Morphological and Trophic Features of the Invasive Babka gymnotrachelus (Gobiidae) in the Plain and Mountainous Ecosystems of the Dniester Basin: Spatiotemporal Expansion and Possible Threats to Native Fishes
Abstract
1. Introduction
2. Material and Methods
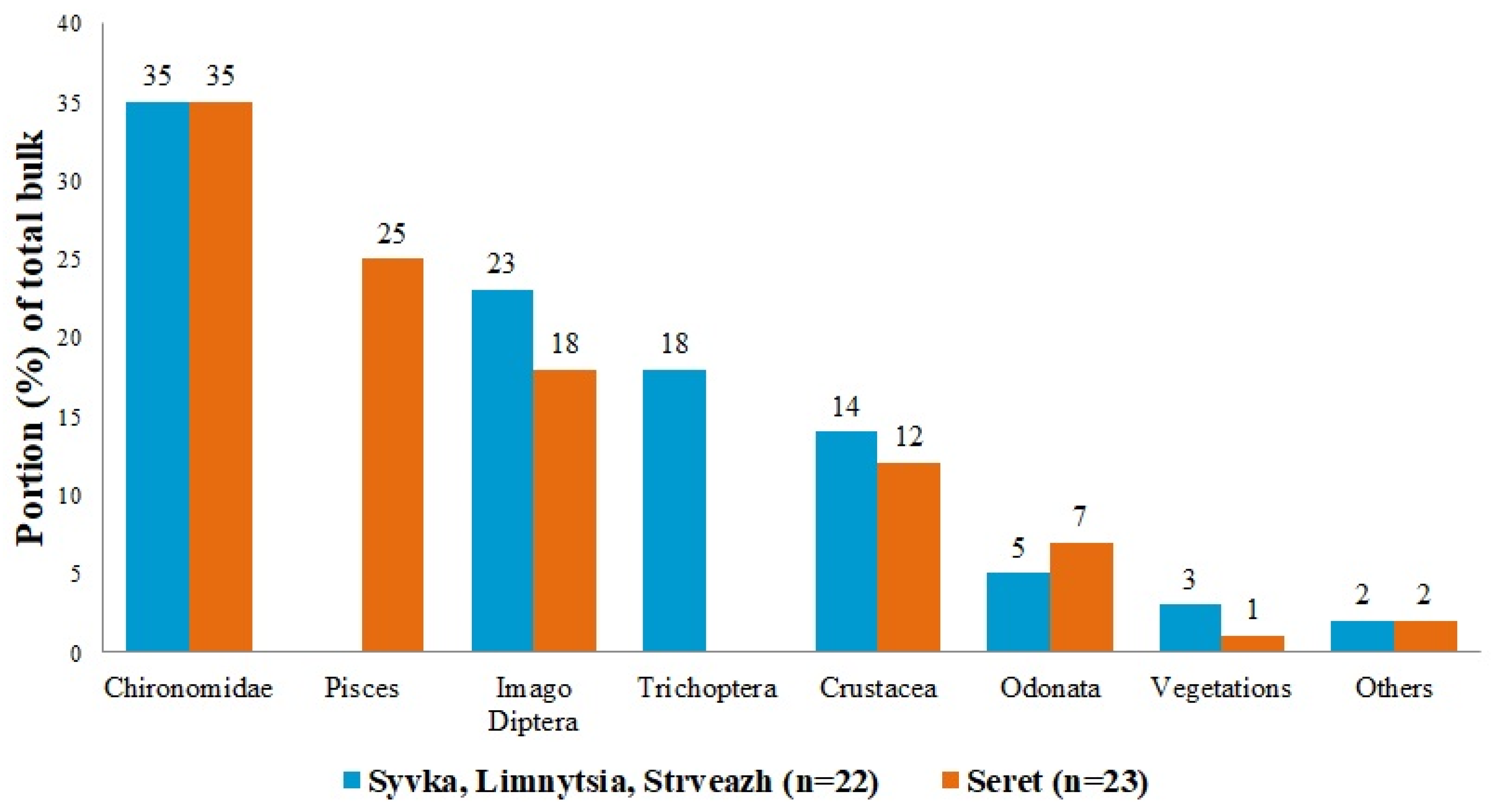
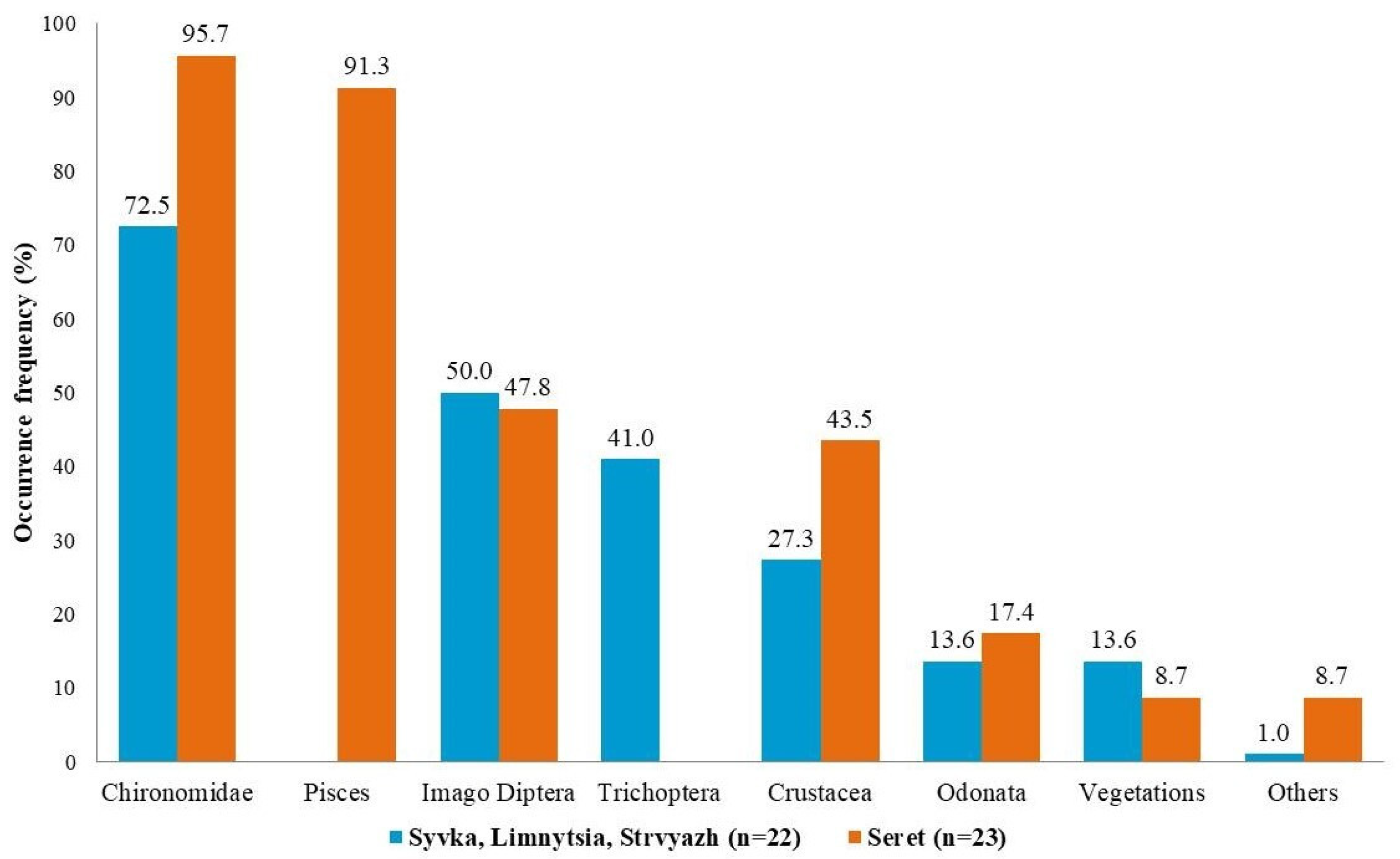
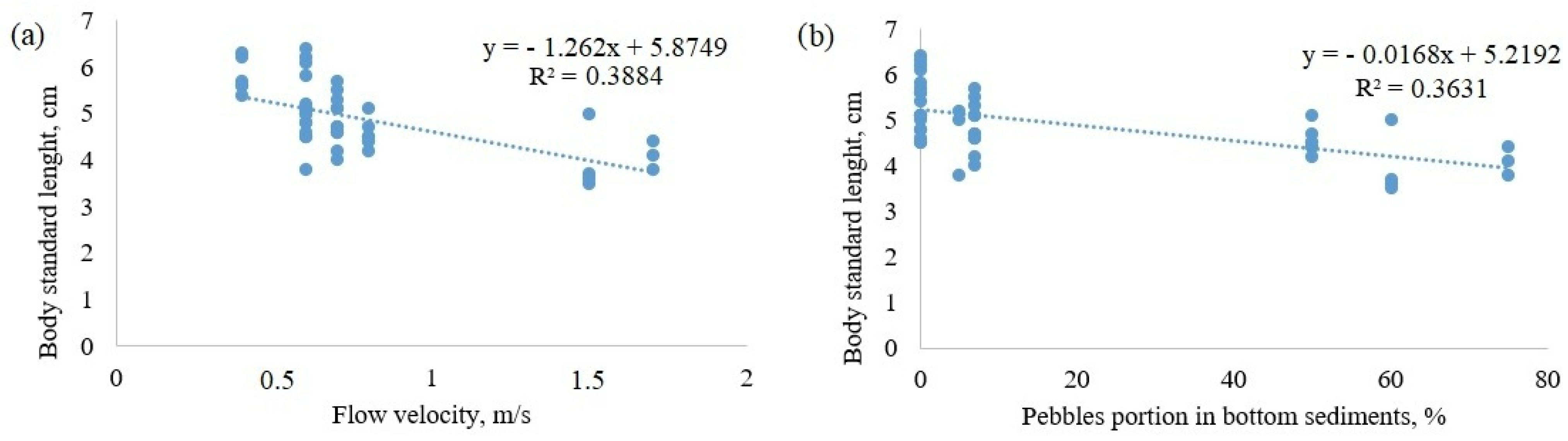
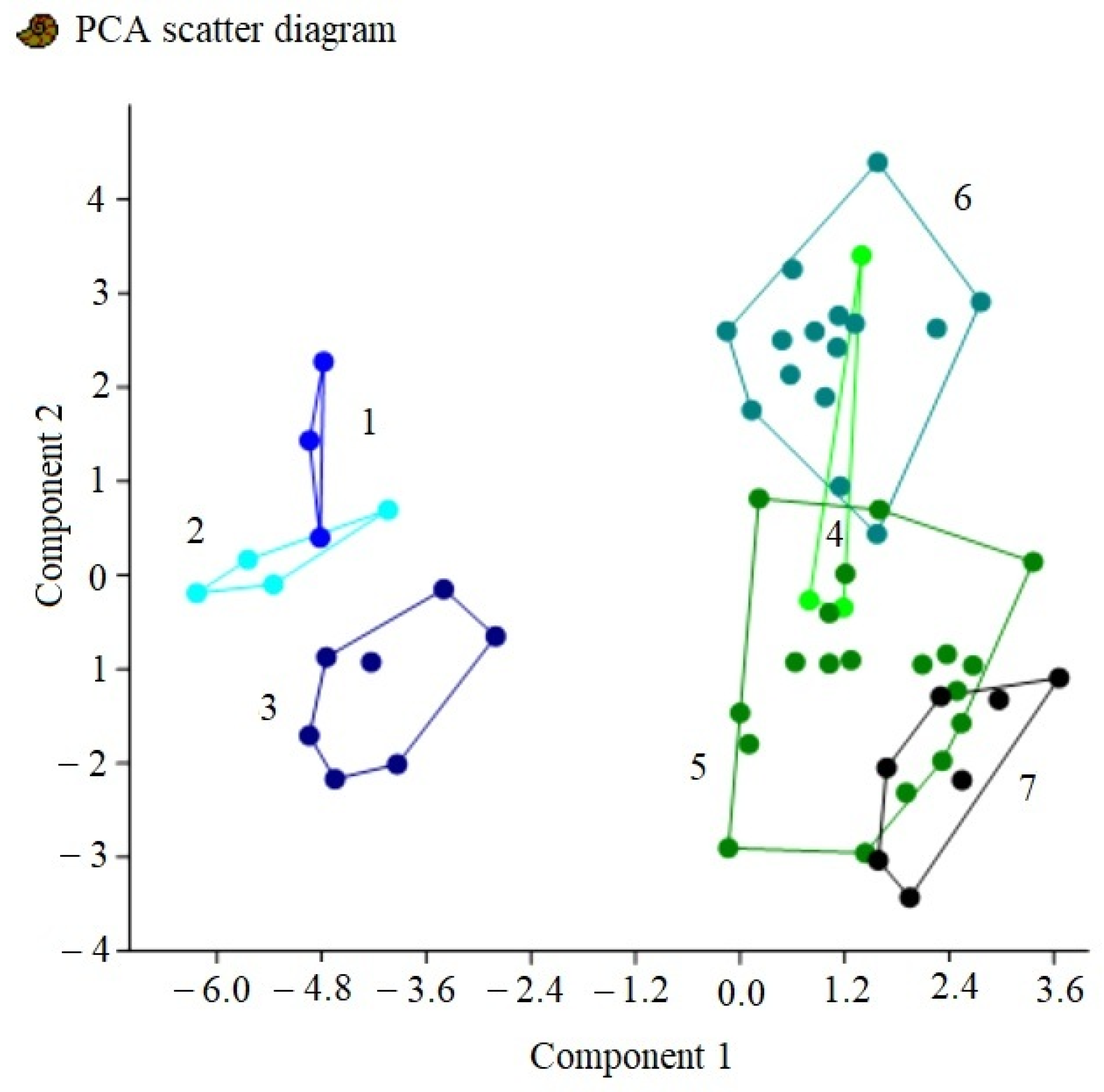
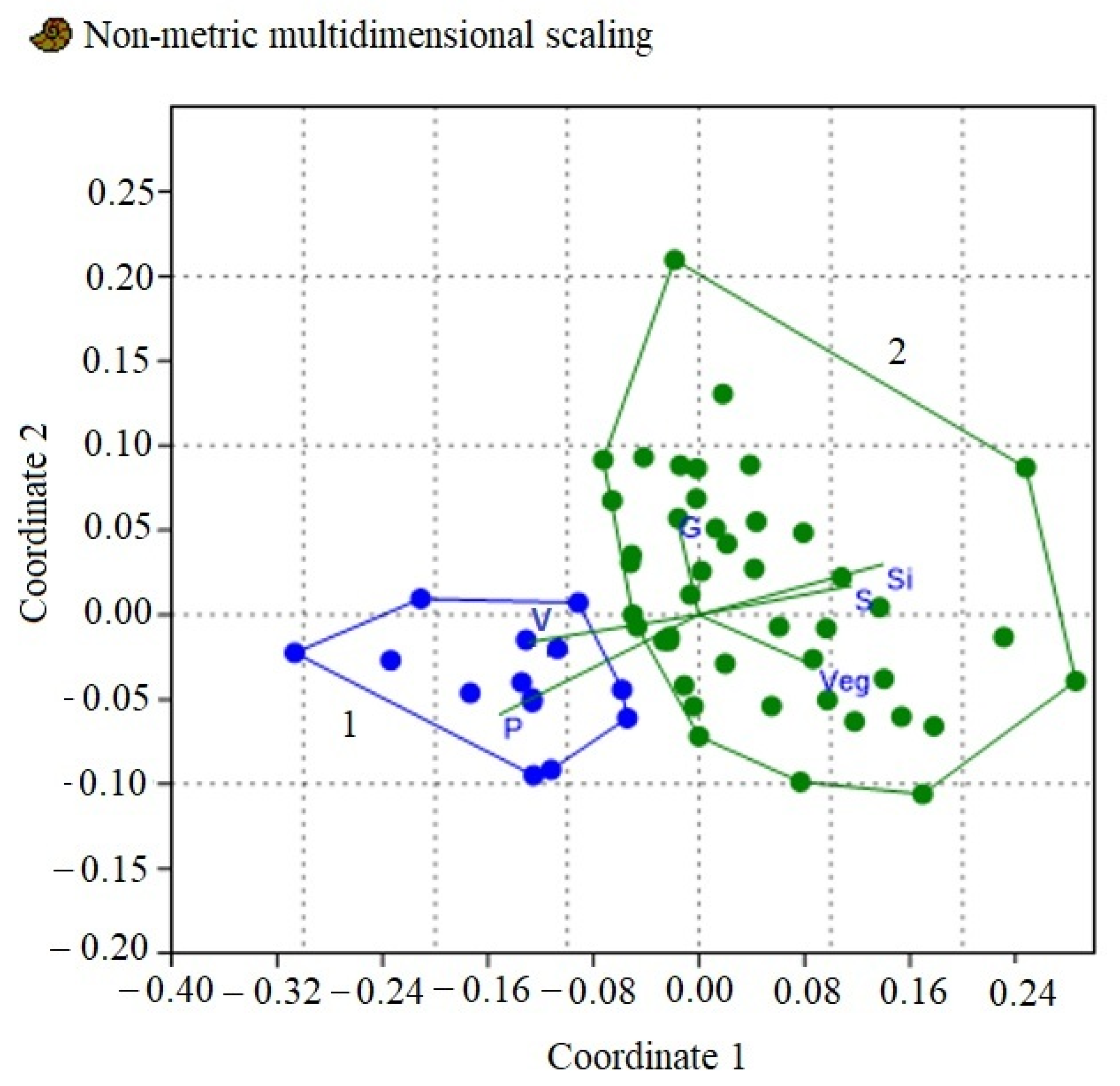
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Priscoli, J.D. Waterandcivilization:Usinghistorytoreframewaterpolicydebatesandtobuildanewecologicalrealism. Water Policy 2000, 1, 623–636. [Google Scholar] [CrossRef]
- Hosseiny, S.H.; Bozorg-Haddad, O.; Bocchiola, D. Water, culture, civilization, and history. Econ. Political Soc. Issues Water Resour. 2021, 189–216. [Google Scholar] [CrossRef]
- Kostrzewa, J.; Grabowski, M. Opportunistic feeding strategy as factor promoting the expansion of racer goby (Neogobius gymnotrachelus Kessler, 1857) in the Vistulabasin. Lauterbornia 2003, 48, 91–100. [Google Scholar]
- Lodge, D.M.; Stein, R.A.; Brown, K.M.; Covich, A.P.; Bronmark, C.; Garvey, J.E.; Klosiewskt, S.P. Predicting impact of freshwater exotic species on native biodiversity: Challenges in spatial scaling. Austral Ecol. 1998, 23, 53–67. [Google Scholar] [CrossRef]
