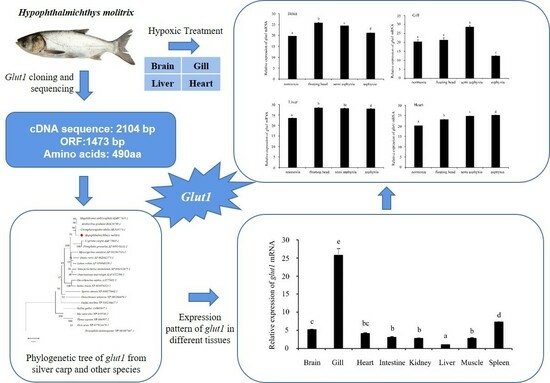
Molecular Characterization and Response of Silver Carp (Hypophthalmichthys molitrix) GLUT1 under Hypoxia Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Fish
2.2. Experimental Design
2.3. Total RNA Extraction and cDNA Synthesis
2.4. Glut1 Cloning and Sequencing
2.5. Bioinformatics Analysis of glut1
2.6. Fluorescence Quantitative PCR (qPCR) Analysis
2.7. Statistical Analysis
3. Results
3.1. Glut1 Sequence Analysis
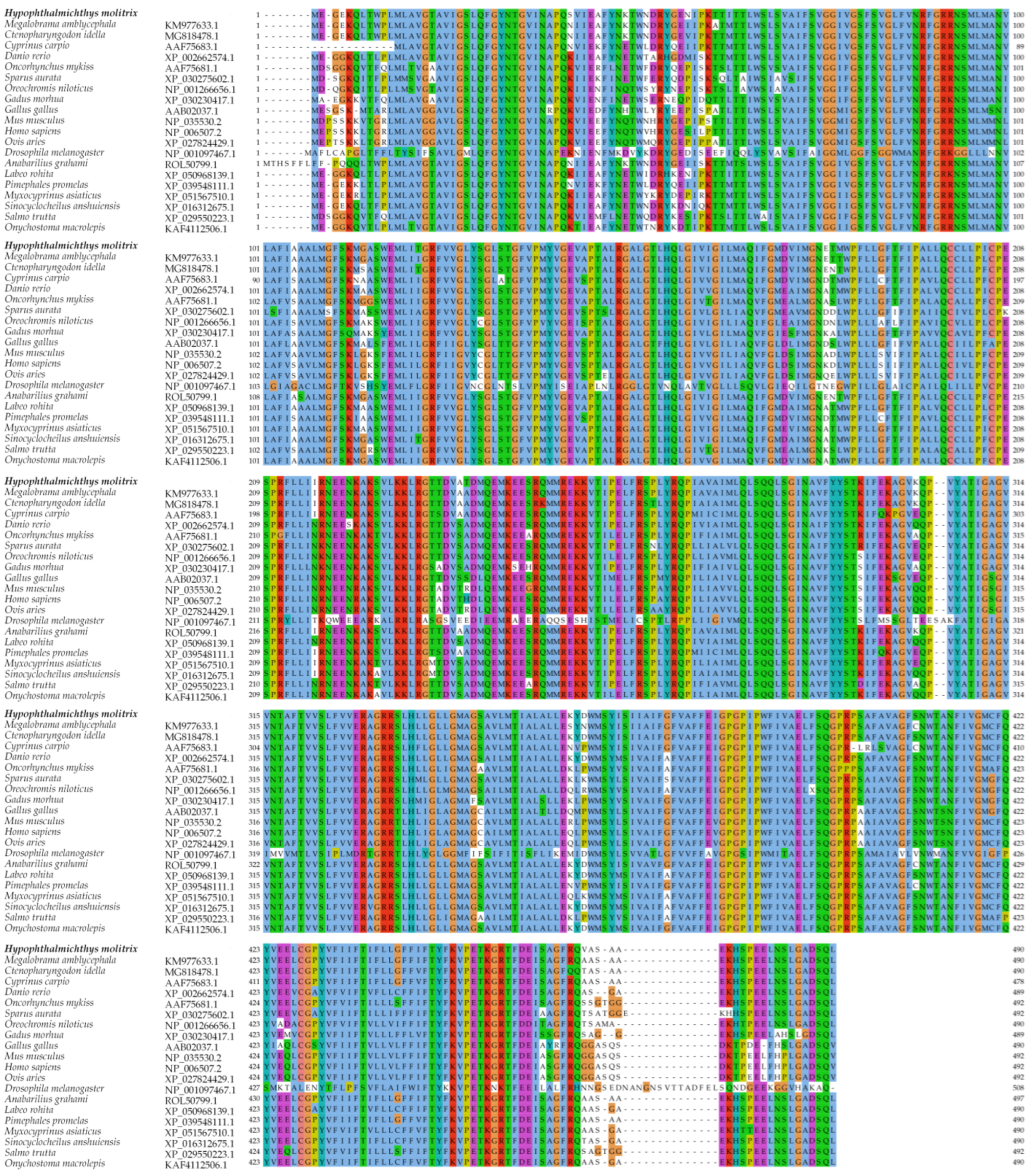
3.2. Homology and Phylogenetic Tree Analysis of Silver Carp glut1
3.3. Expression Pattern of glut1 in Different Tissues of Silver Carp
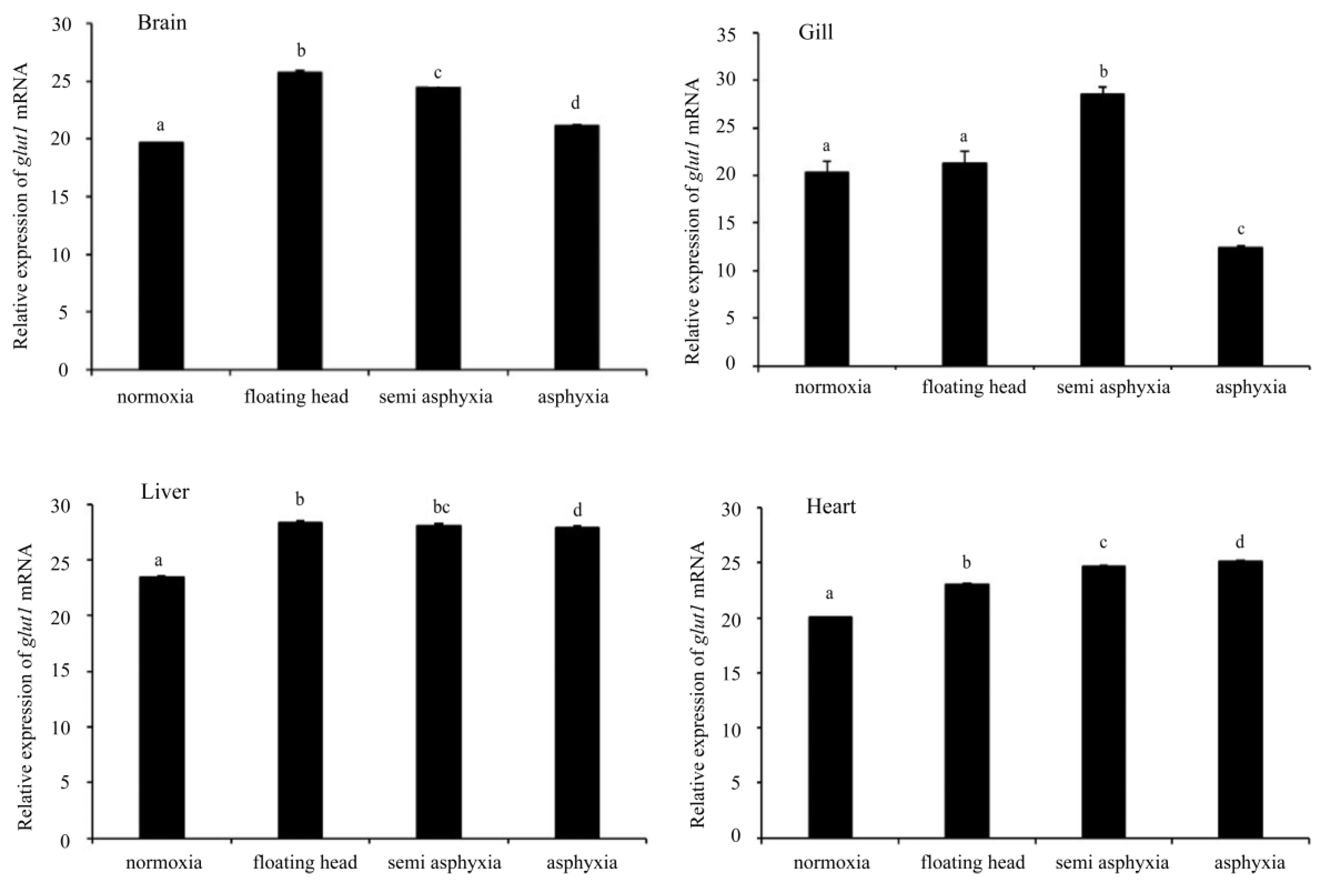
3.4. Analysis of glut1 Expression in Tissues under Different Oxygen Concentrations
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fisheries and Fisheries Administration Bureau of the Ministry of Agriculture and Rural Affairs. 2022 China Fisheries Statistical Yearbook; China Agricultural Publishing House: Beijing, China, 2022. [Google Scholar]
- Guo, L.; Wang, Q.; Xie, P.; Tao, M.; Zhang, J.; Niu, Y.; Ma, Z. A non-classical biomanipulation experiment in Gonghu Bay of Lake Taihu: Control of Microcystis blooms using silver and bighead carp. Aquac. Res. 2015, 46, 2211–2224. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, L.; Fu, J.; Xu, X.; Yue, G.H.; Li, J. Population structure, demographic history and local adaptation of the grass carp. BMC Genom. 2019, 20, 467. [Google Scholar] [CrossRef]
- Liu, J.; Xiong, B.; Wang, J.; Cheng, Z.; Wang, D. Analysis on the Ecological Function of Silver Carp and Bighead Carp in Aquaculture Water. Reserv. Fish. 2008, 158, 8–10. [Google Scholar]
- Li, C.; Wang, J.; Chen, J.; Schneider, K.; Veettil, R.K.; Elmer, K.R.; Zhao, J. Native bighead carp Hypophthalmichthys nobilis and silver carp Hypophthalmichthys molitrix populations in the Pearl River are threatened by Yangtze River introductions as revealed by mitochondrial DNA. J. Fish Biol. 2020, 96, 651–662. [Google Scholar] [CrossRef]
- Li, X.; Zou, G.; Liang, H.; He, X.; Sha, H.; Feng, C.; Cui, F. Effects of continuous hypoxia stress on blood indicators, tissue antioxidant enzymes activity and genes expression in Changfeng silver carp. J. Hunan Agric. Univ. 2022, 6, 723–729. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. Lipid peroxidation, oxygen radicals, cell damage, and antioxidant therapy. Lancet 1984, 1, 1396–1397. [Google Scholar] [CrossRef]
