Correlations between Environmental Factors and the Distribution of Juvenile Hucho bleekeri in the Taibai River, Shaanxi, China
Abstract
1. Introduction
2. Materials and Methods
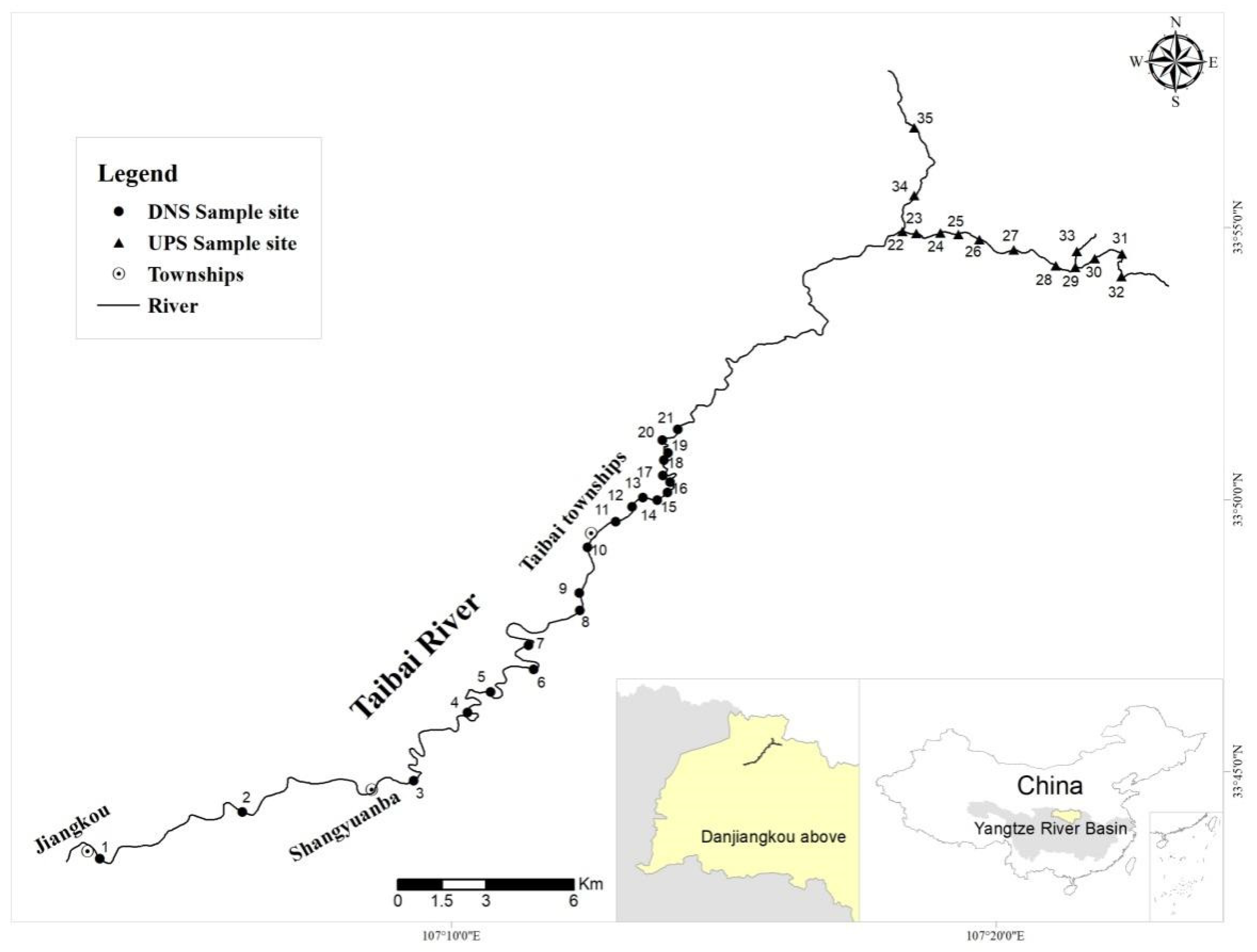
2.1. Watershed Environmental Survey
2.2. Reach Characteristics and Juvenile Fish Distribution
2.2.1. Juvenile Fish Collection
2.2.2. Measurement of Habitat Variables
2.3. Microhabitat Characteristics of Juvenile Fish
2.3.1. Underwater Shot
2.3.2. Measurement of Microhabitat Variables
3. Results
3.1. Environmental Characteristics of the Taibai River
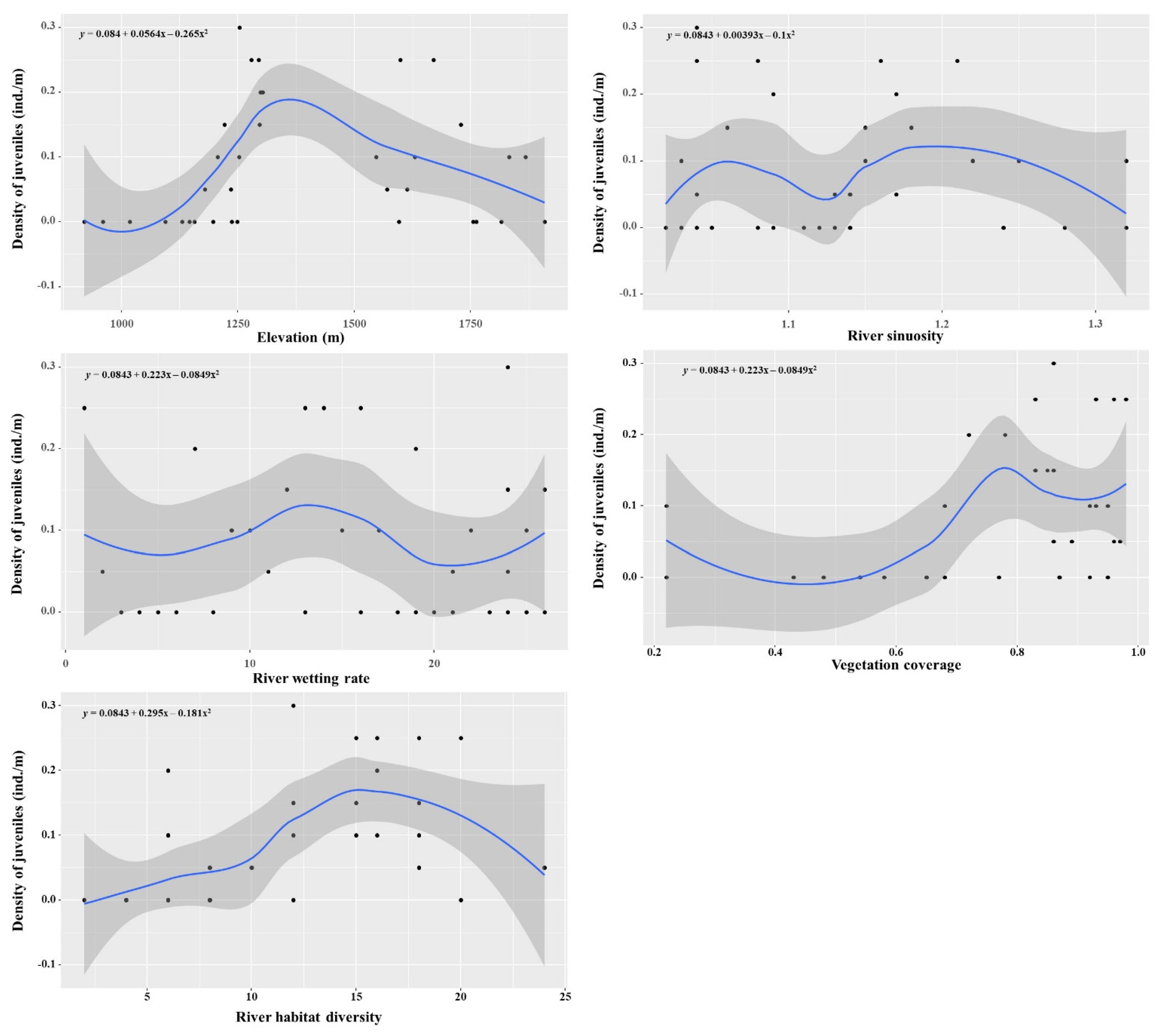
3.2. Correlation between the Juvenile H. bleekeri Distribution and Environmental Characteristics in the Reach
3.3. Microhabitat Selection Preference of Juvenile H. bleekeri
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Ma, K.P. Hot topics for biodiversity science. Biodivers. Sci. 2016, 24, 1. (In Chinese) [Google Scholar] [CrossRef]
- Ye, S.W.; Li, Z.J.; Zhang, T.L.; Liu, J.S.; Xie, S.G. Assessing fish distribution and threats to fish biodiversity in the Yangtze River Basin, China. Ichthyol. Res. 2014, 61, 183–188. [Google Scholar] [CrossRef]
