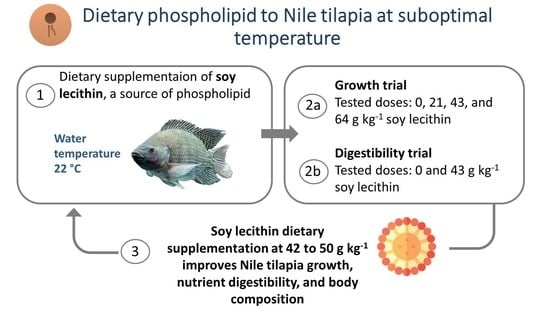
Soy Lecithin Supplementation Promotes Growth and Increases Lipid Digestibility in GIFT Nile Tilapia Raised at Suboptimal Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Diets
2.2. Experimental Procedures
2.3. Fish Biometrics and Sample Collection
2.4. Digestibility Trial
2.5. Chemical Analyses
2.6. Statistical Analysis
3. Results
3.1. Growth Performance, Protein Retention, and Somatic Indices
3.2. Body and Fillet Composition
3.3. Whole-Body Fatty Acid Profiles
3.4. Selected Nutrient Digestibility and Digestible Intake
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. World Fisheries and Aquaculture; FAO: Rome, Italy, 2022; ISBN 9789251072257. [Google Scholar]
- Azaza, M.S.; Dhraïef, M.N.; Kraïem, M.M. Effects of water temperature on growth and sex ratio of juvenile Nile tilapia Oreochromis niloticus (Linnaeus) reared in geothermal waters in southern Tunisia. J. Therm. Biol. 2008, 33, 98–105. [Google Scholar] [CrossRef]
- Nobrega, R.O.; Banze, J.F.; Batista, R.O.; Fracalossi, D.M. Improving winter production of Nile tilapia: What can be done? Aquac. Rep. 2020, 18, 100453. [Google Scholar] [CrossRef]
- da Silva, B.C.; Pereira, A.; Marchiori, N.d.C.; Mariguele, K.H.; Massago, H.; Klabunde, G.H.F. Cold tolerance and performance of selected Nile tilapia for suboptimal temperatures. Aquac. Res. 2021, 52, 1071–1077. [Google Scholar] [CrossRef]
- Ibarz, A.; Padrós, F.; Gallardo, M.Á.; Fernández-Borràs, J.; Blasco, J.; Tort, L. Low-temperature challenges to gilthead sea bream culture: Review of cold-induced alterations and “Winter Syndrome”. Rev. Fish Biol. Fish. 2010, 20, 539–556. [Google Scholar] [CrossRef]
- Lima De Almeida, A.; Charlle Kally, C.; De Almeida, L.; De Fátima, E.; Martins, F.; Maria, Â.; Pimentel, A.; Tatiane, R.; Fortes-silva, R. Effect of the dietary linoleic/α -linolenic ratio (n6/n3) on histopathological alterations caused by suboptimal temperature in tilapia (Oreochromis niloticus). J. Therm. Biol. 2019, 85, 102386. [Google Scholar] [CrossRef] [PubMed]
- Shi, G.C.; Dong, X.H.; Chen, G.; Tan, B.P.; Yang, Q.H.; Chi, S.Y.; Liu, H.Y. Physiological responses and HSP70 mRNA expression of GIFT strain of Nile tilapia (Oreochromis niloticus) under cold stress. Aquac. Res. 2015, 46, 658–668. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y.; Niu, M.; Hu, L.; Chen, L. Cold Acclimation for Enhancing the Cold Tolerance of Zebrafish Cells. Front. Physiol. 2022, 12, 813451. [Google Scholar] [CrossRef]
- Zhou, T.; Gui, L.; Liu, M.; Li, W.; Hu, P.; Duarte, D.F.C.; Niu, H.; Chen, L. Transcriptomic responses to low temperature stress in the Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2019, 84, 1145–1156. [Google Scholar] [CrossRef]
