Evaluation of a Prefabricated Fish Passage Design for Great Plains Fishes
Abstract
:1. Introduction
2. Materials and Methods
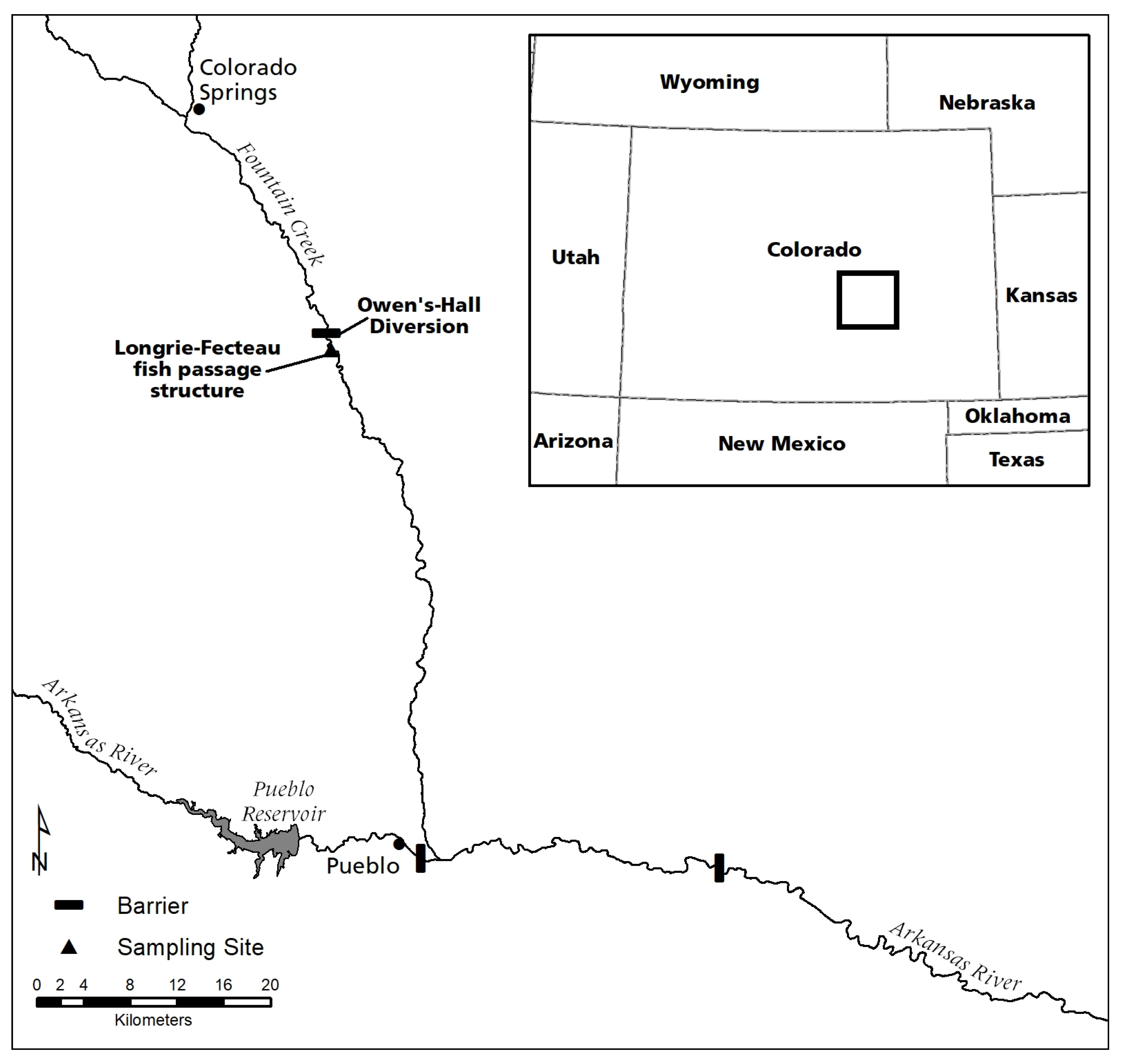
2.1. Study Area
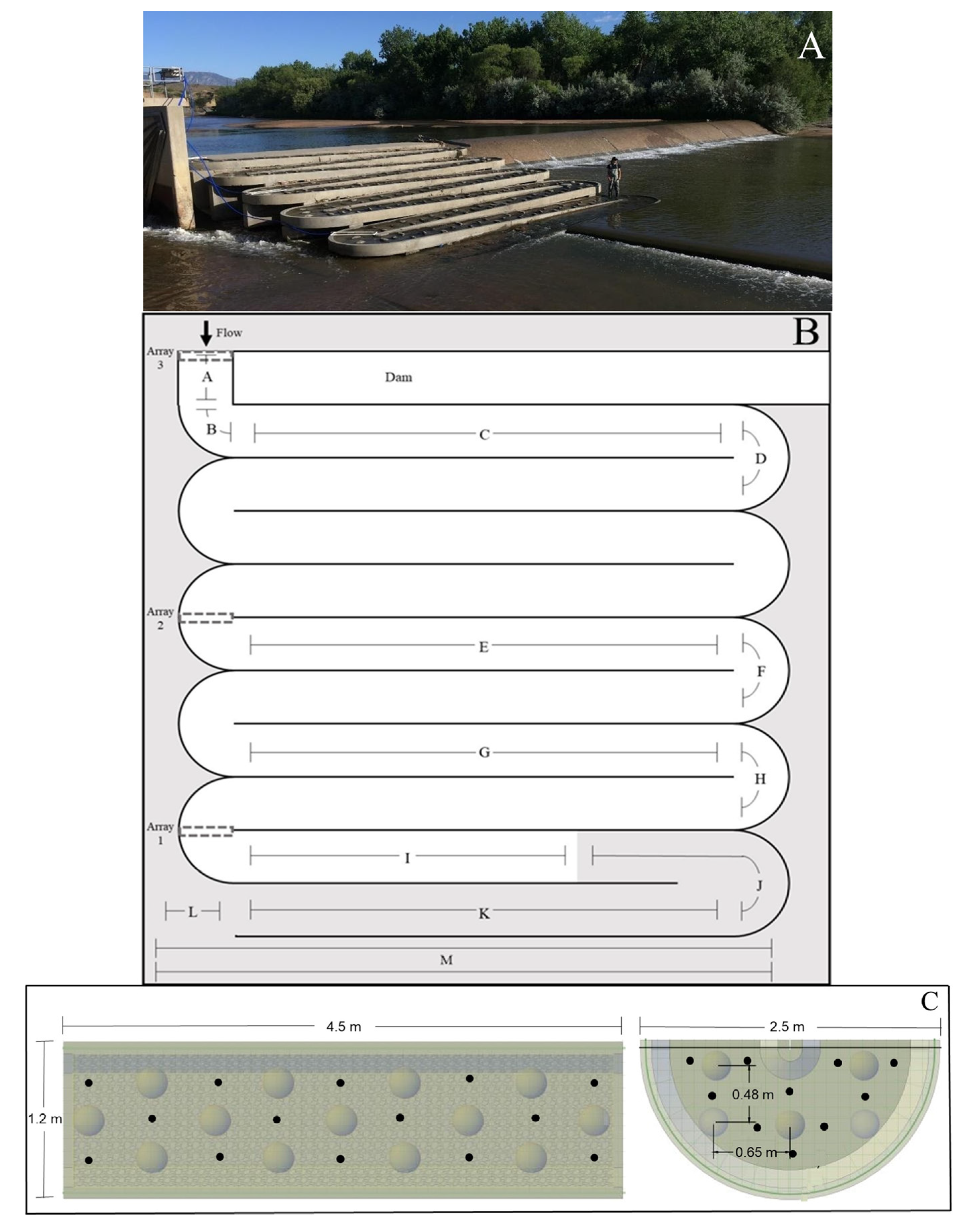
2.2. Fishway Design and Construction
2.3. Fish Sampling Methods
2.4. Antenna Construction and Operation
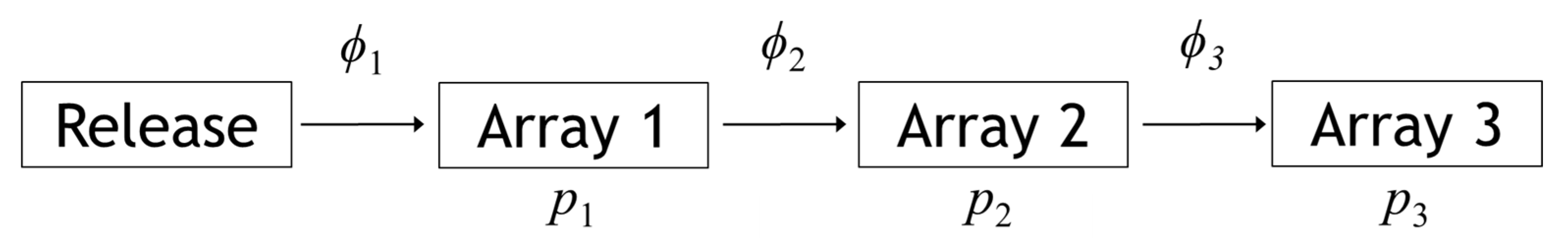
2.5. Cormack–Jolly–Seber Model
2.6. Timing of Movements
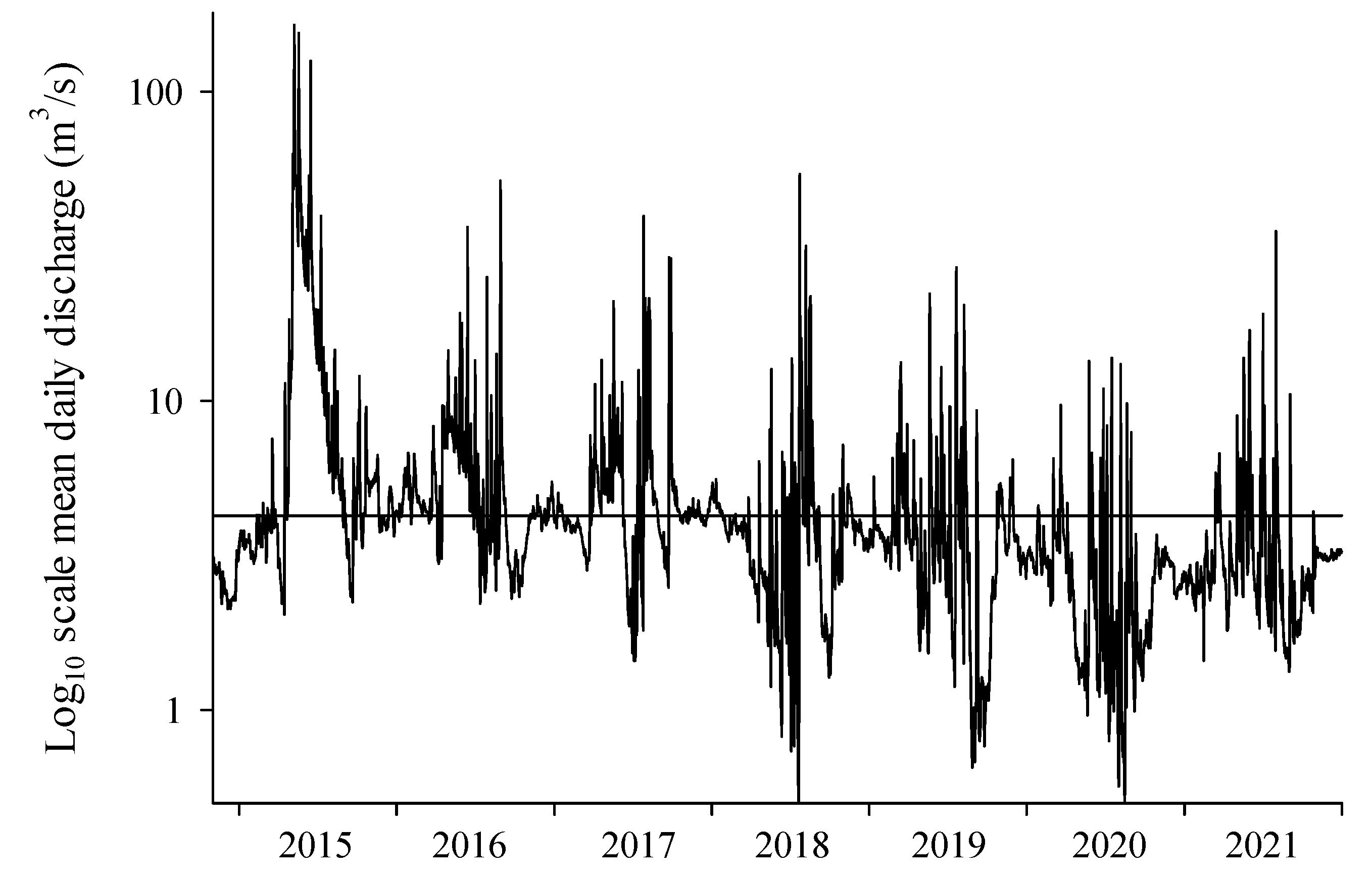
2.7. Hydraulic Measurements
3. Results
3.1. Tagging Summary
3.2. Cormack–Jolly–Seber Results
3.3. Timing of Movements
3.4. Hydraulic Measurements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schlosser, I.J.; Angermeier, P.L. Spatial variation in demographic processes of lotic fishes: Conceptual models, empirical evidence, and implications for conservation. Am. Fish. Soc. Symp. 1995, 17, 392–401. [Google Scholar]
- Gido, K.B.; Whitney, J.E.; Perkin, J.S.; Turner, T.F. Fragmentation, Connectivity and Fish Species Persistence in Freshwater Ecosystems. In Conservation of Freshwater Fishes; Chapter 10; Cambridge University Press: Cambridge, UK, 2016. [Google Scholar]
- Bowler, D.E.; Benton, T.G. Causes and consequences of animal dispersal strategies: Relating individual to spatial dynamics. Biol. Rev. 2005, 80, 205–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falke, J.A.; Fausch, K.D. From metapopulations to metacommunities: Linking theory with empirical observations of the spatial population dynamics of stream fishes. Am. Fish. Soc. Symp. 2010, 73, 207–233. [Google Scholar]