- Liu, J.K.; Cao, W.X. Fish resources of the Yangtze River basin and the tactics for their conservation. Resour. Env. Yangtze Basin 1992, 1, 17–23. (In Chinese) [Google Scholar]
- Brown, J.H. On the relationship between abundance and distribution of species. Am. Nat. 1984, 124, 255–279. [Google Scholar] [CrossRef]
- Taylor, C.M.; Winston, M.R.; Matthews, W.J. Fish species-environment and abundance relationships in a great plains river system. Ecography 1993, 16, 16–23. [Google Scholar] [CrossRef]
- Lopes, L.F.G.; Catmo, J.S.A.D.; Cortes, R.M.V.; Oliveira, D. Hydrodynamics and water quality modelling in a regulated river segment: Application on the instream flow definition. Ecol. Model. 2004, 173, 194–218. [Google Scholar] [CrossRef]
- Yi, Y.J.; Wang, Z.Y.; Lu, Y.J. Habitat suitability index model for Chinese Sturgeon in the Yangtze River. Adv. Water Sci. 2007, 18, 538–543. (In Chinese) [Google Scholar]
- Monaaghan, K.A.; Soares, A.M. The bioassessment of fish and macro invertebrates in a Mediterranean-Atlantic climate: Habitat assessment and concordane between contrasting ecological samples. Ecol. Indic. 2010, 10, 184–191. [Google Scholar] [CrossRef]