- Shi, X.; Zhou, B. The role of Nrf2 and MAPK pathways in PFOS-induced oxidative stress in zebrafish embryos. Toxicol. Sci. Off. J. Soc. Toxicol. 2010, 115, 391–400. [Google Scholar] [CrossRef]
- Zhang, Z.W.; Li, Z.; Liang, H.W.; Li, L.; Luo, X.Z.; Zou, G.W. Molecular cloning and differential expression patterns of copper/zinc superoxide dismutase and manganese superoxide dismutase in Hypophthalmichthys molitrix. Fish Shellfish. Immunol. 2011, 30, 473–479. [Google Scholar] [CrossRef]
- Li, X.; Feng, C.; Sha, H.; Zhou, T.; Zou, G.; Liang, H. Tandem Mass Tagging-Based Quantitative Proteomics Analysis Reveals Damage to the Liver and Brain of Hypophthalmichthys molitrix Exposed to Acute Hypoxia and Reoxygenation. Antioxidants 2022, 11, 589. [Google Scholar] [CrossRef]
- Li, X.; Li, F.; Zou, G.; Feng, C.; Sha, H.; Liu, S.; Liang, H. Physiological responses and molecular strategies in heart of silver carp (Hypophthalmichthys molitrix) under hypoxia and reoxygenation. Comp. Biochem. Physiology. Part D Genom. Proteom. 2021, 40, 100908. [Google Scholar] [CrossRef]
- Ling, C. Study on the Body and Molecular Response of Silver Carp to Hypoxia Stress. Master’s Thesis, Shanghai Ocean University, Shanghai, China, 2021. [Google Scholar] [CrossRef]
- Denko, N.C. Hypoxia, HIF1 and glucose metabolism in the solid tumour. Nat. Rev. Cancer 2008, 8, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Liberti, M.V.; Locasale, J.W. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem. Sci. 2016, 41, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Osthus, R.C.; Shim, H.; Kim, S.; Li, Q.; Reddy, R.; Mukherjee, M.; Xu, Y.; Wonsey, D.; Lee, L.A.; Dang, C.V. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J. Biol. Chem. 2000, 275, 21797–21800. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Huang, G.; Liu, B.; Fan, S.; Li, Y.; Chen, M.; Yu, D. Cloning of glut1 gene from winged pearl oyster Pteria penguin and its expression in response to glucose challenge. South China Fish. Sci. 2016, 12, 81–89. [Google Scholar]
- Wang, E.F.; Li, X.; Jia, C.S.; Liu, G.W.; Fang, H.X.; Yu, S. ypoxic preconditioning up-regulates the activity and gene expressions of glucose transporters (glut1 and glut3) in cultured rat hippocampal neurons and astrocytes under anoxic condition. Basic Clin. Med. 2009, 29, 1273–1276. [Google Scholar]