- Van Leeuwen, C.H.; Dalen, K.; Museth, J.; Junge, C.; Vøllestad, L.A. Habitat fragmentation has interactive effects on the population genetic diversity and individual behaviour of a freshwater salmonid fish. River Res. Appl. 2018, 34, 60–68. [Google Scholar] [CrossRef]
- Nilsson, C.; Reidy, C.A.; Dynesius, M.; Revenga, C. Fragmentation and flow regulation of the world’s large river systems. Science 2005, 308, 405–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perkin, J.S.; Gido, K.B. Fragmentation alters stream fish community structure in dendritic ecological networks. Ecol. Appl. 2012, 22, 2176–2187. Available online: https://www.jstor.org/stable/41723010 (accessed on 29 November 2011). [CrossRef]
- Perkin, J.S.; Gido, K.B.; Cooper, A.R.; Turner, T.F.; Osborne, M.J.; Johnson, E.R.; Mayes, K.B. Fragmentation and dewatering transform Great Plains stream fish communities. Ecol. Monogr. 2015, 85, 73–92. [Google Scholar] [CrossRef] [Green Version]
- Perkin, J.S.; Gido, K.B.; Costigan, K.H.; Daniels, M.D.; Johnson, E.R. Fragmentation and drying ratchet down Great Plains stream fish diversity. Aquat. Conserv. 2015, 25, 639–655. [Google Scholar] [CrossRef]