- Glencross, B.D. Exploring the nutritional demand for essential fatty acids by aquaculture species. Rev. Aquac. 2009, 1, 71–124. [Google Scholar] [CrossRef]
- Liu, C.; Ge, J.; Zhou, Y.; Thirumurugan, R.; Gao, Q.; Dong, S. Effects of decreasing temperature on phospholipid fatty acid composition of different tissues and hematology in Atlantic salmon (Salmo salar). Aquaculture 2020, 515, 734587. [Google Scholar] [CrossRef]
- Tocher, D.R. Metabolism and functions of lipids and fatty acids in teleost fish. Rev. Fish. Sci. 2003, 11, 107–184. [Google Scholar] [CrossRef]
- Abdel-Ghany, H.M.; El-Sayed, A.F.M.; Ezzat, A.A.; Essa, M.A.; Helal, A.M. Dietary lipid sources affect cold tolerance of Nile tilapia (Oreochromis niloticus). J. Therm. Biol. 2019, 79, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Kostetsky, E.Y.; Velansky, P.V.; Sanina, N.M. Phase transitions of phospholipids as a criterion for assessing the capacity for thermal adaptation in fish. Russ. J. Mar. Biol. 2013, 39, 214–222. [Google Scholar] [CrossRef]
- Nobrega, R.O.; Batista, R.O.; Corrêa, C.F.; Mattioni, B.; Filer, K.; Pettigrew, J.E.; Fracalossi, D.M. Dietary supplementation of Aurantiochytrium sp. meal, a docosahexaenoic-acid source, promotes growth of Nile tilapia at a suboptimal low temperature. Aquaculture 2019, 507, 500–509. [Google Scholar] [CrossRef]
- Fernandes, V.A.G.; Brignol, F.D.; Filler, K.; Pettigrew, J.; Fracalossi, D.M. Aurantiochytrium sp. meal as DHA source in Nile tilapia diet, part I: Growth performance and body composition. Aquac. Res. 2019, 50, 390–399. [Google Scholar] [CrossRef]
- Ng, W.K.; Sigholt, T.; Bell, J.G. The influence of environmental temperature on the apparent nutrient and fatty acid digestibility in Atlantic salmon (Salmo salar L.) fed finishing diets containing different blends of fish oil, rapeseed oil and palm oil. Aquac. Res. 2004, 35, 1228–1237. [Google Scholar] [CrossRef]
- Olsen, R.E. The influence of temperature on the apparent nutrient and fatty acid digestibility of Arctic charr, Salvelinus alpinus L. Aquac. Res. 1998, 29, 147–158. [Google Scholar] [CrossRef]
- Corrêa, C.F.; Nobrega, R.O.; Mattioni, B.; Turchini, G.M.; Fracalossi, D.M. LC-PUFA endogenous biosynthesis is improved in Nile tilapia fed plant-oil sources at cold suboptimal temperature. Aquaculture 2023, 562, 738832. [Google Scholar] [CrossRef]
- Corrêa, C.F.; Nobrega, R.O.; Mattioni, B.; Block, J.M.; Fracalossi, D.M. Dietary lipid sources affect the performance of Nile tilapia at optimal and cold, suboptimal temperatures. Aquac. Nutr. 2017, 23, 1016–1026. [Google Scholar] [CrossRef]