- Anastasiu, P.; Preda, C.; Bănăduc, D.; Cogălniceanu, D. Alien species of EUC on cern in Romania. Transylv. Rev. Syst. Ecol. Res. 2017, 19, 93–106. [Google Scholar] [CrossRef]
- Bănăduc, D.; Simić, V.; Cianfaglione, K.; Barinova, S.; Afanasyev, S.; Öktener, A.; McCall, G.; Simić, S.; Curtean-Bănăduc, A. Freshwater as a sustainable resource and generator of secondary resources in the 21st century: Stressors, threats, risks, management and protection strategies, and conservation approaches. Int. J. Environ. Res. Public Health 2022, 19, 16570. [Google Scholar] [CrossRef]
- Lipej, L.; Kovačić, M.; Dulčić, J. An analysis of adriatic ichthyofauna—Ecology, zoogeography, and conservation status. Fishes 2022, 7, 58. [Google Scholar] [CrossRef]
- Yick, J.L.; Wisniewski, C.; Diggle, J.; Patil, J.G. Eradication of the invasive Common Carp, Cyprinus Carpio from a Large Lake: Lessons and Insights from the Tasmanian Experience. Fishes 2021, 6, 6. [Google Scholar] [CrossRef]
- Bănăduc, D.; Afanasyev, S.; Akeroyd, J.R.; Năstase, A.; Năvodaru, I.; Tofan, L.; Curtean-Bănăduc, A. The Danube Delta: The Achilles heel of Danube River–Danube Delta–Black Sea region fish diversity under a Black sea impact scenario due to sea level rise—A prospective review. Fishes 2023, 8, 355. [Google Scholar] [CrossRef]