- Hu, M.H.; Wang, Y.J.; Cao, L.; Xiong, B.X. Threatened fishes of the world: Hucho bleekeri Kimura, 1934 (Salmonidae). Environ. Biol. Fish. 2008, 82, 385–386. [Google Scholar] [CrossRef]
- Ding, R.H. The Fishes of Sichuan, China; Sichuan Publishing House of Science and Technology: Chengdu, China, 1994; p. 641. [Google Scholar]
- Yue, P.Q.; Chen, Y.Y. China Red Data Book of Endangered Animals (Pisces); Science Press: Beijing, China, 1998. [Google Scholar]
- Shen, Z.X.; Tang, W.J.; Li, K.M. The analysis of population dynamics of Hucho bleekeri in Markehe River, Qinghai Province. Reserv. Fish. 2006, 26, 71–73. (In Chinese) [Google Scholar]
- Yang, D.G.; Wei, Q.W.; Li, X.X.; Zhang, X.Q.; Cheng, B.L. The distributing actuality and protecting countermeasure of rare aquatic animals in Xushui River of Qinling Mountains. J. Fish. Sci. China 1999, 6, 123–125. (In Chinese) [Google Scholar]
- Song, Z. Hucho bleekeri. The IUCN Red List of Threatened Species. 2012. Version 2014. 3. Available online: https://www.iucnredlist.org/species/13151680/174797529 (accessed on 19 June 2012).