- Refaey, M.M.; Mehrim, A.I.; Zenhom, O.A.; Mansour, A.T. Effect of fatty acids manipulation on survival and physiological response of hybrid red tilapia under chronic cold stress. Aquaculture 2022, 561, 738663. [Google Scholar] [CrossRef]
- Corrêa, C.F.; Nobrega, R.O.; Block, J.M.; Fracalossi, D.M. Mixes of plant oils as fish oil substitutes for Nile tilapia at optimal and cold suboptimal temperature. Aquaculture 2018, 497, 82–90. [Google Scholar] [CrossRef]
- Batista, R.O.; Nobrega, R.O.; Schleder, D.D.; Pettigrew, J.E.; Fracalossi, D.M. Aurantiochytrium sp. Meal improved body fatty acid profile and morphophysiology in nile tilapia reared at low temperature. Fishes 2021, 6, 45. [Google Scholar] [CrossRef]
- Tocher, D.R.; Bendiksen, E.Å.; Campbell, P.J.; Bell, J.G. The role of phospholipids in nutrition and metabolism of teleost fish. Aquaculture 2008, 280, 21–34. [Google Scholar] [CrossRef] [Green Version]
- Van Nieuwenhuyzen, W.; Tomás, M.C. Update on vegetable lecithin and phospholipid technologies. Eur. J. Lipid Sci. Technol. 2008, 110, 472–486. [Google Scholar] [CrossRef]
- EFSA Panel on Additives and Products or Substances used in Animal Feed (FEEDAP). Safety and efficacy of lecithins for all animal species. EFSA J. 2016, 14, 4561. [Google Scholar] [CrossRef]
- Jobling, M. National Research Council (NRC): Nutrient requirements of fish and shrimp. Aquac. Int. 2012, 20, 601–602. [Google Scholar] [CrossRef]
- Furuya, W.M.; Furuya, V.R.B.; Nagae, M.Y.; Graciano, T.S.; Michelato, M.; Xavier, T.O.; Vidal, L.V. Nutrição de Tilápias no Brasil. Sci. Agrar. Parana. 2012, 11, 19–34. [Google Scholar] [CrossRef]
- Popma, T.J.; Lovshin, L.L. Worldwide Prospects of Commercial Production of Tilapia; Auburn University: Auburn, AL, USA, 1995. [Google Scholar]
- Rodrigues, A.P.O.; Gominho-Rosa, M.D.C.; Cargnin-Ferreira, E.; de Francisco, A.; Fracalossi, D.M. Different utilization of plant sources by the omnivores jundiá catfish (Rhamdia quelen) and Nile tilapia (Oreochromis niloticus). Aquac. Nutr. 2012, 18, 65–72. [Google Scholar] [CrossRef]
- Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International. Volume I, Agricultural Chemicals, Contaminants, Drugs, 17th ed.; Horwitz, W., Ed.; AOAC International: Arlington, VA, USA, 2000; pp. 44–46. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Juaneda, P.; Rocquelin, G. Rapid and convenient separation of pentachlorophenol from human fat using silica Sep-PakTM cartridges. J. Chromatogr. A 1985, 346, 435–439. [Google Scholar] [CrossRef]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