- Copp, G.H.; Bianco, P.G.; Bogutskaya, N.G.; Eros, T.; Falka, I.; Ferreira, M.T.; Fox, M.G.; Freyhof, J.; Gozlan, R.E.; Grabowska, J.; et al. To be, or not to be, a non-native freshwater fish? J. Appl. Ichthyol. 2005, 21, 242–262. [Google Scholar] [CrossRef]
- Movchan, Y.V. Fishes of Ukraine; Determinative Key and Reference Book; Zoloti Vorota: Kyiv, Ukraine, 2011; 420p. [Google Scholar]
- Turianyn, I.I. Fishes of Carpathian Water Bodies; Karpaty: Uzhgorod, Ukraine, 1982; 144p. [Google Scholar]
- Afanasyev, S.A.; Gupalo, Y.A.; Manturova, O.V. Distribution and Peculiarities of Biology of the Pumpkinseed Lepomis gibbosus (Perciformes: Centrarchidae) in the Water Bodies of Kyiv City. Hydrobiol. J. 2017, 53, 14–25. [Google Scholar] [CrossRef]
- Kvach, Y.; Kutsokon, Y. The non-indigenous fishes in the fauna of Ukraine: A potentia ad actum. BioInvasions Rec. 2017, 6, 269–279. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. (Eds.) FishBase. World Wide Web Electronic Publication. Version (12/2019). Available online: www.fishbase.org (accessed on 27 February 2023).