- Wang, H.; Chanson, H. Modelling upstream fish passage in standard box culverts: Interplay between turbulence, fish kinematics, and energetics. River Res. Appl. 2018, 34, 244–252. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, P.A.; Leng, X.; Von Brandis-Marini, J.; Chanson, H. Hybrid modelling of low velocity zones in an asymmetrical channel with sidewall longitudinal rib to assist fish passage. River Res. Appl. 2020, 36, 807–818. [Google Scholar] [CrossRef]
- Leng, X.; Chanson, J. Hybrid modelling of low velocity zones in box culverts to assist fish passage: Why simple is better! River Res. Appl. 2020, 36, 1765–1777. [Google Scholar] [CrossRef]
- Perkin, J.S.; Gido, K.B. Stream fragmentation thresholds for a reproductive guild of Great Plains fishes. Fisheries 2011, 36, 371–383. [Google Scholar] [CrossRef]
- Perkin, J.S.; Starks, T.A.; Pennock, C.A.; Gido, K.B.; Hopper, G.W.; Hedden, S.C. Extreme drought causes fish recruitment failure in a fragmented Great Plains riverscape. Ecohydrology 2019, 12, e2120. [Google Scholar] [CrossRef]
- Dudley, R.K.; Platania, S.P. Flow regulation and fragmentation imperil pelagic-spawning riverine fishes. Ecol. Appl. 2007, 17, 2074–2086. [Google Scholar] [CrossRef] [PubMed]
- Worthington, T.A.; Echelle, A.A.; Perkin, J.S.; Mollenhauer, R.; Farless, N.; Dyer, J.J.; Logue, D.; Brewer, S.K. The emblematic minnows of the North American Great Plains: A synthesis of threats and conservation opportunities. Fish Fish. 2018, 19, 271–307. [Google Scholar] [CrossRef]
- Prenosil, E.; Koupal, K.; Grauf, J.; Schoenebeck, C.; Hoback, W.W. Swimming and jumping ability of 10 Great Plains fish species. J. Freshw. Ecol. 2016, 31, 123–130. [Google Scholar] [CrossRef]