- O’Fallon, J.V.; Busboom, J.R.; Nelson, M.L.; Gaskins, C.T. A direct method for fatty acid methyl ester synthesis: Application to wet meat tissues, oils, and feedstuffs. J. Anim. Sci. 2007, 85, 1511–1521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cahu, C.L.; Infante, J.L.Z.; Barbosa, V. Effect of dietary phospholipid level and phospholipid:neutral lipid value on the development of sea bass (Dicentrarchus labrax) larvae fed a compound diet. Br. J. Nutr. 2003, 90, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Daprà, F.; Geurden, I.; Corraze, G.; Bazin, D.; Zambonino-Infante, J.L.; Fontagné-Dicharry, S. Physiological and molecular responses to dietary phospholipids vary between fry and early juvenile stages of rainbow trout (Oncorhynchus mykiss). Aquaculture 2011, 319, 377–384. [Google Scholar] [CrossRef]
- Sivaramakrishnan, T.; Ambasankar, K.; Kumaraguru Vasagam, K.P.; Syama Dayal, J.; Sandeep, K.P.; Bera, A.; Makesh, M.; Kailasam, M.; Vijayan, K.K. Effect of dietary soy lecithin inclusion levels on growth, feed utilization, fatty acid profile, deformity and survival of milkfish (Chanos chanos) larvae. Aquac. Res. 2021, 52, 5366–5374. [Google Scholar] [CrossRef]
- Zhao, J.; Ai, Q.; Mai, K.; Zuo, R.; Luo, Y. Effects of dietary phospholipids on survival, growth, digestive enzymes and stress resistance of large yellow croaker, Larmichthys crocea larvae. Aquaculture 2013, 410–411, 122–128. [Google Scholar] [CrossRef]
- Pagheh, E.; Agh, N.; Marammazi, J.G.; Nouri, F.; Sepahdari, A.; Gisbert, E.; Torfi Mozanzadeh, M. Dietary soybean lecithin affects growth performance, fillet biochemical composition and digestive enzyme activity in Sparidentex hasta juvenile. J. Appl. Anim. Res. 2019, 47, 24–33. [Google Scholar] [CrossRef] [Green Version]
- Taylor, J.F.; Martinez-Rubio, L.; Del Pozo, J.; Walton, J.M.; Tinch, A.E.; Migaud, H.; Tocher, D.R. Influence of dietary phospholipid on early development and performance of Atlantic salmon (Salmo salar). Aquaculture 2015, 448, 262–272. [Google Scholar] [CrossRef] [Green Version]
- Geurden, I.; Coutteau, P.; Sorgeloos, P. Increased docosahexaenoic acid levels in total and polar lipid of European sea bass (Dicentrarchus labrax) postlarvae fed vegetable or animal phospholipids. Mar. Biol. 1997, 129, 689–698. [Google Scholar] [CrossRef]
- Chen, Y.P.; Jiang, W.D.; Liu, Y.; Jiang, J.; Wu, P.; Zhao, J.; Kuang, S.Y.; Tang, L.; Tang, W.N.; Zhang, Y.A.; et al. Exogenous phospholipids supplementation improves growth and modulates immune response and physical barrier referring to NF-κB, TOR, MLCK and Nrf2 signaling factors in the intestine of juvenile grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2015, 47, 46–62. [Google Scholar] [CrossRef]