- Kukuła, K.; Ortyl, B.; Bylak, A. Habitat selection patterns of a species at the edge—Case study of the native racer goby population in Central Europe. Sci. Rep. 2019, 9, 19670. [Google Scholar] [CrossRef]
- Bănăduc, D.; Joy, M.; Olosutean, H.; Afanasyev, S.; Curtean-Bănăduc, A. Natural and anthropogenic driving forces as key elements in the Lower Danube Basin–South-Eastern Carpathians–North-Western Black Sea coast area lakes: A broken stepping stones for fish in a climatic change scenario? Environ. Sci. Eur. 2020, 32, 73. [Google Scholar] [CrossRef]
- Bănăduc, D.; Rey, S.; Trichkova, T.; Lenhardt, M.; Curtean-Bănăduc, A. The Lower Danube River–Danube Delta–North West Black Sea: A pivotal area of major interest for the past, present and future of its fish fauna—A short review. Sci. Total Environ. 2016, 545–546, 137–151. [Google Scholar] [CrossRef]
- Bănăduc, D.; Bănăduc, A.; Lenhardt, M.; Guti, G. “Porţile de Fier/Iron Gates” Gorges Area (Danube) Fish Fauna. Transylv. Rev. Syst. Ecol. Res. “Iron Gates” Natural Park 2014, 16, 171–196. [Google Scholar] [CrossRef][Green Version]
- Bănăduc, D.; Bakhshalizadeh, S.; Curtean-Bănăduc, A. Natura 2000 A Panacea? Natura 2000 Site Oltul Mijlociucibin-Hârtibaciu(ROSCI0132)—A Local Extinction of a Native Fish Species and a New Alien Fish Arrival Case Study. Transylv. Rev. Syst. Ecol. Res. 2023, 25, 81–100. [Google Scholar] [CrossRef]
- Curtean-Bănăduc, A.; Mihuţ, C.; Burcea, A.; McCall, G.S.; Matei, C.; Bănăduc, D. Screening for Microplastic Uptake in an Urbanized Freshwater Ecosystem: Chondrostoma nasus (Linnaeus, 1758) Case Study. Water 2023, 15, 1578. [Google Scholar] [CrossRef]
- Boeraş, I.; Burcea, A.; Coman, C.; Bănăduc, D.; Curtean-Bănăduc, A. Bacterial microbiomes in the sediments of lotic systems ecologic drivers and role: A case study from the Mureş River, Transylvania, Romania. Water 2021, 13, 3518. [Google Scholar] [CrossRef]
- Bănăduc, D.; Sas, A.; Cianfaglione, K.; Barinova, S.; Curtean-Bănăduc, A. The role of aquatic refuge habitats for fish, and threats in the context of climate change and human impact, during seasonal hydrological drought in the Saxon Villages area (Transylvania, Romania). Atmosphere 2021, 12, 1209. [Google Scholar] [CrossRef]
- Curtean-Bănăduc, A.; Burcea, A.; Mihuţ, C.-M.; Bănăduc, D. The benthic trophic corner stone compartment in POPs transfer from abiotic environment to higher trophic levels—Trichoptera and Ephemeroptera pre-alert indicator role. Water 2021, 13, 1778. [Google Scholar] [CrossRef]
- Bănăduc, D.; Curtean-Bănăduc, A.; Cianfaglione, K.; Akeroyd, J.; Cioca, L.-I. Proposed environmental risk management elements in a Carpathian valley basin, within the Roşia Montană European historical mining area. Int. J. Environ. Res. Public Health 2021, 18, 4565. [Google Scholar] [CrossRef] [PubMed]
- Costea, G.; Pusch, M.T.; Bănăduc, D.; Cosmoiu, D.; Curtean-Bănăduc, A. A review of hydropower plants in Romania: Distribution, current knowledge, and their effects on fish in headwater streams. Renew. Sustain. Energy Rev. 2021, 145, 111003. [Google Scholar] [CrossRef]
- Burcea, A.; Boeraş, I.; Mihuţ, C.-M.; Bănăduc, D.; Matei, C.; Curtean-Bănăduc, A. Adding the Mureş River Basin (Transylvania, Romania) to the list of hotspots with high contamination with pharmaceuticals. Sustainability 2020, 12, 10197. [Google Scholar] [CrossRef]
- Voicu, R.; Voicu, L.; Radecki-Pawlik, A.; Bănăduc, D.; Plesiński, K. A new concept of frontal migration system for fish—For overflow weirs and river sills. Transylv. Rev. Syst. Ecol. Res. 2022, 24, 95–104. [Google Scholar] [CrossRef]
- Bylak, A.; Kukuła, K. Importance of peripheral basins: Implications for the conservation of fish assemblages. Aquat. Conserv. Mar. Freshw. Ecosyst. 2018, 28, 1055–1066. [Google Scholar] [CrossRef]
- Kvach, Y.; Zamorov, V.; Pupins, M. Review of Invasive Ponto-Caspian Gobiids: Current Range and History of Expansion; Daugavpils University Academic Press “Saule”: Daugavpils, Latvia, 2021; 92p. [Google Scholar]
- Kukuła, K.; Bylak, A. Rodzima populacja babki łysej Neogobius gymnotrachelus w Polsce? (The native population of racer goby Neogobius gymnotrachelus in Poland?). Chrońmy Przyr. Ojczystą 2013, 69, 61–65. [Google Scholar]
- Sabaniejew, L.P. Ryby Rosyji. Zhizn’ i lowlya (uzhené) nashykh présnowodnykh ryb; Fizkultura i Sport: Moskwa, Russia, 1982; Volume 1. [Google Scholar]
- Kessler, K. Description of fish belonging to families common to the Black and Caspian Seas. Proc. StPetersburg Nat. Hist. Soc. 1874, 5, 191–324. [Google Scholar]
- Berg, L.S. Freshwater Fishes of the U.S.S.R. and Adjacent Countries: Guide to the Fauna of the U.S.S.R., 4th ed.; USSR Academy of Sciences Press: Moscow, Russia, 1949; Volume 3, pp. 928–1382. [Google Scholar]
- Grabowski, M.; Hupało, K.; Bylak, A.; Kukuła, K.; Grabowska, J. Double origin of the racer goby (Babka gymnotrachelus) in Poland revealed with mitochondrial marker. Possible implications for the species alien/native status. J. Limnol. 2015, 75, 101–108. [Google Scholar] [CrossRef]
- Sokolov, N. Catalogue of a collection of cyclostomata and fishes of the State Natural History Museum of NAS of Ukraine. Proc. State Nat. Hist. Mus. 2004, 19, 15–28. [Google Scholar]
- Yaroshenko, M.F. Hydrofauna of the Dniester River; USSR Academy of Sciences Press: Moscow, Russia, 1957; 168p. [Google Scholar]
- Vaynshtein, A.S. Ichthyofauna of the upper Dniester basin. Pratsi In-tu gidrobiologii AN UkrRSR 1958, 34, 48–60. [Google Scholar]
- Tatarynov, K.A. Fishes. In Ukranian Carpathians. Nature; Holubets, M.A., Honchar, M.T., Komendar, V.I., Kucheriaviy, A., Odynak, Y.P., Eds.; Nauk. Dumka: Kyiv, Ukraine, 1988; pp. 156–161. [Google Scholar]
- Transboundary Dniester River Basin: Ecological State, Reference Conditions, Management; Afanasyev, S., Manturova, O., Eds.; Kafedra: Kyiv, Ukraine, 2021; 384p. [Google Scholar]
- Fauna of Ukraine, Fishes; Smirnov, A.I., Ed.; Nauk. Dumka: Kyiv, Ukraine, 1986; Volume 8, 320p. [Google Scholar]
- Movchan, Y.V.; Manilo, L.G.; Smirnov, A.I.; Shcherbukha, A.Y. Catalogue of Collections of Zoological Museum of the National Natural History Museum of NAS of Ukraine; Zoomuzey NNPM NAN Ukrainy: Kyiv, Ukraine, 2003; 241p. [Google Scholar]
- Surface Waters Sidebar. Available online: http://geoportal.davr.gov.ua:81/ (accessed on 28 June 2023).