- Ficke, A.D.; Myrick, C.A.; Jud, N. The swimming and jumping ability of three small Great Plains fishes: Implications for fishway design. Trans. Am. Fish. Soc. 2011, 140, 1521–1531. [Google Scholar] [CrossRef]
- Fausch, K.D.; Bestgen, K.R. Ecology of fishes indigenous to the central and southwestern Great Plains. In Ecology and Conservation of Great Plains Vertebrates; Knopf, F.L., Samson, F.B., Eds.; Springer: New York, NY, USA, 1997; pp. 131–166. [Google Scholar]
- Katopodis, C.; Williams, J.G. The development of fish passage research in a historical context. Ecol. Eng. 2012, 48, 8–18. [Google Scholar] [CrossRef]
- Knapp, M.; Montgomery, J.; Whittaker, C.; Franklin, P.; Baker, C.; Friedrich, H. Fish passage hydrodynamics: Insights into overcoming migration challenges for small-bodied fish. J. Ecohydraul. 2019, 4, 43–55. [Google Scholar] [CrossRef]
- Nikora, V.I.; Aberle, J.; Biggs, B.J.F.; Jowett, I.G.; Sykes, J.R.E. Effects of fish size, time-to-fatigue and turbulence on swimming performance: A case study of Galaxis maculatus. J. Fish Biol. 2003, 63, 1365–1382. [Google Scholar] [CrossRef]
- Nichols, S.; Berghuis, A.; Lay, C.; Mallen-Cooper, M. Fishway options for weirs of the Northern Murray-Darling basin. NSW Dep. Prim. Ind. 2012, 11687, 1–22. Available online: https://www.dpi.nsw.gov.au/fishing/habitat/publications/pubs/fishway-options-for-weirs-in-the-northern-murray-darling-basin (accessed on 10 February 2022).