- Du, H.; Li, L.X.; Wei, Q.W.; Zhang, S.H.; Wang, C.Y.; Sun, Q.L.; Yang, X.G.; Li, L. The rediscovery of Hucho bleekeri in the Taibai River, the upper tributary of the Han River, China. Chin. J. Zool. 2014, 3, 414. (In Chinese) [Google Scholar]
- Wu, W.R. Ecological niche of Hucho bleekeri Kimura and aquatic lifes in Dachuan River. Chin. J. Fish. 1989, 1, 23–33. (In Chinese) [Google Scholar]
- Zhou, Y.J.; Wu, W.R. A preliminary research for the conditions of spawning ground and the spawning habit of Hucho bleekeri kimura in the Da Chuan River. Chin. J. Fish. 1988, 1, 67–73. (In Chinese) [Google Scholar]
- Ding, R.H. Protection biology of Hucho bleekeri V. The ecology habitat characteristics and the protective measures. Sichuan J. Zool. 1995, 14, 144–146. [Google Scholar]
- Burnett, K.M.; Reeves, G.H.; Miller, D.J.; Clarke, S.; Vance-Borland, K.; Christiansen, K. Distribution of salmon-habitat potential relative to landscape characteristics and implications for conservation. Ecol. Appl. 2007, 17, 66–80. [Google Scholar] [CrossRef] [PubMed]
- Kondolf, G.M.; Vick, J.C.; Ramirez, T.M. Salmon spawning habitat rehabilitation on the Merced River, California: An evaluation of project planning and performance. Trans. Am. Fish. Soc. 1996, 125, 899–912. [Google Scholar] [CrossRef]
- Guay, J.C.; Boisclair, D.; Rioux, D.; Leclerc, M.; Lapointe, M.; Legendre, P. Development and validation of numerical habitat models for juveniles of Atlantic salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 2000, 57, 2065–2075. [Google Scholar] [CrossRef]
- Meng, W.; Zhang, Y.; Qu, X.D. Techniques and Methods of River Ecological Survey; Science Press: Beijing, China, 2011; pp. 92–123. [Google Scholar]
- Bisson, P.A.; Nielsen, J.L.; Palmason, R.A.; Grove, L.E. A system of naming habitat types in small streams, with examples of habitat utilization by salmonids during low streamflow. In Proceedings of the Acquisition and Utilization of Aquatic Habitat Inventory Information, Portland, OR, USA, 28–30 October 1981. [Google Scholar]
- Cummins, K.W.; Lauff, G.H. The influence of substrate particle size on the microdistribution of stream macrobenthos. Hydrobiologia 1969, 34, 145–181. [Google Scholar] [CrossRef]
- Dong, C.Z.; Li, H.M.; Zhao, C.G.; Gong, X.F.; Hong, X. Study on protective biology of precious Hucho bleekeri which is in imminent danger. Chin. J. Fish. 1998, 11, 65–70. (In Chinese) [Google Scholar]
- Ding, R.H.; Gui, L.H.; Li, M.; Luo, Q.H. Protective Biology of Hucho bleekeri in the Upper Changjiang River, China. J. Guangxi Norm. Univ. 2010, 28, 96–102. (In Chinese) [Google Scholar]