- Order of the State Committee of Fisheries of Ukraine No. 19 of February 15, 1999. Available online: https://zakon.rada.gov.ua/laws/show/z0269-99#Text (accessed on 24 February 2019).
- Afanasyev, S.O. Development of European approaches to biological assessment of the state of hydroecosystems and their application to the monitoring of ukrainian rivers. Hydrobiol. J. 2002, 38, 130–148. [Google Scholar] [CrossRef]
- Pravdin, I.F. Guide to the Study of Fish; Pishhevaja promyshlennost: Moscow, Russia, 1966; 375p. [Google Scholar]
- Pylypenro, Y.V.; Shevchenko, P.G.; Tsedyk, V.V.; Korniyenko, V.O. Methods of Ichthyological Research (Tutorial); OLDI-PLUS: Kherson, Ukraine, 2017; 432p. [Google Scholar]
- Zabroda, T.A.; Dyrypasko, O.A. Estimation of sex differences in morphometric characteristics of the round goby Neogobius melanostomus (Pallas, 1814) of the Sea of Azov. Bull. Zaporizhzhya Natl. Univ. 2009, 2, 41–47. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 4. Available online: http://palaeo-electronica.org/2001_1/past/issue1_01.htm (accessed on 18 April 2019).
- Grabowska, J.; Zięba, G.; Przybylski, M.; Smith, C. The role of intraspecific competition in the dispersal of an invasive fish. Freshw. Biol. 2019, 64, 933–941. [Google Scholar] [CrossRef]
- Zogaris, S.; Ntakis, A.; Barbieri, R. The Racer Goby, Babka gymnotrachelus (Kessler, 1857) Invades the Evros River: Evidence of Recent Establishment in Greece. Knowl. Manag. Aquat. Ecosyst. 2019, 420, 13. [Google Scholar] [CrossRef]
- Ohayon, J.L.; Stepien, C.A. Genetic and biogeographic relationships of the racer goby Neogobius gymnotrachelus (Gobiidae: Teleostei) from introduced and native Eurasian locations. J. Fish Biol. 2007, 71, 360–370. [Google Scholar] [CrossRef]
- Afanasyev, S.O.; Gupalo, O.O.; Lietytska, O.M.; Tymoshenko, N.V.; Roman, A.M.; Abramiuk, I.I.; Golub, O.O. Alien Fish Species of the Ukrainian Part of the Dniester River Basin: Distribution and Dynamics of Settlement. Hydrobiol. J. 2022, 58, 52–66. [Google Scholar] [CrossRef]
- Jakubčinová, K.; Simonović, P.; Števove, B.; Čanak Atlagić, J.; Kováč, V. What can morphology tell us about ecology of four invasive goby species? J. Fish Biol. 2017, 90, 1999–2019. [Google Scholar] [CrossRef] [PubMed]
- Popa, G.-O.; Dudu, A.; Bănăduc, D.; Curtean-Bănăduc, A.; Burcea, A.; Ureche, D.; Nechifor, R.; Georgescu, S.E.; Costache, M. Genetic Analysis of Populations of Brown Trout (Salmo trutta L.) from the Romanian Carpathians. Aquat. Living Resour. 2019, 32, 23. [Google Scholar] [CrossRef]
