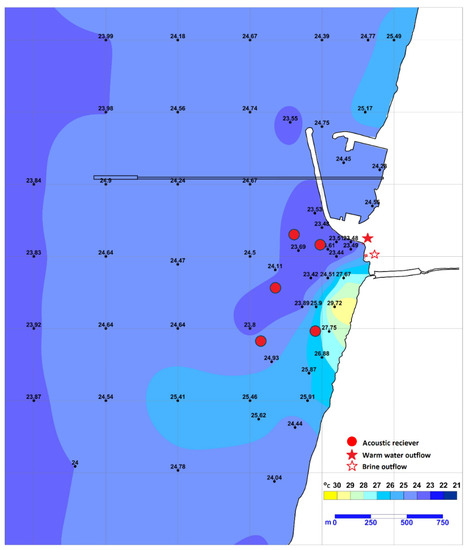
Figure 1.
Map of the study site. Temperature is shown as measured by IEC staff on 22 May 2018, at 2 m depth. Circles (●) represent receiver locations; star (★) represents the discharge point for warm water and star with no fill (✩) for warm saline water. Adapted from the IEC monitoring report 2018.
In addition to OR, a desalination plant operates on-site and discharges its brine into the same effluent. As a result, the mixed water reaches the sea with a salinity about 3 PPT higher than the ambient seawater. The bottom depth at the discharge site ranges from 0 to 4 m in most places and reaches a maximum depth of 7.5 m in a certain area excavated by the discharge current.
2.2. Shark Tagging and Receivers’ Deployment
Carcharhinid sharks were caught and tagged on-site in the warm effluent, between November 2017 and April 2018, using baited drumlines. The sharks were pulled close to the boat and were strapped around the tail base and behind the pectoral fins. Once secured, the sharks were measured, sexed (according to appearance or absence of claspers), and fitted with an external Floy tag in the dorsal fin. HP16 tags equipped with a depth sensor (Thelma Biotel, Trondheim, Norway; 69 kHz; delay range: 30–90 s; depth range: 0–51 m; resolution: 0.2 m; battery life: 90 months) were surgically implanted into the peritoneal cavity of 4 C. plumbeus and 9 C. obscurus sharks. Transmitters were set to nominally transmit every 60 s.
An acoustic receiver (VR2W, Vemco Ltd., Halifax, NS, Canada) was placed in the effluent on 15 January 2017, and four additional receivers (TBR 700, Thelma Biotel, Trondheim, Norway) were added on 7 March 2018.
2.3. Data Analysis
Data aggregation of a two-minute time frame was chosen to reduce the effect of the transition from one receiver to five and to even the number of detections. Mean depth (DM) was calculated for each aggregated data point. Detections from the first 24 h after tagging were discarded for each individual to eliminate tagging’s effect on the movement analysis [9].
Day in the season (DIS) was used to describe the number of days starting 1 November for each season (an arbitrary date before the start of the tagging season), and a daily mean ambient seawater temperature (SWT) was calculated to check thermal changes. Time of day (TOD) was defined using the SUNCALC package [10], with the day divided into four time segments—Day, Night, Dawn, and Dusk—such that dawn was defined as the time between night’s end (morning astronomical twilight start) to the end of the golden hour, approximately two hours later, and dusk was defined as between the beginning of the evening’s golden hour and the beginning of night (dark enough for astronomical observations), which was approximately two hours later, as well. Lunar phase (LP) was added from the Lunar package. Total length (TL) represents the measured length of the shark on tagging. Data were then aggregated once more per Shark, DIS, and TOD. An aggregated data line based on three data points or less was removed, and the median (DM) value was chosen to describe the depth. Finally, a linear mixed model (LM, lmer function, Package lme4) was used to determine which factors affected the DM choice of the sharks. The model included interactions between the species and the TOD, and a random effect was included for individuals in order to control for possible dependences. A scale function was used to transform data to fit the same scale for all factors.
DM Median ~ Species × TOD + SWT + DIS + LP +TL + (1|Shark)
Model selection was made by the Dredge function (Package MuMIn) with 5000 bootstrap resamples, showing 3 models with delta AIC < 2. Hedges G test was performed as post hoc for the model-chosen factors. Data analysis was performed in R (v. 1.8–12; R Foundation for Statistical Computing, Vienna, Austria).
3. Results
Sharks of the two species were caught along the tagging period and often in the same tagging event (three out of the four C. plumbeus sharks were tagged in the same event as a dusky shark. Table 1), providing proof of coexistence and mutual use of the heated area. All tagged C. plumbeus sharks were males, and all C. obscurus were females considerably larger than the C. plumbeus males (mean length ± SE: 298.2 ± 12.5 cm vs. 180 ± 4.5 cm respectively). These findings correspond with additional sharks caught and measured on site (Table A1, Appendix A) and with photographed observations showing mainly large female C. obscurus and smaller male C. plumbeus (unpublished data).