- Ye, L.; Lu, K.; Zhang, D.; Qin, S. Insulin promotes glucose uptake via PlgK-AKT pathway in H9c2 myocardial cells. J. Third Mil. Med. Univ. 2015, 37, 538–542. [Google Scholar]
- Li, S.; Li, Z.; Sang, C.; Zhang, J.; Chen, N.-S.; Huang, X. Glucose transporters in pearl gentian grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus ♂): Molecular cloning, characterization, tissue distribution and their expressions in response to dietary carbohydrate level. Aquac. Res. 2018, 49, 253–264. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, R.S.; Mok, H.O.; Wang, Y.; Poon, W.W.; Cheng, S.H.; Kong, R.Y. Isolation, characterization and expression analysis of a hypoxia-responsive glucose transporter gene from the grass carp, Ctenopharyngodon idellus. Eur. J. Biochem. 2003, 270, 3010–3017. [Google Scholar] [CrossRef]
- Miller Neilan, R.; Rose, K. Simulating the effects of fluctuating dissolved oxygen on growth, reproduction, and survival of fish and shrimp. J. Theor. Biol. 2014, 343, 54–68. [Google Scholar] [CrossRef]
- Kuhlicke, J.; Frick, J.S.; Morote-Garcia, J.C.; Rosenberger, P.; Eltzschig, H.K. Hypoxia inducible factor (HIF)-1 coordinates induction of Toll-like receptors TLR2 and TLR6 during hypoxia. PLoS ONE 2007, 2, e1364. [Google Scholar] [CrossRef]
- Balmaceda-Aguilera, C.; Martos-Sitcha, J.A.; Mancera, J.M.; Martínez-Rodríguez, G. Cloning and expression pattern of facilitative glucose transporter 1 (GLUT1) in gilthead sea bream Sparus aurata in response to salinity acclimation. Comp. Biochem. Physiology. Part A Mol. Integr. Physiol. 2012, 163, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.R.; MacCormack, T.J.; Barry, C.A.; Driedzic, W.R. Sequence and expression of a constitutive, facilitated glucose transporter (GLUT1) in Atlantic cod Gadus morhua. J. Exp. Biol. 2004, 207, 4697–4706. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morris, S. Neuroendocrine regulation of osmoregulation and the evolution of air-breathing in decapod crustaceans. J. Exp. Biol. 2001, 204, 979–989. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Jiang, S.; Li, Y.; Yang, Q.; Jiang, S.; Yang, L.; Huang, J.; Zhou, F. Molecular cloning and expression pattern analysis of GLUT1 in black tiger shrimp (Penaeus monodon). South China Fish. Sci. 2019, 15, 72–82. [Google Scholar]