- Fang, J.; Ding, R.H. Protection biology of Hucho bleekeri IV. Estimating of its resource and cause of being faced with danger of extinction. Sichuan J. Zool. 1995, 14, 101–104. (In Chinese) [Google Scholar]
- Ding, R.H.; Qin, Z.P. Protection biology of Hucho bleekeri I. Distribution area and its transition. Sichuan J. Zool. 1995, 14, 152–154. (In Chinese) [Google Scholar]
- Schneider, M.; Noack, M.; Gebler, T. Handbook for the Habitat Simulation Model. Casimir. Module Casimir-Fish. Base Version; Institut fur Wasserbau, Universitat Stuttgart: Stuttgart, Germany, 2010; p. 12. [Google Scholar]
- Zhou, L.G.; Yue, N.Y.; Wang, W.Y.; Zhao, W.C.; Xu, T.Q.; Liu, C.G. Species diversity and protective suggestion of fish in Shaanxi Niubeiliang nature reserve. J. Shaanxi Norm. Univ. 2003, 31, 1–4. (In Chinese) [Google Scholar]
- Dong, Z.R. Diversity of river morphology and diversity of bio-communities. J. Hydraul. Eng. 2003, 34, 1–6. (In Chinese) [Google Scholar]
- Kang, B.; He, D.M. Research Progress of Biodiversity of Fish Species in the Lancangjiang River. Resour. Sci. 2007, 29, 195–200. (In Chinese) [Google Scholar]
- Lee, T.Y.; Huang, J.C.; Kao, S.J.; Liao, L.Y.; Tzeng, C.S. Modeling the effects of riparian planting strategies on stream temperature: Increasing suitable habitat for endangered Formosan Land-locked Salmon in Shei-Pa National Park, Taiwan. Hydrol. Process. 2012, 26, 3635–3644. [Google Scholar] [CrossRef]
- Hsu, C.B.; Tzeng, C.S.; Yen, C.H.; Kuan, W.H.; Lin, H.J. Habitat use by the Formosan landlocked salmon Oncorhynchus masou formosanus. Aquat. Boil. 2010, 10, 227–239. [Google Scholar] [CrossRef]
- Michael, B.; Frank, J.R. Assessing habitat requirements of Young Colorado River cutthroat trout by use of macrohabitat and microhabitat analyses. Trans. Am. Fish. Soc. 2011, 120, 571–581. [Google Scholar]
- Nakamura, Y.; Shibuno, T.; Lecchini, D.; Watanabe, Y. Habitat selection by emperor fish larvae. Aquat. Biol. 2009, 6, 61–65. [Google Scholar] [CrossRef]
- Horstkotte, J.; Plath, M. Divergent evolution of feeding substrate preferences in a phylogenetically young species flock of pupfish (Cyprinodon spp.). Naturwissenschaften 2008, 95, 1175–1180. [Google Scholar] [CrossRef]
- Bona, F.; Falasco, E.; Fenoglio, S.; Iorio, L.; Badino, G. Response of macroinvertebrate and diatom communities to human-inducedphysical alteration in mountain streams. River Res. Appl. 2008, 24, 1068–1081. [Google Scholar] [CrossRef]