- Adel, M.; Gholaghaie, M.; Khanjany, P.; Citarasu, T. Effect of dietary soybean lecithin on growth parameters, digestive enzyme activity, antioxidative status and mucosal immune responses of common carp (Cyprinus carpio). Aquac. Nutr. 2017, 23, 1145–1152. [Google Scholar] [CrossRef]
- Kasper, C.S.; Brown, P.B. Growth Improved in Juvenile Nile Tilapia Fed Phosphatidylcholine; Growth Improved in Juvenile Nile Tilapia Fed Phosphatidylcholine. N. Am. J. Aquac. 2003, 65, 39–43. [Google Scholar] [CrossRef]
- Tian, J.; Wen, H.; Lu, X.; Liu, W.; Wu, F.; Yang, C.G.; Jiang, M.; Yu, L.J. Dietary phosphatidylcholine impacts on growth performance and lipid metabolism in adult Genetically Improved Farmed Tilapia (GIFT) strain of Nile tilapia Oreochromis niloticus. Br. J. Nutr. 2018, 119, 12–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.J.; Hosseindoust, A.R.; Choi, Y.H.; Kumar, A.; Jeon, S.M.; Lee, S.H.; Jung, B.Y.; Kill, D.Y.; Chae, B.J. An evaluation of metabolizable energy content of main feed ingredients for growing pigs when adding dietary lysophospholipids. Livest. Sci. 2018, 210, 99–103. [Google Scholar] [CrossRef]
- Wangkahart, E.; Bruneel, B.; Wisetsri, T.; Nontasan, S.; Martin, S.A.M.; Chantiratikul, A. Interactive effects of dietary lipid and nutritional emulsifier supplementation on growth, chemical composition, immune response and lipid metabolism of juvenile Nile tilapia (Oreochromis niloticus). Aquaculture 2022, 546, 737341. [Google Scholar] [CrossRef]
- Lu, K.L.; Xu, W.N.; Li, X.F.; iu, W.B.; Wang, L.N.; Zhang, C.N. Hepatic triacylglycerol secretion, lipid transport and tissue lipid uptake in blunt snout bream (Megalobrama amblycephala) fed high-fat diet. Aquaculture 2013, 408–409, 160–168. [Google Scholar] [CrossRef]
- Xie, S.; Yin, P.; Tian, L.; Liu, Y.; Niu, J. Lipid metabolism and plasma metabolomics of juvenile largemouth bass Micropterus salmoides were affected by dietary oxidized fish oil. Aquaculture 2020, 522, 735158. [Google Scholar] [CrossRef]
- Feng, S.; Cai, Z.; Zuo, R.; Mai, K.; Ai, Q. Effects of dietary phospholipids on growth performance and expression of key genes involved in phosphatidylcholine metabolism in larval and juvenile large yellow croaker, Larimichthys crocea. Aquaculture 2017, 469, 59–66. [Google Scholar] [CrossRef]
- Lin, S.-M.; Li, F.-J.; Yuangsoi, B.; Doolgindachbaporn, S. Effect of dietary phospholipid levels on growth, lipid metabolism, and antioxidative status of juvenile hybrid snakehead (Channa argus×Channa maculata). Fish Physiol. Biochem. 2018, 44, 401–410. [Google Scholar] [CrossRef]
- Oboh, A.; Kabeya, N.; Carmona-Antoñanzas, G.; Castro, L.F.C.; Dick, J.R.; Tocher, D.R.; Monroig, O. Two alternative pathways for docosahexaenoic acid (DHA, 22:6n-3) biosynthesis are widespread among teleost fish. Sci. Rep. 2017, 7, 3889. [Google Scholar] [CrossRef] [Green Version]