- Huhta, S. Watson Lake Fish Passage and Screening: 100% Design Report; OneFish Engineering, LLC: Lafayette, CO, USA, 2018. [Google Scholar]
- Richer, E.E.; Fetherman, E.R.; Krone, E.A.; Wright, F.B., III; Kondratieff, M.C. Multispecies fish passage evaluation at a rock-ramp fishway in a Colorado transition zone stream. N. Am. J. Fish. Manag. 2020, 40, 1510–1522. [Google Scholar] [CrossRef]
- Venus, T.E.; Smialek, N.; Pander, J.; Harby, A.; Geist, J. Evaluating cost trade-offs between hydropower and fish passage mitigation. Sustainability 2020, 12, 8520. [Google Scholar] [CrossRef]
- Katopodis, C. Developing a toolkit for fish passage, ecological flow management and fish habitat works. J. Hydrol. Res. 2005, 43, 451–467. [Google Scholar] [CrossRef]
- Silva, A.T.; Lucas, M.C.; Castro-Santos, T.; Katopodis, C.; Baumgartner, L.J.; Thiem, J.D.; Aarestrup, K.; Pompeu, P.S.; O’Brien, G.C.; Braun, D.C.; et al. The future of fish passage science, engineering, and practice. Fish Fish. 2017, 19, 340–362. [Google Scholar] [CrossRef] [Green Version]
- Griffith, M.B.; McManus, M.G. Consideration of spatial and temporal scales in stream restorations and biotic monitoring to assess restoration outcomes: A literature review part 1. River Res. Appl. 2020, 36, 1385–1397. [Google Scholar] [CrossRef]
- Griffith, M.B.; McManus, M.G. Consideration of spatial and temporal scales in stream restorations and biotic monitoring to assess restoration outcomes: A literature review part 2. River Res. Appl. 2020, 36, 1398–1415. [Google Scholar] [CrossRef] [PubMed]
- Noonen, M.J.; Grant, W.A.; Jackson, C.D. A quantitative assessment of fish passage efficiency. Fish Fish. 2012, 13, 450–464. [Google Scholar] [CrossRef]
- Bunt, C.M.; Katopodis, C.; McKinley, R.S. Attraction and passage efficiency of white suckers and smallmouth bass by two denil fishways. N. Am. J. Fish. Manag. 2011, 19, 793–803. [Google Scholar] [CrossRef]
- Mulligan, K.B.; Towler, H.B.; Sojkowski, B.; Noreika, J. Fishway entrance gate experiments with adult American Shad. Water Resour. Res. 2019, 55, 10839–10855. [Google Scholar] [CrossRef]
- Cooke, S.J.; Hinch, S.G. Improving the reliability of fishway attraction and passage efficiency estimates to inform fishway engineering, science, and practice. Ecol. Eng. 2013, 58, 123–132. [Google Scholar] [CrossRef]
- Bunt, C.M. Fishway entrance modifications enhance fish attraction. Fish. Manag. Ecol. 2001, 8, 95–105. [Google Scholar] [CrossRef]
- Li, W.; Bao, J.; Zhang, C.; Mi, X.; Zhang, D.; Jiang, H.; Twardek, W.M.; Cooke, S.J.; Duan, M. Group size influences light-emitting diode light colour and substrate preference of David’s Schizothoracin (Schizothorax davidi): Relevance for design of fish passage facilities. River Res. Appl. 2022, 38, 280–292. [Google Scholar] [CrossRef]
- Agostinho, A.A.; Agostinho, C.S.; Pelicice, F.M.; Marques, E.E. Fish ladders: Safe fish passage or hotspot for predation? Neotrop. Ichthyol. 2012, 10, 687–696. [Google Scholar] [CrossRef] [Green Version]
- Edelmann, P.; Serguson, S.A.; Stogner Sr, R.W.; August, M.; Payne, W.F.; Bruce, J.F. Evaluation of Water Quality, Suspended Sediment, and Stream Morhpohology with an Emphasis on Stormwater on Fountain and Monument Creek Basin, Colorado Springs and Vicinity, Colorado, 1981–2001. U.S. Geological Survey Water Resource Investigation Report 0204. 2002. Available online: http://pubs.usgs.gov/wri/wri024104/pdf/wrir02-4104.pdf (accessed on 23 August 2021).