Table 1.
Summary of biological and detection data for sharks tagged with depth sensors at the warm effluent of Orot Rabin (OR) power station, ordered by the tagging date. Detection rate stands for the number of detections per hour per receiver.
A linear mixed-model analysis found movement in DM best explained by three top models, which included the species, time of day (TOD), and day in the season (DIS). The model did not find the ambient temperature, lunar phase, or the shark’s total length to significantly affect the DM. Residuals distribution for the model appears in Figure A2.
The Akaike information criterion (AIC) was similar to the first 3 models (delta < 2), and all 3 models were able to account for 58% of the variance (Table 2).

Table 2.
Model selection results only include models with ∆AIC < 2. DM represents median depth, TOD represents the category time of day, DIS represents day in season, and Shark represents an individual shark.
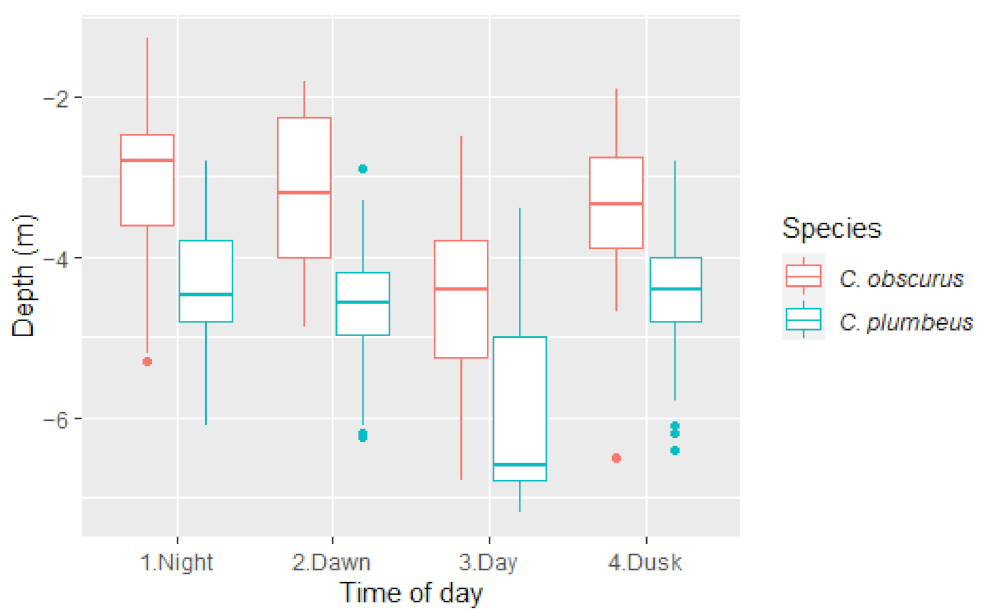
C. plumbeus were deeper than C. obscurus at all times of the day, with a mean difference of 1.5 m during the day and at night (Figure 2). In crepuscular times, this number changes towards a higher number (1.8 m) at dawn and a lower number (1.26 m) at dusk, suggesting C. plumbeus might start the movement earlier than C. obscurus, thus creating a bigger gap in the morning and a smaller one going back up at night.
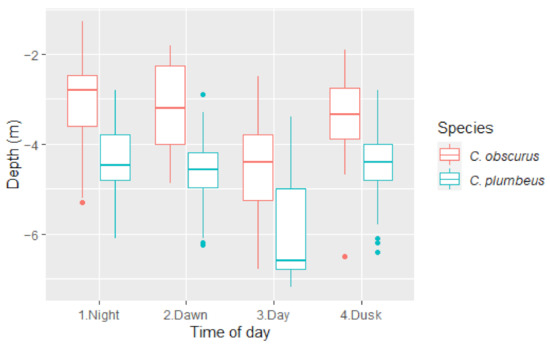
Figure 2.
Median depth by the time of day for C. obscurus and C. plumbeus. The upper and lower limits of boxes represent the 75th and 25th percentiles, respectively. Horizontal lines represent the median value.
This result was repeated when comparing DM at the different TOD within each species. Compared to DM at night, C. obscurus ventured 1.39 m deeper during the day (there was no significant difference between DM at night and the transient times), whereas C. plumbeus changed their DM significantly early at dawn and continued moving 2 m deeper for the day. DM at dusk was not significantly different from the night (Table 3).