- Turchini, G.M.; Torstensen, B.E.; Ng, W.K. Fish oil replacement in finfish nutrition. Rev. Aquac. 2009, 1, 10–57. [Google Scholar] [CrossRef]

| Ingredients 1, g kg −1 Dry Diet | Diets | |||
|---|---|---|---|---|
| 0SL 5 | 21SL | 43SL | 64SL | |
| Soybean meal | 450.0 | 450.0 | 450.0 | 450.0 |
| Broken rice | 387.0 | 380.0 | 371.0 | 357.0 |
| Poultry by-product meal | 100.0 | 100.0 | 100.0 | 100.0 |
| Corn oil | 34.0 | 20.0 | 7.0 | 0.0 |
| Soy lecithin | 0.0 | 21.0 | 43.0 | 64.0 |
| Others 2 | 29.0 | 29.0 | 29.0 | 29.0 |
| Analyzed Composition, g 100 g −1 Dry Weight | ||||
| Dry matter | 91.14 | 91.30 | 91.21 | 90.36 |
| Crude energy (kcal kg−1) | 4632 | 4674 | 4744 | 4812 |
| Crude protein | 33.04 | 33.10 | 33.63 | 32.97 |
| Ether extract | 6.61 | 7.53 | 7.83 | 8.74 |
| Polar lipids | 2.59 | 3.87 | 4.87 | 5.97 |
| Mineral matter | 6.61 | 6.81 | 6.98 | 7.55 |
| C16:0 3 | 0.89 | 1.00 | 1.06 | 1.11 |
| C18:1 n-9 | 1.56 | 1.29 | 1.17 | 1.01 |
| C18:2 n-6 | 1.96 | 2.10 | 2.23 | 2.35 |
| C18:3 n-3 | nd 6 | 0.02 | 0.03 | 0.03 |
| SFA 4 | 1.12 | 1.22 | 1.35 | 1.43 |
| MUFA | 1.74 | 1.66 | 1.55 | 1.41 |
| PUFA | 1.96 | 2.10 | 2.26 | 2.39 |
| PUFA n-6 | 1.96 | 2.10 | 2.23 | 2.36 |
| PUFA n-3 | 0.00 | 0.02 | 0.03 | 0.03 |
| Ingredients 1, g kg−1 Dry Diet | Diets | |
|---|---|---|
| 0SLD 5 | 43SLD | |
| Soybean meal | 450.0 | 450.0 |
| Broken rice | 386.0 | 370.0 |
| Poultry by-product meal | 100.0 | 100.0 |
| Corn oil | 34.0 | 7.0 |
| Soy lecithin | 0.0 | 43.0 |
| Others 2 | 29.0 | 29.0 |
| Yttrium oxide | 1.0 | 1.0 |
| Analyzed Composition, g 100 g−1 Dry Weight | ||
| Dry matter | 90.79 | 91.12 |
| Crude energy (kcal kg−1) | 4670 | 4676 |
| Crude protein | 31.39 | 30.41 |
| Ether extract | 6.21 | 7.84 |
| Polar lipids | 2.27 | 4.91 |
| Mineral matter | 6.48 | 6.82 |
| C16:0 3 | 0.85 | 1.15 |
| C18:1 n-9 | 1.48 | 1.18 |
| C18:2 n-6 | 1.89 | 2.12 |
| C18:3 n-3 | nd 6 | 0.03 |
| SFA 4 | 1.09 | 1.43 |
| MUFA | 1.75 | 1.58 |
| PUFA | 1.89 | 2.16 |
| PUFA n-6 | 1.89 | 2.13 |
| PUFA n-3 | 0.00 | 0.03 |
| Variables | Diets | Pooled SEM 3 | p Value 4 | |||
|---|---|---|---|---|---|---|
| 0SL 2 | 21SL | 43SL | 64SL | |||
| Initial weight, g | 12.22 | 12.23 | 12.26 | 12.22 | 0.08 | 0.580 |
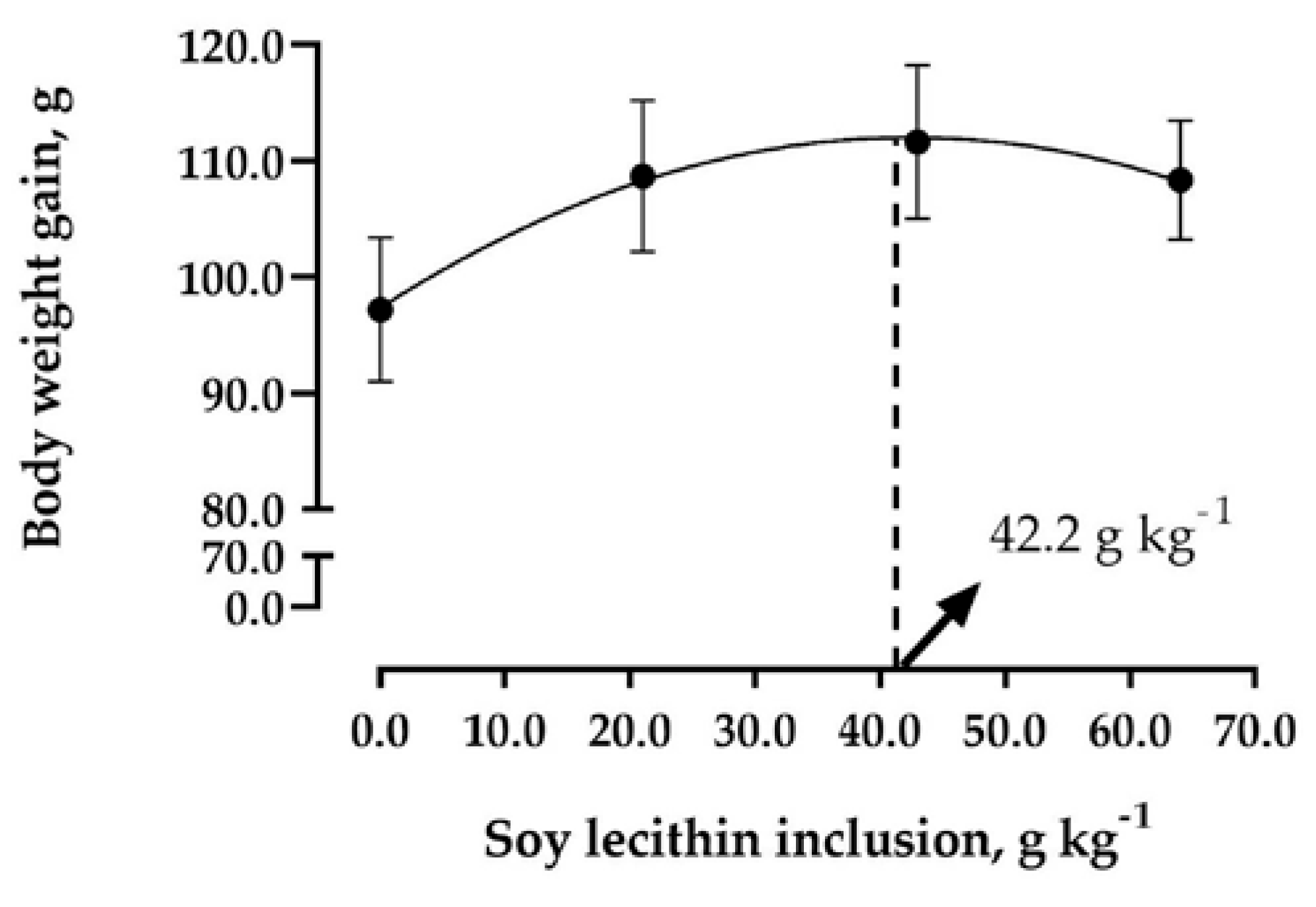
| Weight gain, g | 97.19 | 108.67 | 111.62 | 108.34 | 7.37 | 0.005 |
| Daily weight gain, g day−1 | 1.08 | 1.21 | 1.24 | 1.20 | 0.08 | 0.005 |
| Specific growth rate, % day−1 | 2.43 | 2.54 | 2.57 | 2.54 | 0.07 | 0.004 |
| Daily feed intake, % live weight day−1 | 2.91 | 2.77 | 2.73 | 2.73 | 0.06 | <0.0001 |