- Mau, D.P.; Stogner Sr, R.W.; Edelmann, P. Characterization of Stormflows and Wastewater Treatment-Plant Effluent Discharges on Water Quality, Suspended Sediment, and Stream Morphology for Fountain and Monument Creek Watersheds, Colorado, 1981–2006. U.S. Geological Survey Scientific Investigations Report 2007–5104, 1–76. 2007. Available online: https://pubs.usgs.gov/sir/2007/5104/ (accessed on 23 August 2021).
- Colorado Parks and Wildlife. Aquatics Database. Available online: https://cpw.state.co.us (accessed on 5 January 2022).
- Colorado Parks and Wildlife. State Wildlife Action Plan: A Strategy for Conserving Wildlife in Colorado. Colorado Parks and Wildlife, Denver. 2015. Available online: https://cpw.state.co.us/aboutus/Pages/StateWildlifeActionPlan.aspx (accessed on 8 November 2021).
- Walters, D.M.; Zuellig, R.E.; Crockett, H.J.; Bruce, J.F.; Lukacs, P.M.; Fitzpatrick, R.M. Barriers impede upstream spawning migration of flathead chub. Trans. Am. Fish. Soc. 2014, 143, 17–25. [Google Scholar] [CrossRef]
- Bestgen, K.R.; Crockett, H.J.; Haworth, M.R.; Fitzpatrick, R.M. Production of nonadhesive eggs by flathead chub and implications for downstream transport and conservation. J. Fish. Wildl. Manag. 2016, 7, 434–443. [Google Scholar] [CrossRef]
- Haworth, M.R.; Bestgen, K.R. Daily increment validation and effects of streamflow variability and water temperature on growth of age-0 Flathead Chub. N. Am. J. Fish. Manag. 2016, 36, 744–753. [Google Scholar] [CrossRef]
- Haworth, M.R.; Bestgen, K.R. Flow and water temperature affect reproduction and recruitment of a Great Plains cyprinid. Can. J. Fish. Aquat. Sci. 2017, 74, 853–863. [Google Scholar] [CrossRef]
- Swarr, T.R. Improving Rock Ramp Fishways for Small-Bodied Great Plains Fishes. Master’s Thesis, Colorado State University, Fort Collins, CO, USA, 2018. Available online: https://www.proquest.com/pagepdf/2118684443?accountid=10223 (accessed on 5 February 2019).
- Ficke, A.D.; Myrick, C.A.; Kondratieff, M.C. The effects of PIT tagging on the swimming performance and survival of three nonsalmonid freshwater fishes. Ecol. Eng. 2012, 48, 86–91. [Google Scholar] [CrossRef]
- Aarestrup, K.; Lucas, M.C.; Hansen, J.A. Efficiency of a nature-like bypass channel for sea trout (Salmo trutta) ascending a small Danish stream studied by PIT telemetry. Ecol. Freshw. Fish 2003, 12, 160–168. [Google Scholar] [CrossRef]
- White, G.C.; Burnham, K.P. Program MARK: Survival estimation from populations of marked animals. Bird Study 1999, 46, S120–S138. [Google Scholar] [CrossRef]
- Anderson, D.R. Model Based Inference for the Life Sciences: A Primer on Evidence; Springer LLC: New York, NY, USA, 2008. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002. [Google Scholar]
- Chow, V.T. Open-Channel Hydraulics; The Blackburn Press: Caldwell, NJ, USA, 1959. [Google Scholar]
- Yu, S.L.; Peters, E. Use of Froude number to determine habitat selection by fish. Rivers 1997, 6, 10–18. [Google Scholar]
- Huhta, S.; Engen, C. Whitney and Bh Eaton Fish Passage Improvement Feasibility Study: Final Report; OneFish Engineering, LLC: Lafayette, CO, USA; Flywater Inc.: Fort Collins, CO, USA, 2016. [Google Scholar]
- Bunt, C.M.; Castro-Santos, T.; Haro, A. Performance of fish passage structures at upstream barriers to migration. River Res. Appl. 2012, 28, 457–478. [Google Scholar] [CrossRef]
- Kolden, E.; Fox, B.D.; Beldsoe, B.P.; Kondratieff, M.C. Modelling whitewater park hydraulics and fish habitat in Colorado. River Res. Appl. 2016, 32, 1116–1127. [Google Scholar] [CrossRef]