Table 3.
Unpaired Hedges’ g test among species and time of day groups.
4. Discussion
The aggregation of sharks at OR’s effluent provides a unique opportunity to examine how human development causes a change in the movement and behaviour of certain shark species, as well as the behavioural adaptations of the sharks to the new conditions in terms of competition and use of space. In this study, we describe this aggregation behaviour, and the vertical movement patterns within it, at an individual level, as well as offer a possible explanation for the observed coexistence between these species at the site.
Clear and constant diel vertical movement was found for both species at the site. All sharks swam in the upper water column at night and ventured deeper during the day, although the shift of DM between day and night was characterized by a seemingly minor difference for sharks of that size (i.e., a change of no more than 2 m for 2–3.5 m long sharks). A distinct difference in utilised DM was found between the species, showing C. plumbeus swam deeper than C. obscurus, displaying spatial partitioning of the species. Moreover, the only place within the heated area to reach a depth greater than 5 m is underneath the discharge current, where C. plumbeus sharks have been documented repeatedly (Figure 3). The utilised DM (for each species) was not related to the ambient SWT, the lunar phase, or the individual size of the sharks, suggesting a species-specific spatial partitioning at the study site. These results are further reinforced by the swimming profile recorded by an archival tag attached to one of the C. plumbeus sharks (Figure A1).

Figure 3.
Carcharhinus plumbeus swimming under the current at 7 m. (The photo was reprinted with permission from Ilan Elgrably).
The idea of spatial partitioning is further supported by the order of magnitude that was found in the difference in the detection rate of C. plumbeus (Table 1), suggesting different utilization of the space by C. obscurus and C. plumbeus at the study site. The number of detections, however, may be affected by the acoustic noise the artificial current causes in shallower waters.
In this study, the scale of DM variation was very small (due to the nature of the study site), as was the difference in sizes within each species. All C. obscurus individuals were considerably larger than the C. plumbeus individuals, and therefore, it is impossible to fully determine whether the daily changes in spatial occupation were due to individual size, species, or sex. Here we observed a few dozen sharks of each species coexisting in “close quarters”, seemingly facilitated by a daily “shift-change” in terms of time and DM locations. Recently, temporal shifts have been shown between sharks of different species in Tampa, Florida, demonstrating robust temporal partitioning of foraging times [7]. This might also be the case here, with C. plumbeus waiting their turn to feed.
Diel movement may be driven by prey behaviour [8,11]. C. plumbeus and C. obscurus mainly feed on teleost fish and cephalopods [12,13,14,15] and are considered to be at the same trophic level (4.1 for C. plumbeus and 4.2 for C. obscurus, Cortés, 1999), but size differences between the species at the study site could be driving differences in feeding preferences, as has been suggested for other species [16,17,18].
Inter-species competition can also explain the difference between the movements of the sharks. The larger C. obscurus spent time at the site freely during the day, while the smaller C. plumbeus entered the “preferred” depth at night when C. obscurus individuals were not there. The slight change in the timing of the transition between deep and shallow supports the theory that one species “responds” to the movement of the other species.
The idea of division in depth utilization according to sharks’ size has been suggested by [19], where smaller sixgill sharks (Hexanchus griseus) used shallower sites than larger individuals; however, this was only observed in individuals of the same species. In this study, the total length of individual sharks was not significant, but it could be overshadowed compared to the size variation between the two species.
Salinity has also been found to be a driver in shark movement. Reference [5] found two species of river sharks segregated spatially along a salinity gradient. This possibility should be further explored at the study site in terms of salinity tolerance and/or preference for both species and whether it plays a part in the species’ depth distribution.
The unique circumstances provided by the shark aggregations at OR allow us to examine changes in DM on a scale that is rarely possible. It seems that diel vertical movement was maintained, even though functionally, the differences in depth are considered minor compared to the vertical movement reported for sharks of the same species in different areas. These findings may suggest that vertical diel movement is an inherently basic behaviour in sharks of these species and is maintained, even in cases when it is not essential.
Author Contributions
Conceptualization, A.B. and D.T.; data curation, A.B., A.S., E.B., Z.Z.S. and S.M.; formal analysis, A.B.; investigation, A.B.; methodology, A.B. and A.S.; supervision, D.T.; visualization, A.B.; writing—original draft, A.B.; writing—review & editing, A.S. and S.M. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the Morris Kahn Marine Research Station, Department of Marine Biology, Leon H. Charney School of Marine Sciences, University of Haifa, Israel.