- Popa, G.-O.; Curtean-Bănăduc, A.; Bănăduc, D.; Florescu, I.E.; Burcea, A.; Dudu, A.; Georgescu, S.E.; Costache, M. Molecular markers reveal reduced genetic diversity in Romanian populations of Brown Trout, Salmo trutta L., 1758 (Salmonidae). Acta Zool. Bulg. 2016, 68, 399–406. [Google Scholar]
- Bănăduc, D. The Hucho hucho (Linnaeus) 1758 (Salmoniformes, Salmonidae) species, monitoring in the Vişeu River (Maramureş, Romania). Transylv. Rev. Syst. Ecol. Res. 2008, 5, 183–188. [Google Scholar]
- Voicu, R.; Bănăduc, D.; Greenberg, L.; Curtean-Bănăduc, A. Caraş River Gorge aspects of Salmonids’ communities management—Technical solutions. Manag. Sustain. Dev. 2018, 10, 5–12. [Google Scholar] [CrossRef]
- Bănăduc, D.; Răchită, R.; Curtean-Bănăduc, A.; Gheorghe, L. The species Hucho hucho (Linnaeus, 1758), (Salmoniformes, Salmonidae) in Ruscova River (Northern Romanian Carpathians). Acta Oecol. Carp. 2013, VI, 149–166. [Google Scholar]
- Curtean-Bănăduc, A.; Bănăduc, D. Thymallus thymallus (Linnaeus, 1758), ecological status in Maramureş Mountains Nature Park (Romania). Transylv. Rev. Syst. Ecol. Res. 2016, 18, 53–68. [Google Scholar] [CrossRef]
- Kakareko, T.; Zbikowski, J.; Zytkowicz, J. Diet partitioning in summer of two syntopic neogobiids from two different habitats of the lower Vistula River, Poland. J. Appl. Ichthyol. 2005, 21, 292–295. [Google Scholar] [CrossRef]
- Reid, D.F.; Orlova, M.I. Geological and evolutionary underpinnings for the success of Ponto-Caspian species invasions in the Baltic Sea and North American Great Lakes. Can. J. Fish. Aquat. Sci. 2002, 59, 1144–1158. [Google Scholar] [CrossRef]
- Alieva, A.K.; Nasibulina, B.M.; Bakhshalizadeh, S.; Kurochkina, T.F.; Popov, N.N.; Barbol, B.I.; Bănăduc, D.; Jussupbekova, N.M.; Kuanysheva, G.A.; Ali, A.M. The Low Ontogenetic Diet Diversity and Flexibility of the Pike-Perch, Sander lucioperca (Linnaeus, 1758) (Osteichthyes, Percidae): A Case Study. Fishes 2023, 8, 395. [Google Scholar] [CrossRef]
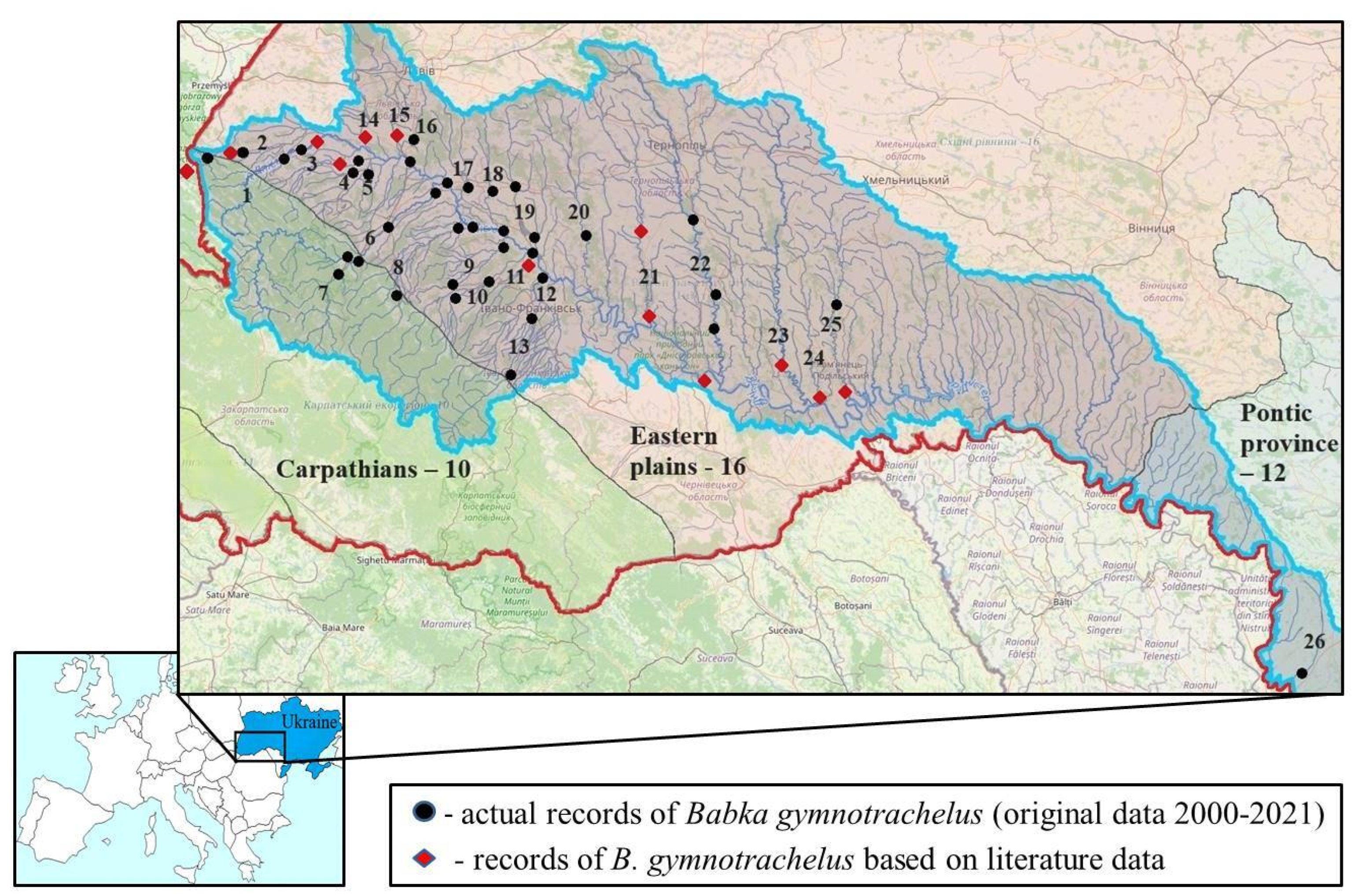
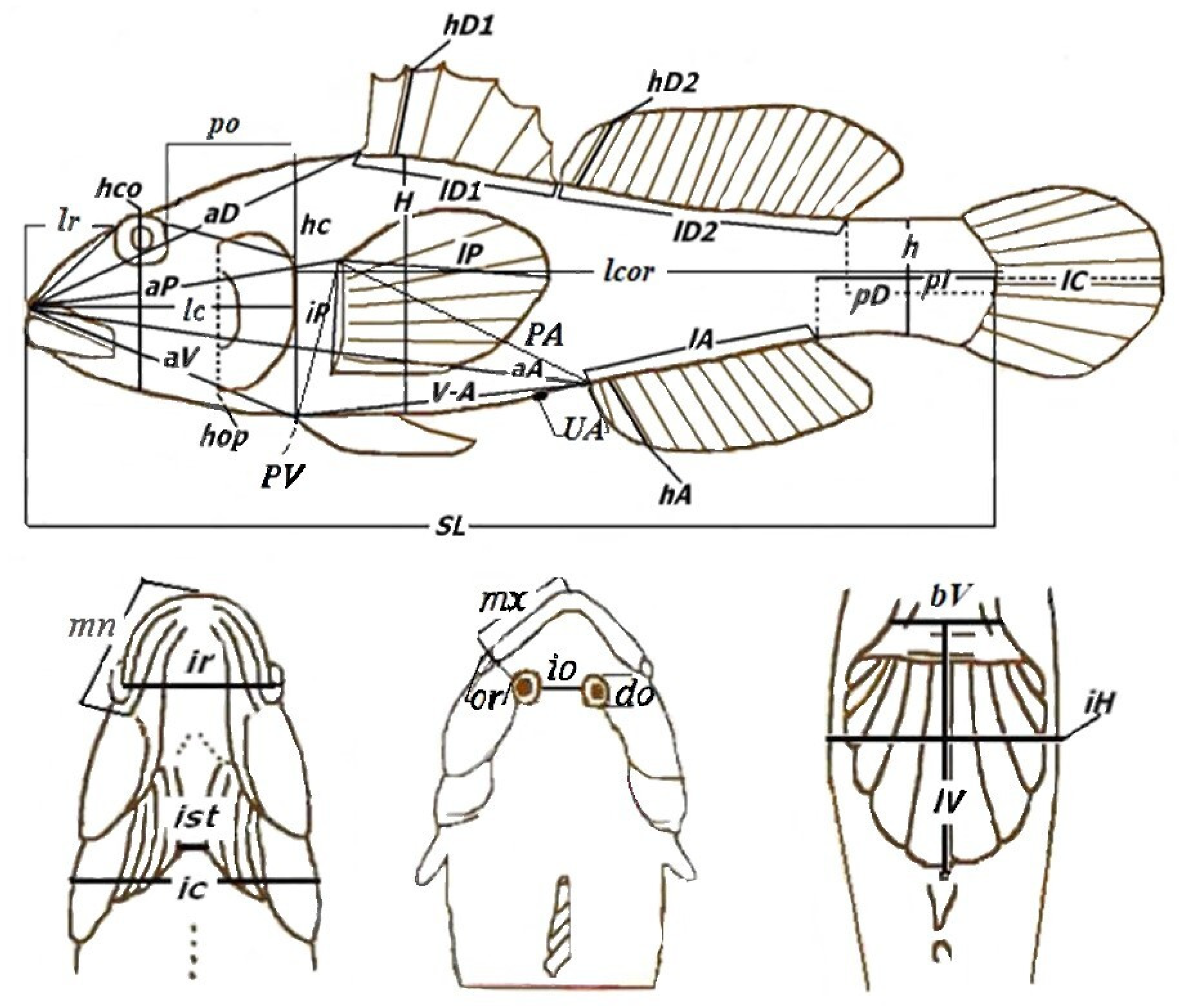
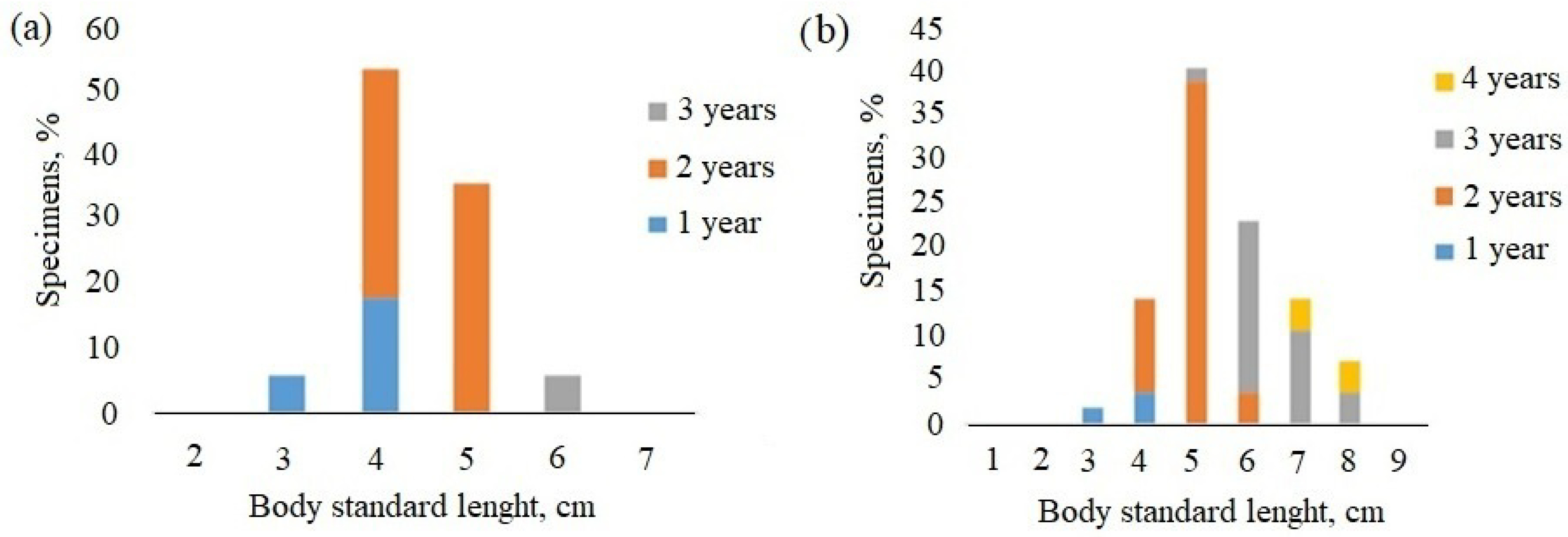
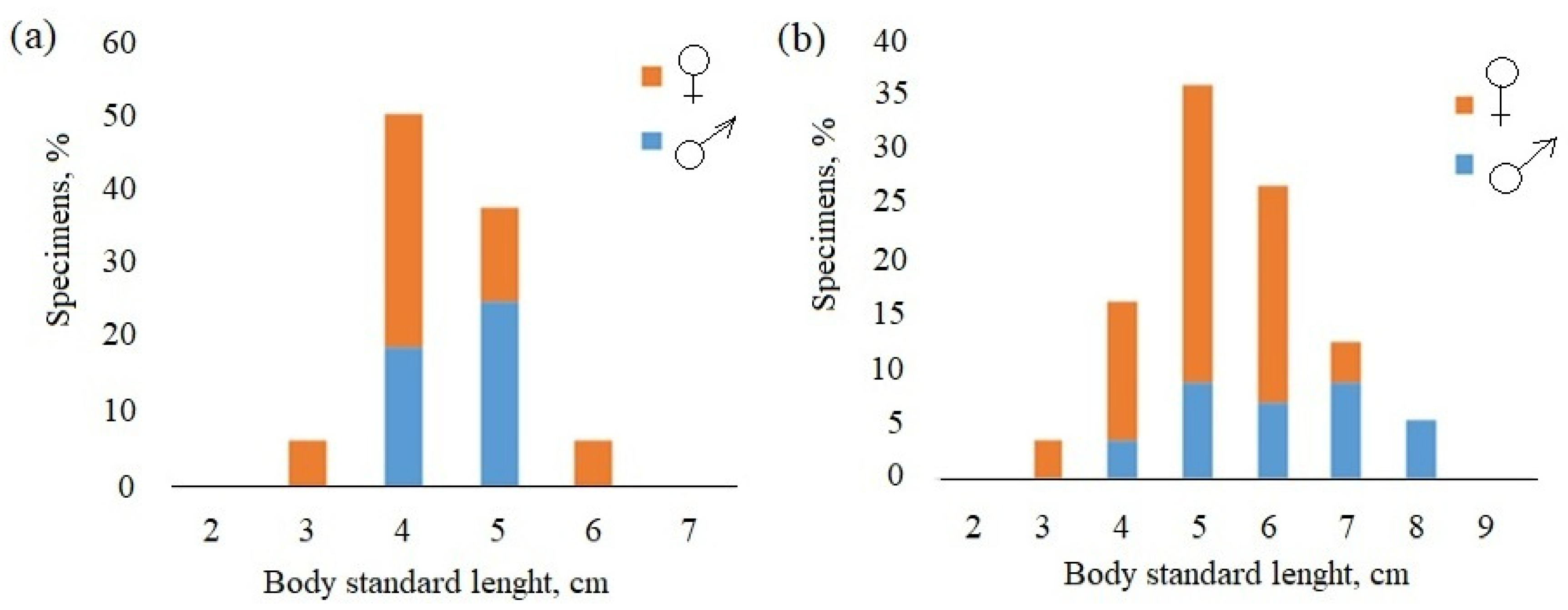
| Site | River | n of Specimens | Date | Height m a.s.l. |
|---|---|---|---|---|