- Stephens, T.A.; Bledsoe, B.P.; Fox, B.D.; Kolden, E.; Kondratieff, M.C. Effects of whitewater parks on fish passage: A spatially explicit hydraulic analysis. Ecol. Eng. 2015, 83, 305–318. [Google Scholar] [CrossRef] [Green Version]
- Clay, C.H. Design of Fishways and Other Fish Facilities; Lewis Publishers: Ann Arbor, MI, USA, 1995. [Google Scholar]
- Schütz, C.; Henning, M.; Czerny, R.; Herbst, M.; Pitsch, M. Addition of auxiliary discharge into a fishway—A contribution to fishway design at barrages of large rivers. Ecol. Eng. 2021, 167, 106287. [Google Scholar] [CrossRef]
- Katopodis, C.; Kells, J.A.; Acharya, M. Nature-like and conventional fishways: Alternative concepts? Can. Water Resour. J. 2001, 26, 211–232. [Google Scholar] [CrossRef] [Green Version]
- Nau, G.S.; Spares, A.D.; Andrews, S.N.; Mallory, M.L.; McLellan, N.R.; Stokesbury, M.J.W. Body size, experiences, and sex do matter: Multiyear study shows improved passage rates for alewife (Alosa pseudoharengus) through a small-scale Denil and pool-and-weir fishways. River Res. Appl. 2017, 33, 1472–1483. [Google Scholar] [CrossRef]
- Fraser, D.F.; Gilliam, J.F.; Daley, M.J.; Le, A.N.; Skalski, G.T. Explaining leptokurtic movement distributions: Intrapopulation variation in boldness and exploration. Am. Nat. 2001, 158, 124–135. [Google Scholar] [CrossRef]
- Skalski, G.T.; Gilliam, J.F. Modeling diffusive spread in a heterogeneous population: A movement study with stream fish. Ecology 2000, 81, 1685–1700. [Google Scholar] [CrossRef]
- Radinger, J.; Wolter, C. Patterns and predictors of fish dispersal in rivers. Fish Fish. 2014, 15, 456–473. [Google Scholar] [CrossRef]
- Booth, M.T.; Hairston, N.G., Jr.; Flecker, A.S. Consumer movement dynamics as hidden drivers of stream habitat structure: Suckers as ecosystem engineers on the night shift. Oikos 2020, 129, 194–208. [Google Scholar] [CrossRef]
- Brittain, C. How Does Rock-Ramp Fishway Surface Texture Affect The Passage Success of Small-Bodied Great Plains Fishes? Master’s Thesis, Colorado State University, Fort Collins, CO, USA, 2022. Available online: https://hdl.handle.net/10217/235555 (accessed on 3 July 2022).
- Vowles, A.S.; Anderson, J.J.; Gessel, M.H.; Williams, J.G.; Kemp, P.S. Effects of avoidance behaviour on downstream fish passage through areas of accelerating flow when light and dark. Anim. Behav. 2014, 92, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Pennock, C.A.; Cathcart, C.N.; Hedden, S.C.; Weber, R.E.; Gido, K.B. Fine-scale movement and habitat use of a prairie stream fish assemblage. Oecologia 2018, 186, 831–842. [Google Scholar] [CrossRef]
- Richer, E.; Krone, E.; Wright, F.; Kondratieff, M.; Treble, A. Evaluation of a Rock Ramp Fishway at the Fossil Creek Reservoir Inlet Diversion (FCRID) on the Cache la Poudre River; Colorado Parks and Wildlife: Fort Collins, CO, USA, 2018. [Google Scholar]
- Scott, M.L.; Magoulick, D.D. Swimming performance of five warmwater stream fish species. Trans. Am. Fish. Soc. 2011, 137, 209–215. [Google Scholar] [CrossRef]
- Underwood, Z.E.; Myrick, C.A.; Compton, R.I. Comparative swimming performance of five Catostomus species and Roundtail Chub. N. Am. J. Fish. Manag. 2014, 34, 753–763. [Google Scholar] [CrossRef]
- Billman, E.J.; Pyron, M. Evolution of form and function: Morphology and swimming performance in North American minnows. J. Freshw. Ecol. 2005, 20, 221–232. [Google Scholar] [CrossRef]
- Zhang, G.; Chanson, H. Numerical Investigations of Box Culvert Hydrodynamics with Smooth, Unequally Roughened and Baffled Barrels to Enhance Upstream Fish Passage; The University of Queensland, School of Engineering: Brisbane, Australia, 2018. [Google Scholar]