- Honda, K.; Kagiwada, H.; Tojo, N.; Miyashita, K. Riverine environmental characteristics and seasonal habitat use by adult Sakhalin taimen Hucho perryi. J. Fish Biol. 2010, 77, 1526–1541. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.D.; Kim, J.H.; Jo, H.B.; Yeom, M.A.; Heo, W.M.; Joo, G.J.; Jang, M.H. Seasonal habitat utilization and movement patterns of the threatened Brachymystax lenok tsinlingensis in a Korean river. Environ. Biol. Fish. 2015, 98, 225–236. [Google Scholar] [CrossRef]
- Wang, W.; Li, W.C.; Ma, X.Z.; He, L.; Ye, W.F. Effects of water temperature and light intensity on the behaviours of Peltebagrus vachellu fingerlings. Chin. J. Ecol. 2008, 27, 791–796. [Google Scholar]
- Feng, X.B.; Zhu, Y.J.; Li, X.; He, Y.F.; Zhao, J.H.; Yang, D.G. Habiat suitability index model and minimum habitat area of young procypris rabaudi (Tahang): A simulation experiment in laboratory. Chin. J. Appl. Ecol. 2013, 24, 227–234. [Google Scholar]
| Species | Individual Number | Species Number | Proportion (%) | |
|---|---|---|---|---|
| Fish | Phoxinus lagowskii | 380 | 51.77 | |
| Triplophysa sp. | 211 | 28.75 | ||
| Brachymystax lenok tsinlingensis | 58 | 7.9 | ||
| Cobitis sinensis | 49 | 6.68 | ||
| Hucho bleekeri | 36 | 4.9 | ||
| Zooplankton | Protozoan | 7 | 53.85 | |
| Copepoda | 3 | 23.08 | ||
| Rotifer | 2 | 15.38 | ||
| Cladocera | 1 | 7.69 | ||
| Phytoplankton | Bacillariophyta | 33 | 70.21 | |
| Chlorophyta | 8 | 17.02 | ||
| Cyanophyta | 2 | 4.26 | ||
| Chrysophyta | 2 | 4.26 | ||
| Xanthophyta | 1 | 2.13 | ||
| Euglenophyta | 1 | 2.13 | ||
| Benthic animals | Oligochaeta | 1 | 3.57 | |
| Gastropoda | 2 | 7.14 | ||
| Insecta | 23 | 82.14 | ||
| Turbellaria | 1 | 3.57 | ||
| Nematoda | 1 | 3.57 | ||
| Spring | Summer | Autumn | Winter | Average | |
|---|---|---|---|---|---|
| Zooplankton (mg/L) | 0.17 ± 0.09 | 0.21 ± 0.08 | 0.16 ± 0.09 | 0.25 ± 0.08 | 0.20 ± 0.09 |
| Phytoplankton (mg/L) | 1.37 ± 0.37 | 2.34 ± 0.52 | 1.62 ± 0.23 | 0.89 ± 0.16 | 1.55 ± 0.63 |
| Benthic animals (g/m2) | 2.88 ± 1.33 | 2.47 ± 1.51 | 2.83 ± 1.07 | 2.83 ± 1.28 | 2.75 ± 1.32 |
| Area | Sampling Site | Juvenile Number | Juvenile Density (ind./m) | Elevation (m) | River Sinuosity | River Wetting Rate | Vegetation Coverage | River Habitat Diversity |
|---|---|---|---|---|---|---|---|---|
| Downstream | 1 | 0 | 0 | 920 | 1.03 | 0.35 | 0.22 | 4 |
| 2 | 0 | 0 | 960 | 1.02 | 0.94 | 0.65 | 4 | |
| 3 | 0 | 0 | 1018 | 1.05 | 0.42 | 0.54 | 6 | |
| 4 | 0 | 0 | 1094 | 1.28 | 0.88 | 0.92 | 8 | |
| 5 | 0 | 0 | 1130 | 1.14 | 0.78 | 0.58 | 4 | |
| 6 | 0 | 0 | 1146 | 1.03 | 0.56 | 0.54 | 6 | |
| 7 | 0 | 0 | 1157 | 1.08 | 0.87 | 0.48 | 4 | |