Institutional Review Board Statement
Shark tagging was conducted under permit numbers 2017/41714 and 2018/42027, issued by The Israeli Nature and Parks Authority (INPA), and according to European ecological standards.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the authors upon reasonable request and with permission of the “Top Predator Lab” at Morris Kahn Marine Research Station, Department of Marine Biology, Leon H. Charney School of Marine Sciences, University of Haifa, Israel.
Acknowledgments
We thank Ilan Elgrably for his photograph (Figure 3), Ran Golan for fruitful discussions, and Kfir Avramzon the maritime lab manager in the engineering projects division, IEC, Israel.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A
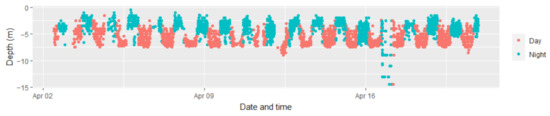
Figure A1.
Depth data as was recorded in an archival tag of a Carcharhinus plumbeus male. Points are coloured according to the time of day (day in red, night in blue).
Figure A1.
Depth data as was recorded in an archival tag of a Carcharhinus plumbeus male. Points are coloured according to the time of day (day in red, night in blue).

Table A1.
Size measurements of untagged sharks captured within the study site between 2016–2017.
Table A1.
Size measurements of untagged sharks captured within the study site between 2016–2017.
| Species | Catch Date | TL (cm) | Sex |
|---|---|---|---|
| C. obscurus | 25 February 2016 | 322 | Female |
| C. obscurus | 25 February 2016 | 328 | Female |
| C. obscurus | 23 March 2016 | 309 | Female |
| C. obscurus | 23 March 2016 | 325 | Female |
| C. obscurus | 23 March 2016 | 299 | Female |
| C. obscurus | 17 January 2017 | 200 | Female |
| C. obscurus | 20 February 2017 | 250 | Female |
| C. obscurus | 21 February 2017 | 290 | Female |
| C. obscurus | 23 February 2017 | 280 | Female |
| C. obscurus | 6 March 2017 | 280 | Female |
| C. obscurus | 8 March 2017 | 390 | Female |
| C. obscurus | 28 March 2017 | 320 | Female |
| C. obscurus | 19 December 2017 | 283 | Female |
| C. obscurus | 9 January 2018 | 303 | Female |
| C. plumbeus | 8 March 2017 | 170 | Male |
| C. plumbeus | 23 February 2017 | 177 | Male |
| C. plumbeus | 6 April 2017 | 198 | Male |
| C. plumbeus | 6 April 2017 | 179 | Male |
| C. plumbeus | 1 May 2018 | 178 | Male |
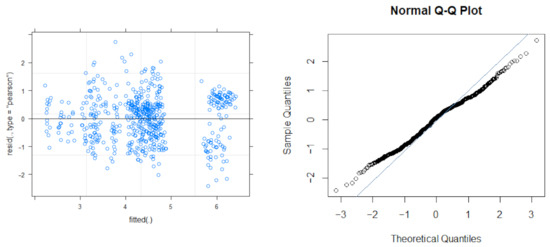
Figure A2.
Residuals distribution for the LM model.
Figure A2.
Residuals distribution for the LM model.
References
- Barash, A.; Pickholtz, R.; Pickholtz, E.; Blaustein, L.; Rilov, G. Seasonal Aggregations of Sharks near Coastal Power Plants in Israel: An Emerging Phenomenon. Mar. Ecol. Prog. Ser. 2018, 590, 145–154. [Google Scholar] [CrossRef]
- Weigmann, S. Annotated Checklist of the Living Sharks, Batoids and Chimaeras (Chondrichthyes) of the World, with a Focus on Biogeographical Diversity. J. Fish Biol. 2016, 88, 837–1037. [Google Scholar] [CrossRef] [PubMed]
- Cortés, E. Standardized Diet Compositions and Trophic Levels of Sharks. ICES J. Mar. Sci. 1999, 56, 707–717. [Google Scholar] [CrossRef]
- Finke, D.L.; Snyder, W.E. Niche Partitioning Increases Resource Exploitation by Diverse Communities. Science 2008, 321, 1488–1490. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, R.G.; Campbell, H.A.; Cramp, R.L.; Burke, C.L.; Micheli-Campbell, M.A.; Pillans, R.D.; Lyon, B.J.; Franklin, C.E. Niche Partitioning between River Shark Species Is Driven by Seasonal Fluctuations in Environmental Salinity. Funct. Ecol. 2020, 34, 2170–2185. [Google Scholar] [CrossRef]