- Salatas, J.H.; Gard, N.H.; Wickwire, T.; Menzie, C.A. Stressor analysis approaches for endangered species assessments. Nat. Sci. 2013, 5, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Inter-Fleuve. Fishway Assessment and Cost Analysis Report: Royal River; Yarmouth, M.E., Ed.; University of Southern Maine: Portland, ME, USA, 2018. [Google Scholar]
- Freestone Aquatics, Inc. Fish Passage and Ditch Diversion Improvement Fraser River; Freestone Aquatics, Inc.: Denver, CO, USA, 2019. [Google Scholar]


| Species | # PIT Tagged | # Detected | Passage Efficiency | % Nighttime Movement | Total Length (Mean (Range); mm) |
|---|---|---|---|---|---|
| Flathead chub (Platygobio gracilis) | 816 | 24 | 2.9% | 85% | 100 (63–177) |
| White sucker (Catostomus comersonii) | 367 | 10 | 2.7% | 80% | 117 (70–297) |
| Central stoneroller (Campostoma anomalum) | 65 | 1 | 1.5% | 100% | 84 (67–144) |
| Longnose dace (Rhinichthys cataractae) | 32 | 1 | 3.1% | 100% | 73 (61–99) |
| Creek chub (Semotilus atromaculatus) | 1 | 1 | 100% | 0% | 109 |
| Longnose sucker (Catostomus catostomus) | 41 | 0 | 0% | - | 113 (83–187) |
| Fathead minnow (Pimephales promelas) | 4 | 0 | 0% | - | 62 (59–66) |
| Sand shiner (Notropis stramineus) | 1 | 0 | 0% | - | 63 |
| Total | 1327 | 37 | - | 104 (59–297) |
| Model | AICc | ΔAICc | wi | Likelihood | K | −2Log(L) |
|---|---|---|---|---|---|---|
| ϕ(array) p(TL) | 389.1491 | 0.0000 | 0.36302 | 1.0000 | 5 | 379.1058 |
| ϕ(array) p(.) | 390.5102 | 1.3611 | 0.18381 | 0.5063 | 4 | 382.4815 |
| ϕ(array + TL) p(TL) | 391.1536 | 2.0045 | 0.13325 | 0.3671 | 6 | 379.0930 |
| ϕ(array) p(array) | 391.6376 | 2.4885 | 0.10461 | 0.2882 | 6 | 379.5770 |
| ϕ(array + TL) p(.) | 392.5174 | 3.3683 | 0.06738 | 0.1856 | 5 | 382.4742 |
| ϕ(array + TL) p(array + TL) | 392.5990 | 3.4499 | 0.06468 | 0.1782 | 8 | 376.4949 |
| ϕ(array) p(array + TL) | 393.3235 | 4.1744 | 0.04503 | 0.1240 | 7 | 379.2427 |
| ϕ(array + TL) p(array) | 393.6507 | 4.5016 | 0.03823 | 0.1053 | 7 | 379.5699 |
| Label | Description | # of Point Measurements | Depth (m) | Bottom Velocity (m/s) | 60% Velocity (m/s) | Froude # |
|---|---|---|---|---|---|---|
| A | Apron | 42 | 0.28 | 0.27 | 0.33 | 0.20 |
| B | Immediately downstream | 12 | 0.37 | −0.04 | −0.04 | −0.02 |
| C | Submerged straight | 28 | 0.14 | 0.08 | 0.06 | 0.05 |
| D | Submerged corner | 10 | 0.22 | 0.07 | 0.13 | 0.09 |
| E | Entrance | 28 | 0.12 | 0.13 | 0.14 | 0.13 |
| F | Bottom corner | 10 | 0.12 | 0.20 | 0.22 | 0.21 |
| G | Bottom straight | 28 | 0.12 | 0.22 | 0.23 | 0.22 |
| H | Middle corner | 10 | 0.12 | 0.22 | 0.26 | 0.24 |
| I | Middle straight | 28 | 0.12 | 0.20 | 0.21 | 0.19 |
| J | Top corner | 10 | 0.12 | 0.16 | 0.17 | 0.16 |
| K | Top straight | 28 | 0.12 | 0.20 | 0.22 | 0.21 |
| L | 90° corner | 8 | 0.12 | 0.24 | 0.25 | 0.23 |
| M | Exit | 6 | 0.12 | 0.17 | 0.18 | 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fitzpatrick, R.M.; Longrie, D.W.; Friebertshauser, R.J.; Foutz, H.P. Evaluation of a Prefabricated Fish Passage Design for Great Plains Fishes. Fishes 2023, 8, 403. https://doi.org/10.3390/fishes8080403
Fitzpatrick RM, Longrie DW, Friebertshauser RJ, Foutz HP. Evaluation of a Prefabricated Fish Passage Design for Great Plains Fishes. Fishes. 2023; 8(8):403. https://doi.org/10.3390/fishes8080403
Chicago/Turabian StyleFitzpatrick, Ryan M., David W. Longrie, Ryan J. Friebertshauser, and H. Paul Foutz. 2023. "Evaluation of a Prefabricated Fish Passage Design for Great Plains Fishes" Fishes 8, no. 8: 403. https://doi.org/10.3390/fishes8080403