| Zarichchia village 49°30′54.3″ N 22°47′16.7″ E | Strviazh | 5 | 12 June 2000 | 352 |
| Rozgirche village 49°06′56.6″ N 23°40′08.9″ E | Stryi | 2 | 12 July 2001 | 320 |
| Koroscenko village (Poland) 49°28′40″ N, 22°41′15″ E | Strviazh | 2 | 23 July2003 | 395 |
| Broshniv-Ocada village 49°00′16.7″ N 24°11′53.8″ E | Syvka | 3 | 2004 | 330 |
| Vygodivka village 48°59′57.9″ N 23°53′27.0″ E | Svicha | 2 | 2004 | 393 |
| Berlogy village 48°58′13.8″ N 24°13′59.5″ E | Limnytsia | 4 | 2005 | 329 |
| Mezhybrody village 49°06′36.8″ N 23°36′18.6″ E | Stryi | 2 | 2006 | 374 |
| Dubyna village 49°03′25.1″ N 23°32′51.0″ E | Opir | 2 | 2009 | 417 |
| Nadvirna town 48°39′05.7″ N 24°34′48.3″ E | Bystrytsia Nadvirnianska | 1 | 2009 | 406 |
| Density, ind/100 m2 | Body Length, cm, M ± m | Body Weight, g, M ± m | Sexes Ratio, ♂:♀ | n of Specimens | |
|---|---|---|---|---|---|
| Mountainous rivers—Carpathian ecoregion | |||||
| Strviazh | 0.8 | 4.4 ± 0.35 | 1.7 ± 0.43 | 1:3 | 4 |
| Svicha | 1.4 | 4.5 ± 0.11 | 1.7 ± 0.14 | 6:1 | 7 |
| Limnytsia | 1.2 | 4.1 ± 0.49 | 1.5 ± 0.59 | only ♀ | 6 |
| Plain rivers—Eastern plain ecoregion | |||||
| Dniester | 3.8 | 5.6 ± 0.34 | 4.0 ± 0.80 | 1:1 | 19 |
| Seret | 4.6 | 5.6 ± 0.20 | 3.9 ± 044 | 1:2 | 23 |
| Smotrych | 3.0 | 4.1 ± 0.33 | 1.5 ± 0.38 | 1:2 | 6 |
| Yagorlyk | 1.8 | 5.9 ± 0.19 | 4.1 ± 0.46 | only ♀ | 9 |
| River | River Type | Mean Flow Velocity, m/s | Projective Cover of Higher Vegetation, % | Sediments Composition, % | |||
|---|---|---|---|---|---|---|---|
| Silt | Sand | Gravel | Pebbles | ||||
| Strviazh | mountainous | 1.7 | 0 | 0 | 0 | 25 | 75 |
| Svicha | mountainous | 0.8 | 3 | 5 | 5 | 40 | 50 |
| Limnytsia | mountainous | 1.5 | 5 | 3 | 5 | 32 | 60 |
| Dniester | plain | 0.7 | 7 | 30 | 20 | 43 | 7 |
| Seret | plain | 0.6 | 15 | 35 | 40 | 25 | 0 |
| Smotrych | plain | 0.6 | 10 | 30 | 40 | 25 | 5 |
| Yagorlyk | plain | 0.4 | 25 | 50 | 45 | 5 | 0 |
| Traits | Mountainous Rivers, n = 14 | Plain Rivers, n = 34 | Student’s t-Test | Confidence Level, p |
|---|---|---|---|---|
| l, mm | 42.79 ± 1.32 | 51.59 ± 1.12 | −4.52 | >0.0001 |
| l cor | 71.04 ± 0.37 | 71.95 ± 0.23 | −2.12 | 0.0393 |
| pl | 16.33 ± 0.50 | 17.36 ± 0.30 | −1.83 | 0.0738 |
| lC | 21.42 ± 0.29 | 20.83 ± 0.20 | 1.60 | 0.1154 |
| H | 15.58 ± 0.30 | 17.31 ± 0.22 | −4.46 | >0.0001 |
| iH | 13.06 ± 0.34 | 14.90 ± 0.20 | −4.93 | >0.0001 |
| h | 7.62 ± 0.18 | 8.47 ± 0.14 | −3.52 | 0.0010 |