- Klimley, A.P.; Ketchum, J.T.; Lara-Lizardi, F.; Papastamatiou, Y.P.; Hoyos-Padilla, E.M. Evidence for Spatial and Temporal Resource Partitioning of Sharks at Roca Partida, an Isolated Pinnacle in the Eastern Pacific. Environ. Biol. Fishes 2022, 105, 1963–1974. [Google Scholar] [CrossRef]
- Lear, K.O.; Whitney, N.M.; Morris, J.J.; Gleiss, A.C. Temporal Niche Partitioning as a Novel Mechanism Promoting Co-Existence of Sympatric Predators in Marine Systems. Proc. R. Soc. B 2021, 288, 20210816. [Google Scholar] [CrossRef] [PubMed]
- Besnard, L.; le Croizier, G.; Galván-Magaña, F.; Point, D.; Kraffe, E.; Ketchum, J.; Rincon, R.O.M.; Schaal, G. Foraging Depth Depicts Resource Partitioning and Contamination Level in a Pelagic Shark Assemblage: Insights from Mercury Stable Isotopes. Environ. Pollut. 2021, 283, 117066. [Google Scholar] [CrossRef] [PubMed]
- Whitney, N.M.; Lear, K.O.; Gleiss, A.C.; Payne, N.; White, C.F. Advances in the Application of High-Resolution Biologgers to Elasmobranch Fishes. In Shark Research: Emerging Technologies and Applications for the Field and Laboratory; Routledge: London, UK, 2018; pp. 45–70. [Google Scholar]
- Thieurmel, B.; Elmarhraoui, A.; Thieurmel, M.B. Package ‘Suncalc’. 2019. Available online: https://cran.r-project.org/web/packages/suncalc/suncalc.pdf (accessed on 8 January 2021).
- Papastamatiou, Y.P.; Wetherbee, B.M.; Lowe, C.G.; Crow, G.L. Distribution and Diet of Four Species of Carcharhinid Shark in the Hawaiian Islands: Evidence for Resource Partitioning and Competitive Exclusion. Mar. Ecol. Prog. Ser. 2006, 320, 239–251. [Google Scholar] [CrossRef]
- Gelsleichter, J.; Musick, J.A.; Nichols, S. Food Habits of the Smooth Dogfish, Mustelus canis, Dusky Shark, Carcharhinus obscurus, Atlantic Sharpnose Shark, Rhizoprionodon terraenovae, and the Sand Tiger, Carcharias taurus, from the Northwest Atlantic Ocean. Environ. Biol. Fishes 1999, 54, 205–217. [Google Scholar] [CrossRef]
- Simpfendorfer, C.A.; Goodreid, A.; McAuley, R.B. Diet of Three Commercially Important Shark Species from Western Australian Waters. Mar. Freshw. Res. 2001, 52, 975–985. [Google Scholar] [CrossRef]
- Smale, M.J. Occurrence and Feeding of Three Shark Species, Carcharhinus brachyurus, C. obscurus and Sphyrna zygaena, on the Eastern Cape Coast of South Africa. South. Afr. J. Mar. Sci. 1991, 11, 31–42. [Google Scholar] [CrossRef]
- Saïdi, B.; Bradaï, M.N.; Bouaïn, A.; Capapé, C. Feeding Habits of the Sandbar Shark Carcharhinus plumbeus (Chondrichthyes: Carcharhinidae) from the Gulf of Gabès, Tunisia. Cah. Biol. Mar. 2007, 48, 139–144. [Google Scholar]
- Vögler, R.; Milessi, A.C.; Duarte, L.O. Changes in Trophic Level of Squatina guggenheim with Increasing Body Length: Relationships with Type, Size and Trophic Level of Its Prey. Environ. Biol. Fishes 2009, 84, 41–52. [Google Scholar] [CrossRef]
- Malpica-Cruz, L.; Herzka, S.Z.; Sosa-Nishizaki, O.; Escobedo-Olvera, M.A. Tissue-specific Stable Isotope Ratios of Shortfin Mako (Isurus oxyrinchus) and White (Carcharodon carcharias) Sharks as Indicators of Size-based Differences in Foraging Habitat and Trophic Level. Fish Oceanogr. 2013, 22, 429–445. [Google Scholar] [CrossRef]
- Romanuk, T.N.; Hayward, A.; Hutchings, J.A. Trophic Level Scales Positively with Body Size in Fishes. Glob. Ecol. Biogeogr. 2011, 20, 231–240. [Google Scholar] [CrossRef]
- Andrews, K.S.; Williams, G.D.; Farrer, D.; Tolimieri, N.; Harvey, C.J.; Bargmann, G.; Levin, P.S. Diel Activity Patterns of Sixgill Sharks, Hexanchus griseus: The Ups and Downs of an Apex Predator. Anim. Behav. 2009, 78, 525–536. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).