| Feed efficiency | 0.76 | 0.80 | 0.81 | 0.81 | 0.02 | <0.0001 |
| Apparent net protein retention, % | 36.66 | 37.63 | 38.75 | 38.12 | 2.06 | 0.133 |
| Viscerosomatic index, % | 14.90 | 13.78 | 13.66 | 13.12 | 1.32 | 0.025 |
| Hepatosomatic index, % | 3.82 | 3.84 | 3.63 | 3.25 | 0.41 | 0.029 |
| Survival, % | 99.33 | 98.00 | 98.67 | 98.67 | 2.98 | 0.632 |
| Variables | Diets | Pooled SEM 3 | p Value 4 | ||||
|---|---|---|---|---|---|---|---|
| Initial | 0SL 2 | 21SL | 43SL | 64SL | |||
| Body composition, g 100 g−1 wet weight | |||||||
| Dry matter | 26.57 | 34.42 | 34.21 | 34.23 | 34.41 | 1.13 | 0.578 |
| Protein | 14.63 | 15.58 | 15.33 | 15.73 | 15.31 | 0.82 | 0.755 |
| Lipid | 8.23 | 15.29 | 14.44 | 14.50 | 14.31 | 0.81 | 0.019 |
| Ash | 3.46 | 3.59 | 3.46 | 3.68 | 3.65 | 1.36 | 0.557 |
| Fillet composition, g 100 g−1 wet weight | |||||||
| Dry matter | 20.66 | 23.47 | 23.28 | 23.67 | 22.83 | 0.46 | 0.066 |
| Protein | 18.61 | 20.26 | 20.11 | 20.12 | 19.53 | 0.42 | 0.012 |
| Lipid | 0.99 | 2.01 | 1.95 | 1.96 | 1.83 | 0.27 | 0.325 |
| Fatty Acid g 100 g−1 Dry Weight | Diets | Pooled SEM 4 | p Value 5 | ||||
|---|---|---|---|---|---|---|---|
| Initial | 0SL 3 | 21SL | 43SL | 64SL | |||
| C16:0 | 7.53 | 7.26 | 7.13 | 7.42 | 7.27 | 0.32 | 0.71 |
| C18:1 n-9 | 9.87 | 11.67 | 10.88 | 10.51 | 10.14 | 0.34 | <0.001 |
| C18:2 n-6 | 2.99 | 3.20 | 3.22 | 3.32 | 3.58 | 0.10 | 0.030 |
| C20:4 n-6 | 0.12 | 0.25 | 0.30 | 0.29 | 0.30 | 0.02 | 0.022 |
| C18:3 n-3 | 0.04 | 0.05 | 0.04 | 0.04 | 0.04 | 0.01 | 0.29 |
| C20:5 n-3 | 0.08 | 0.02 | 0.03 | 0.03 | 0.02 | 0.02 | 0.56 |
| C22:6 n-3 | 0.03 | 0.05 | 0.07 | 0.07 | 0.08 | 0.01 | <0.001 |
| SFA 2 | 10.58 | 10.62 | 10.40 | 10.93 | 10.69 | 0.38 | 0.572 |
| MUFA | 12.74 | 14.34 | 13.55 | 13.19 | 12.82 | 0.37 | <0.001 |
| PUFA | 4.05 | 4.95 | 5.03 | 4.98 | 5.36 | 0.15 | 0.043 |
| PUFA n-6 | 3.37 | 3.99 | 4.07 | 4.10 | 4.45 | 0.09 | 0.011 |
| LC-PUFA n-6 | 0.38 | 0.76 | 0.87 | 0.84 | 0.87 | 0.06 | 0.030 |
| PUFA n-3 | 0.15 | 0.12 | 0.16 | 0.15 | 0.19 | 0.03 | 0.042 |
| LC-PUFA n-3 | 0.11 | 0.07 | 0.11 | 0.11 | 0.13 | 0.03 | 0.017 |
| Nutrient, % | Diets | p Value | |
|---|---|---|---|
| 0SLD 3 | 43SLD | ||
| Dry matter | 79.44 ± 0.30 | 80.01 ± 1.09 | 0.437 |
| Protein | 87.61 ± 0.14 | 87.99 ± 0.94 | 0.532 |
| Energy | 84.34 ± 0.39 | 85.20 ± 0.50 | 0.200 |
| Lipid | 92.84 ± 0.54 | 95.07 ± 0.74 | 0.014 |
| Polar lipid | 79.14 ± 1.42 | 89.61 ± 0.81 | <0.001 |
| C10:0 | 95.13 ± 0.77 | 98.14 ± 0.17 | 0.003 |
| C16:0 | 92.50 ± 0.58 | 96.05 ± 0.31 | 0.006 |
| C18:0 | 90.93 ± 0.57 | 94.91 ± 0.29 | <0.001 |
| C16:1 n-7 | 95.68 ± 0.15 | 96.46 ± 0.16 | 0.006 |
| C18:1 n-9 | 96.16 ± 0.6 | 97.32 ± 0.48 | 0.075 |