| 8 | 1 | 0.05 | 1179 | 1.17 | 0.93 | 0.86 | 8 | |
| 9 | 0 | 0 | 1197 | 1.12 | 0.63 | 0.43 | 6 | |
| 10 | 2 | 0.1 | 1207 | 1.03 | 0.65 | 0.22 | 6 | |
| 11 | 3 | 0.15 | 1221 | 1.18 | 0.75 | 0.85 | 12 | |
| 12 | 1 | 0.05 | 1235 | 1.14 | 0.72 | 0.97 | 10 | |
| 13 | 0 | 0 | 1237 | 1.04 | 0.49 | 0.87 | 6 | |
| 14 | 0 | 0 | 1249 | 1.05 | 0.85 | 0.68 | 8 | |
| 15 | 2 | 0.1 | 1253 | 1.22 | 0.91 | 0.95 | 15 | |
| 16 | 6 | 0.3 | 1254 | 1.04 | 0.93 | 0.86 | 12 | |
| 17 | 5 | 0.25 | 1279 | 1.08 | 0.78 | 0.93 | 16 | |
| 18 | 5 | 0.25 | 1295 | 1.16 | 0.85 | 0.98 | 18 | |
| 19 | 3 | 0.15 | 1297 | 1.15 | 0.95 | 0.83 | 15 | |
| 20 | 4 | 0.2 | 1299 | 1.09 | 0.61 | 0.72 | 6 | |
| 21 | 4 | 0.2 | 1304 | 1.17 | 0.88 | 0.78 | 16 | |
| Upstream | 22 | 2 | 0.1 | 1547 | 1.22 | 0.94 | 0.92 | 16 |
| 23 | 1 | 0.05 | 1571 | 1.13 | 0.9 | 0.96 | 18 | |
| 24 | 5 | 0.25 | 1599 | 1.04 | 0.79 | 0.96 | 20 | |
| 25 | 1 | 0.05 | 1614 | 1.04 | 0.83 | 0.89 | 24 | |
| 26 | 2 | 0.1 | 1630 | 1.15 | 0.66 | 0.93 | 18 | |
| 27 | 5 | 0.25 | 1670 | 1.21 | 0.79 | 0.83 | 15 | |
| 28 | 3 | 0.15 | 1729 | 1.06 | 0.93 | 0.86 | 18 | |
| 29 | 0 | 0 | 1762 | 1.32 | 0.89 | 0.87 | 20 | |
| 30 | 0 | 0 | 1816 | 1.11 | 0.9 | 0.95 | 12 | |
| 31 | 2 | 0.1 | 1868 | 1.32 | 0.83 | 0.92 | 12 | |
| 32 | 0 | 0 | 1909 | 1.24 | 0.93 | 0.77 | 4 | |
| 33 | 2 | 0.1 | 1833 | 1.25 | 0.86 | 0.68 | 6 | |
| 34 | 0 | 0 | 1596 | 1.09 | 0.95 | 0.54 | 2 | |
| 35 | 0 | 0 | 1755 | 1.13 | 0.92 | 0.65 | 4 | |
| Average | 0.08 ± 0.09 | 1395 ± 278 | 1.13 ± 0.08 | 0.80 ± 0.16 | 0.76 ± 0.20 | 11 ± 6 |
| Individual Size | Water Depth (m) | Average | Flow Velocity (m/s) | Average | Illuminance (Lux) | Average | Offshore Distance (m) | Average |
|---|---|---|---|---|---|---|---|---|
| 5–10 cm | 0.29 | 0.50 ± 0.22 a | 0.25 | 0.32 ± 0.13 a | 7144 | 5313 ± 2836 | 4.25 | 5.58 ± 2.50 a |
| 0.85 | 0.28 | 9608 | 8.6 | |||||
| 0.48 | 0.14 | 8054 | 2.77 | |||||
| 0.6 | 0.42 | 6852 | 6.43 | |||||
| 0.36 | 0.37 | 4559 | 5.16 | |||||
| 0.43 | 0.41 | 2775 | 8.71 | |||||
| 0.52 | 0.27 | 9768 | 2.86 | |||||
| 0.31 | 0.22 | 4972 | 5.05 | |||||
| 0.51 | 0.37 | 1602 | 5.92 | |||||
| 0.89 | 0.49 | 7053 | 0.51 | |||||
| 0.34 | 0.6 | 8030 | 5.05 | |||||
| 0.52 | 0.45 | 6645 | 9.57 | |||||
| 0.66 | 0.22 | 4231 | 5.08 | |||||
| 0.78 | 0.44 | 1721 | 3.1 | |||||
| 0.23 | 0.53 | 1337 | 8.04 | |||||
| 0.78 | 0.43 | 9886 | 9.88 | |||||
| 0.33 | 0.27 | 9662 | 4.9 | |||||
| 0.47 | 0.49 | 2767 | 8.29 | |||||
| 0.47 | 0.34 | 7747 | 2.59 | |||||
| 0.71 | 0.33 | 1287 | 6.55 | |||||
| 0.81 | 0.27 | 2760 | 5.07 | |||||
| 0.83 | 0.14 | 4245 | 8.51 | |||||