| ih | 3.14 ± 0.20 | 3.53 ± 0.12 | −1.72 | 0.0915 |
| aD | 33.59 ± 0.23 | 33.37 ± 0.20 | 0.63 | 0.5329 |
| aA | 53.98 ± 0.40 | 57.82 ± 0.31 | −6.94 | >0.0001 |
| hD1 | 12.79 ± 0.43 | 13.40 ± 0.28 | −1.18 | 0.2434 |
| lD2 | 35.53 ± 0.54 | 34.91 ± 0.29 | 1.09 | 0.2814 |
| hD2 | 13.69 ± 0.49 | 13.75 ± 0.21 | −0.14 | 0.8899 |
| lP | 21.95 ± 0.63 | 22.09 ± 0.26 | −0.25 | 0.8038 |
| lV | 20.07 ± 0.37 | 20.48 ± 0.21 | −1.01 | 0.32 |
| lA | 28.78 ± 0.49 | 28.00 ± 0.39 | 1.12 | 0.2666 |
| lr | 26.52 ± 0.81 | 31.86 ± 0.50 | −5.68 | >0.0001 |
| po | 49.48 ± 0.96 | 48.69 ± 0.59 | 0.72 | 0.4757 |
| do | 29.01 ± 0.62 | 27.13 ± 0.39 | 2.58 | 0.0130 |
| oi | 9.14 ± 0.76 | 10.11 ± 0.41 | −1.21 | 0.2309 |
| hc | 58.31 ± 0.73 | 69.50 ± 0.66 | −9.89 | >0.0001 |
| ic | 71.91 ± 1.79 | 81.14 ± 0.88 | −5.18 | >0.0001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Afanasyev, S.; Hupalo, O.; Tymoshenko, N.; Lietytska, O.; Roman, A.; Manturova, O.; Bănăduc, D. Morphological and Trophic Features of the Invasive Babka gymnotrachelus (Gobiidae) in the Plain and Mountainous Ecosystems of the Dniester Basin: Spatiotemporal Expansion and Possible Threats to Native Fishes. Fishes 2023, 8, 427. https://doi.org/10.3390/fishes8090427
Afanasyev S, Hupalo O, Tymoshenko N, Lietytska O, Roman A, Manturova O, Bănăduc D. Morphological and Trophic Features of the Invasive Babka gymnotrachelus (Gobiidae) in the Plain and Mountainous Ecosystems of the Dniester Basin: Spatiotemporal Expansion and Possible Threats to Native Fishes. Fishes. 2023; 8(9):427. https://doi.org/10.3390/fishes8090427
Chicago/Turabian StyleAfanasyev, Sergey, Olena Hupalo, Nataliia Tymoshenko, Olena Lietytska, Anatolii Roman, Oksana Manturova, and Doru Bănăduc. 2023. "Morphological and Trophic Features of the Invasive Babka gymnotrachelus (Gobiidae) in the Plain and Mountainous Ecosystems of the Dniester Basin: Spatiotemporal Expansion and Possible Threats to Native Fishes" Fishes 8, no. 9: 427. https://doi.org/10.3390/fishes8090427
APA StyleAfanasyev, S., Hupalo, O., Tymoshenko, N., Lietytska, O., Roman, A., Manturova, O., & Bănăduc, D. (2023). Morphological and Trophic Features of the Invasive Babka gymnotrachelus (Gobiidae) in the Plain and Mountainous Ecosystems of the Dniester Basin: Spatiotemporal Expansion and Possible Threats to Native Fishes. Fishes, 8(9), 427. https://doi.org/10.3390/fishes8090427