| C22:1 n-11 | 89.34 ± 0.93 | 92.26 ± 0.51 | 0.015 |
| C18:2 n-6 | 97.28 ± 0.20 | 98.74 ± 0.22 | <0.001 |
| C20:2 n-6 | 92.97 ± 1.00 | 95.16 ± 0.57 | 0.004 |
| C18:3 n-3 | nd 4 | 100.00 ± 0.00 | - |
| SFA 2 | 92.11 ± 0.58 | 95.79 ± 0.32 | 0.008 |
| MUFA | 96.06 ± 0.62 | 97.21 ± 0.40 | 0.061 |
| PUFA | 97.26 ± 0.21 | 98.74 ± 0.22 | <0.001 |
| PUFA n-6 | 97.26 ± 0.21 | 98.72 ± 0.22 | <0.001 |
| PUFA n-3 | nd | 100.00 ± 0.0 | - |
| Nutrient Intake, g Fish | Diets | p Value | |
|---|---|---|---|
| 0SL 3 | 43SL | ||
| Energy | 495.88 ± 28.07 | 543.33 ± 35.46 | 0.036 |
| Protein | 36.44 ± 2.08 | 40.20 ± 2.68 | 0.022 |
| Lipid | 7.73 ± 0.44 | 10.11 ± 0.65 | <0.001 |
| Polar lipid | 2.58 ± 0.14 | 5.93 ± 0.39 | <0.001 |
| C16:0 | 1.04 ± 0.05 | 1.38 ± 0.09 | <0.001 |
| C18:1 n-9 | 1.89 ± 0.10 | 1.55 ± 0.10 | <0.001 |
| C18:2 n-6 | 2.40 ± 0.13 | 2.99 ± 0.20 | <0.001 |
| C18:3 n-3 | nd 4 | 0.04 ± 0.00 | - |
| SFA 2 | 1.30 ± 0.07 | 1.76 ± 0.11 | <0.001 |
| MUFA | 2.10 ± 0.12 | 2.05 ± 0.13 | <0.001 |
| PUFA | 2.40 ± 0.13 | 3.03 ± 0.21 | <0.001 |
| PUFA n-6 | 2.40 ± 0.13 | 2.99 ± 0.08 | <0.001 |
| PUFA n-3 | nd | 0.04 ± 0.00 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batista, R.O.; Richter, B.L.; Banze, J.F.; Schleder, D.D.; Salhi, M.; Nobrega, R.O.; Silva, M.F.O.d.; Mattioni, B.; Pettigrew, J.E.; Fracalossi, D.M. Soy Lecithin Supplementation Promotes Growth and Increases Lipid Digestibility in GIFT Nile Tilapia Raised at Suboptimal Temperature. Fishes 2023, 8, 404. https://doi.org/10.3390/fishes8080404
Batista RO, Richter BL, Banze JF, Schleder DD, Salhi M, Nobrega RO, Silva MFOd, Mattioni B, Pettigrew JE, Fracalossi DM. Soy Lecithin Supplementation Promotes Growth and Increases Lipid Digestibility in GIFT Nile Tilapia Raised at Suboptimal Temperature. Fishes. 2023; 8(8):404. https://doi.org/10.3390/fishes8080404
Chicago/Turabian StyleBatista, Rosana Oliveira, Bianca Leticia Richter, Jorge Filipe Banze, Delano Dias Schleder, Maria Salhi, Renata Oselame Nobrega, Maria Fernanda Oliveira da Silva, Bruna Mattioni, James Eugene Pettigrew, and Débora Machado Fracalossi. 2023. "Soy Lecithin Supplementation Promotes Growth and Increases Lipid Digestibility in GIFT Nile Tilapia Raised at Suboptimal Temperature" Fishes 8, no. 8: 404. https://doi.org/10.3390/fishes8080404
APA StyleBatista, R. O., Richter, B. L., Banze, J. F., Schleder, D. D., Salhi, M., Nobrega, R. O., Silva, M. F. O. d., Mattioni, B., Pettigrew, J. E., & Fracalossi, D. M. (2023). Soy Lecithin Supplementation Promotes Growth and Increases Lipid Digestibility in GIFT Nile Tilapia Raised at Suboptimal Temperature. Fishes, 8(8), 404. https://doi.org/10.3390/fishes8080404