| 0.22 | 0.23 | 6036 | 5.17 | |||||
| 0.19 | 0.33 | 2863 | 8.47 | |||||
| 0.2 | 0.2 | 4430 | 1.4 | |||||
| 0.18 | 0.16 | 6365 | 5.73 | |||||
| 0.65 | 0.12 | 1052 | 3.03 | |||||
| 11–15 cm | 0.4 | 0.73 ± 0.34 ab | 0.64 | 0.63 ± 0.12 b | 5495 | 3966 ± 2506 | 4.83 | 9.17 ± 4.11 b |
| 1.4 | 0.69 | 6624 | 4.36 | |||||
| 0.53 | 0.75 | 891 | 4.76 | |||||
| 0.44 | 0.64 | 2940 | 16.3 | |||||
| 1.29 | 0.65 | 1877 | 9.02 | |||||
| 1 | 0.62 | 3067 | 2.68 | |||||
| 0.6 | 0.7 | 722 | 15.47 | |||||
| 0.68 | 0.8 | 966 | 9.75 | |||||
| 0.39 | 0.76 | 2158 | 10.38 | |||||
| 1.08 | 0.67 | 5639 | 7.82 | |||||
| 0.46 | 0.53 | 7635 | 9.67 | |||||
| 0.74 | 0.34 | 5842 | 13.61 | |||||
| 0.43 | 0.44 | 7703 | 10.5 | |||||
| 16–20 cm | 1.09 | 0.95 ± 0.30 b | 0.64 | 0.81 ± 0.16 c | 4739 | 3517 ± 1641 | 10.31 | 11.32 ± 4.86 b |
| 0.83 | 0.42 | 1329 | 1.17 | |||||
| 1.27 | 0.83 | 3954 | 16.65 | |||||
| 0.44 | 0.93 | 1262 | 6.92 | |||||
| 0.52 | 0.84 | 3620 | 13.7 | |||||
| 1.3 | 0.99 | 2164 | 15.06 | |||||
| 1.21 | 0.97 | 2644 | 5.9 | |||||
| 1 | 0.9 | 5900 | 13.4 | |||||
| 0.66 | 0.79 | 3387 | 13.75 | |||||
| 1.19 | 0.82 | 6178 | 16.33 | |||||
| Total average | 0.65 ± 0.33 | 0.50 ± 0.24 | 4604 ± 2670 | 7.66 ± 4.25 |
| Individual Size | Substrate Type | ||||
|---|---|---|---|---|---|
| Gr | Pe | Co | Bo | Br | |
| 5–10 cm | 40.74% | 18.52% | 18.52% | 14.81% | 7.41% |
| 11–15 cm | 23.08% | 23.08% | 30.77% | 23.08% | |
| 16–20 cm | 10.00% | 50.00% | 20.00% | 10.00% | 10.00% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; Wu, J.; Ye, H.; Xiong, W.; Qu, W.; Leng, X.; Du, H. Correlations between Environmental Factors and the Distribution of Juvenile Hucho bleekeri in the Taibai River, Shaanxi, China. Fishes 2023, 8, 379. https://doi.org/10.3390/fishes8070379
Wu J, Wu J, Ye H, Xiong W, Qu W, Leng X, Du H. Correlations between Environmental Factors and the Distribution of Juvenile Hucho bleekeri in the Taibai River, Shaanxi, China. Fishes. 2023; 8(7):379. https://doi.org/10.3390/fishes8070379
Chicago/Turabian StyleWu, Jinming, Jinping Wu, Huan Ye, Wei Xiong, Wanmin Qu, Xiaoqian Leng, and Hao Du. 2023. "Correlations between Environmental Factors and the Distribution of Juvenile Hucho bleekeri in the Taibai River, Shaanxi, China" Fishes 8, no. 7: 379. https://doi.org/10.3390/fishes8070379
APA StyleWu, J., Wu, J., Ye, H., Xiong, W., Qu, W., Leng, X., & Du, H. (2023). Correlations between Environmental Factors and the Distribution of Juvenile Hucho bleekeri in the Taibai River, Shaanxi, China. Fishes, 8(7), 379. https://doi.org/10.3390/fishes8070379