- Zhang, Z.; Li, X.; Yang, F.; Chen, C.; Liu, P.; Ren, Y.; Sun, P.; Wang, Z.; You, Y.; Zeng, Y.X.; et al. DHHC9-mediated GLUT1 S-palmitoylation promotes glioblastoma glycolysis and tumorigenesis. Nat. Commun. 2021, 12, 5872. [Google Scholar] [CrossRef]
- Vinagre, A.S.; Silva, R.S.M.D. Effects of fasting and refeeding on metabolic processes in the crab Chasmagnathus granulata (Dana, 1851). Can. J. Zool. 2002, 80, 1413–1421. [Google Scholar] [CrossRef]
- Teng, J.; Xiao, J. Research progress on glucose transporter 1. Biol. Teach. 2015, 40, 2–3. [Google Scholar]
- Wang, X.; Ye, K.; Wang, Z.; Han, F. Cloning and expression analysis of spleen tyrosine kinase gene in Larimichthys crocea. Hunan Agric. Univ. (Nat. Sci. Ed.) 2017, 43, 662–668. [Google Scholar]
- Sánchez-Paz, A.; García-Carreño, F.; Hernández-López, J.; Muhlia-Almazán, A.; Yepiz-Plascencia, G. Effect of short-term starvation on hepatopancreas and plasma energy reserves of the Pacific white shrimp (Litopenaeus vannamei). J. Exp. Mar. Biol. Ecol. 2007, 340, 184–193. [Google Scholar] [CrossRef]
- Bunn, H.F.; Poyton, R.O. Oxygen sensing and molecular adaptation to hypoxia. Physiol. Rev. 1996, 76, 839–885. [Google Scholar] [CrossRef]
- Lin, Z.; Weinberg, J.M.; Malhotra, R.; Merritt, S.E.; Holzman, L.B.; Brosius, F.C., 3rd. GLUT-1 reduces hypoxia-induced apoptosis and JNK pathway activation. Am. J. Physiol. Endocrinol. Metab. 2000, 278, E958–E966. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, E.; Xu, Z.; Li, T.; Xu, C.; Chen, L. Molecular Response of Carbohydrate Metabolism to Dietary Carbohydrate and Acute Low Salinity Stress in Pacific White Shrimp Litopenaeus vannamei. Turk. J. Fish. Aquat. Sci. 2017, 17, 153–169. [Google Scholar]
- Chen, S. Golden pompano (Trachinotus ovatus) liver, gills physiological and pathological changes and related gene expression under hypoxia; Shanghai Ocean University: Shanghai, China, 2016. [Google Scholar]
- Sun, M.; Jiang, J.; Wang, L. Structural Changes in Gill, Kidney and Intestine of Juvenile Takifugu rubripes under Low Salinity Treatment. J. Zhanjiang Ocean. Univ. 2016, 36, 38–43. [Google Scholar]



| Primer Name | Primer Sequence (5′–3′) | Function |
|---|---|---|
| Glut1-1 | F: CCCTTGGTTTCTGGGACTT | ORF amplification |
| R: ACTGGACGGTTCGTGGTAG | ||
| Glut1-3GSP1 | ACTATCGGAGCTGGAGTCGTCA | 3RACE |
| Glut1-3GSP2 | CTGGACCGCAAACTTTATCGTG | 3RACE |
| UPM | CTAATACGACTCACTATAGGGCAAGCAGTGGTATCAACGCAGAGT | |
| UPM Short | CTAATACGACTCACTATAGGGC | |
| Glut1-qPCR-F | TTGCCTTGCTGGAGAAATACG | qPCR |
| Glut1-qPCR-R | CCCACGATAAAGTTTGCGGTC | qPCR |
| β-actin-F | GAACCCCAAAGCCAACAG | qPCR |
| β-actin-R | CAGAGTCCATCACGATACCAG | qPCR |
| Species | Accession Number | Sequence Identity |
|---|---|---|
| Megalobrama amblycephala | KM977633.1 | 97.3% |
| Ctenopharyngodon idella | MG818478.1 | 96.7% |
| Sinocyclocheilus anshuiensis | XP_016312675.1 | 94.7% |
| Anabarilius grahami | ROL50799.1 | 94.4% |
| Labeo rohita | XP_050968139.1 | 93.7% |
| Onychostoma macrolepis | KAF4112506.1 | 93.3% |
| Pimephales promelas | XP_039548111.1 | 92.9% |
| Myxocyprinus asiaticus | XP_051567510.1 | 91.8% |
| Danio rerio | XP_002662574.1 | 91.2% |
| Cyprinus carpio | AAF75683.1 | 89.4% |
| Salmo trutta | XP_029550223.1 | 87.2% |
| Oncorhynchus mykiss | AAF75681.1 | 86.8% |
| Oreochromis niloticus | NP_001266656.1 | 81.8% |
| Sparus aurata | XP_030275602.1 | 81.5% |
| Gadus morhua | XP_030230417.1 | 81.4% |
| Mus musculus | NP_035530.2 | 80.3% |
| Homo sapiens | NP_006507.2 | 79.9% |
| Ovis aries | XP_027824429.1 | 79.5% |
| Gallus gallus | AAB02037.1 | 78.8% |
| Drosophila melanogaster | NP_001097467.1 | 46% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Li, X.; Zou, G.; Liang, H. Molecular Characterization and Response of Silver Carp (Hypophthalmichthys molitrix) GLUT1 under Hypoxia Stress. Fishes 2023, 8, 425. https://doi.org/10.3390/fishes8080425
Zhang Z, Li X, Zou G, Liang H. Molecular Characterization and Response of Silver Carp (Hypophthalmichthys molitrix) GLUT1 under Hypoxia Stress. Fishes. 2023; 8(8):425. https://doi.org/10.3390/fishes8080425
Chicago/Turabian StyleZhang, Zewen, Xiaohui Li, Guiwei Zou, and Hongwei Liang. 2023. "Molecular Characterization and Response of Silver Carp (Hypophthalmichthys molitrix) GLUT1 under Hypoxia Stress" Fishes 8, no. 8: 425. https://doi.org/10.3390/fishes8080425
APA StyleZhang, Z., Li, X., Zou, G., & Liang, H. (2023). Molecular Characterization and Response of Silver Carp (Hypophthalmichthys molitrix) GLUT1 under Hypoxia Stress. Fishes, 8(8), 425. https://doi.org/10.3390/fishes8080425