Estimates of the Effective Population Size and Genetic Structure of the Critically Endangered Ship Sturgeon (Acipenser nudiventris) in the Chinese Section of the Ili River
Abstract
1. Introduction
2. Materials and Methods
2.1. Genome Re-Sequencing and SNP Calling
2.2. Analysis of the Genomic Structure of the Population and Individual Kinship
2.3. Estimation of Ancestral and Contemporary Effective Population Sizes
3. Results
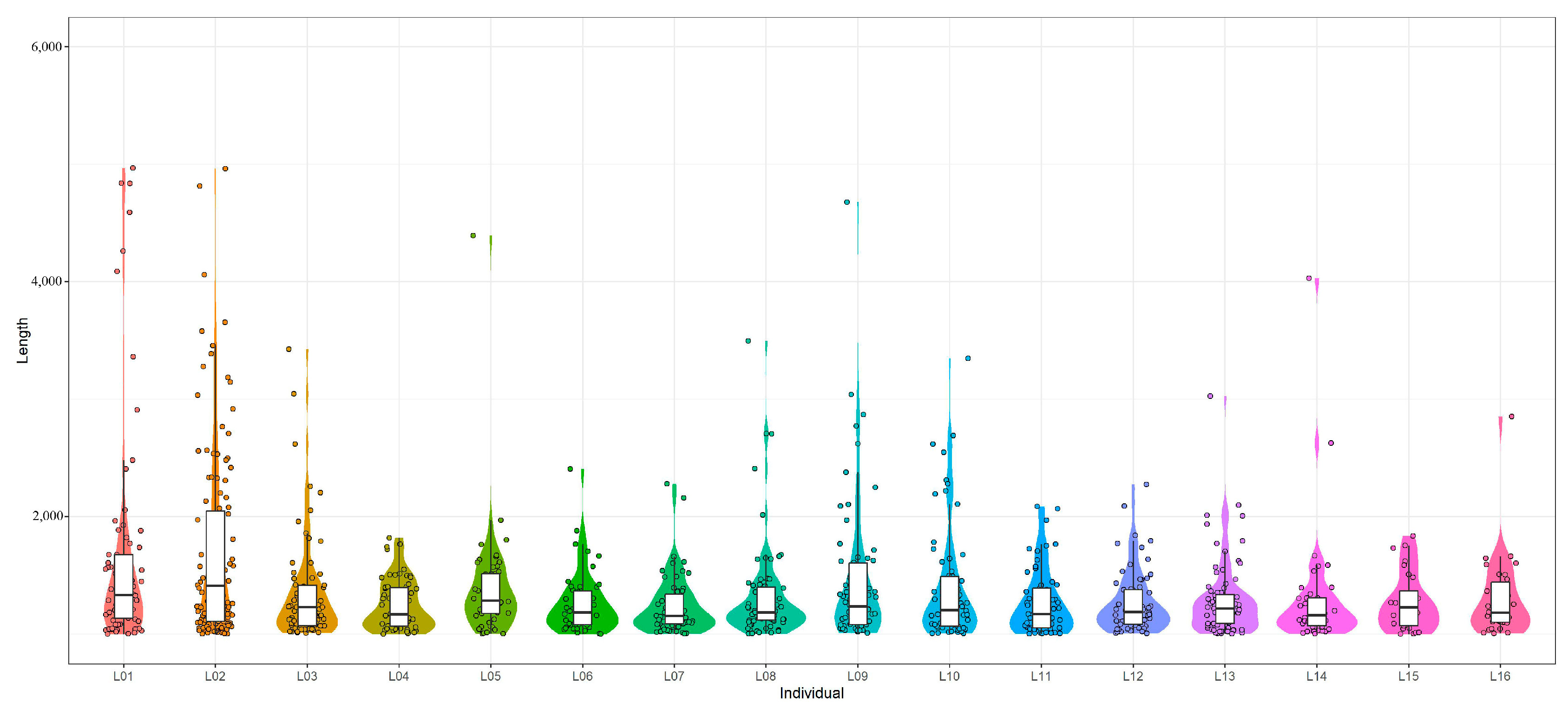
3.1. Statistics and Evaluation of the Sequencing Data
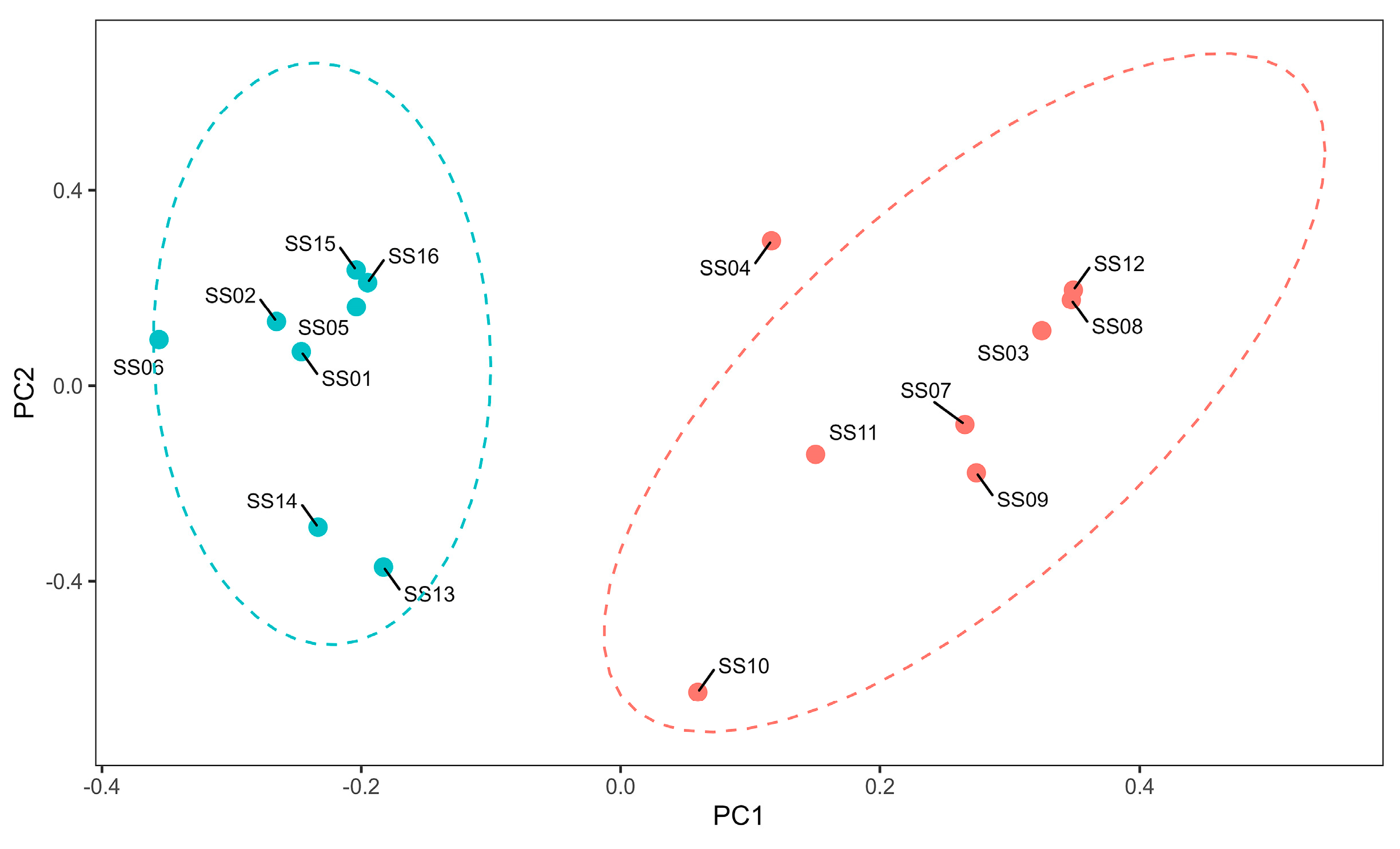
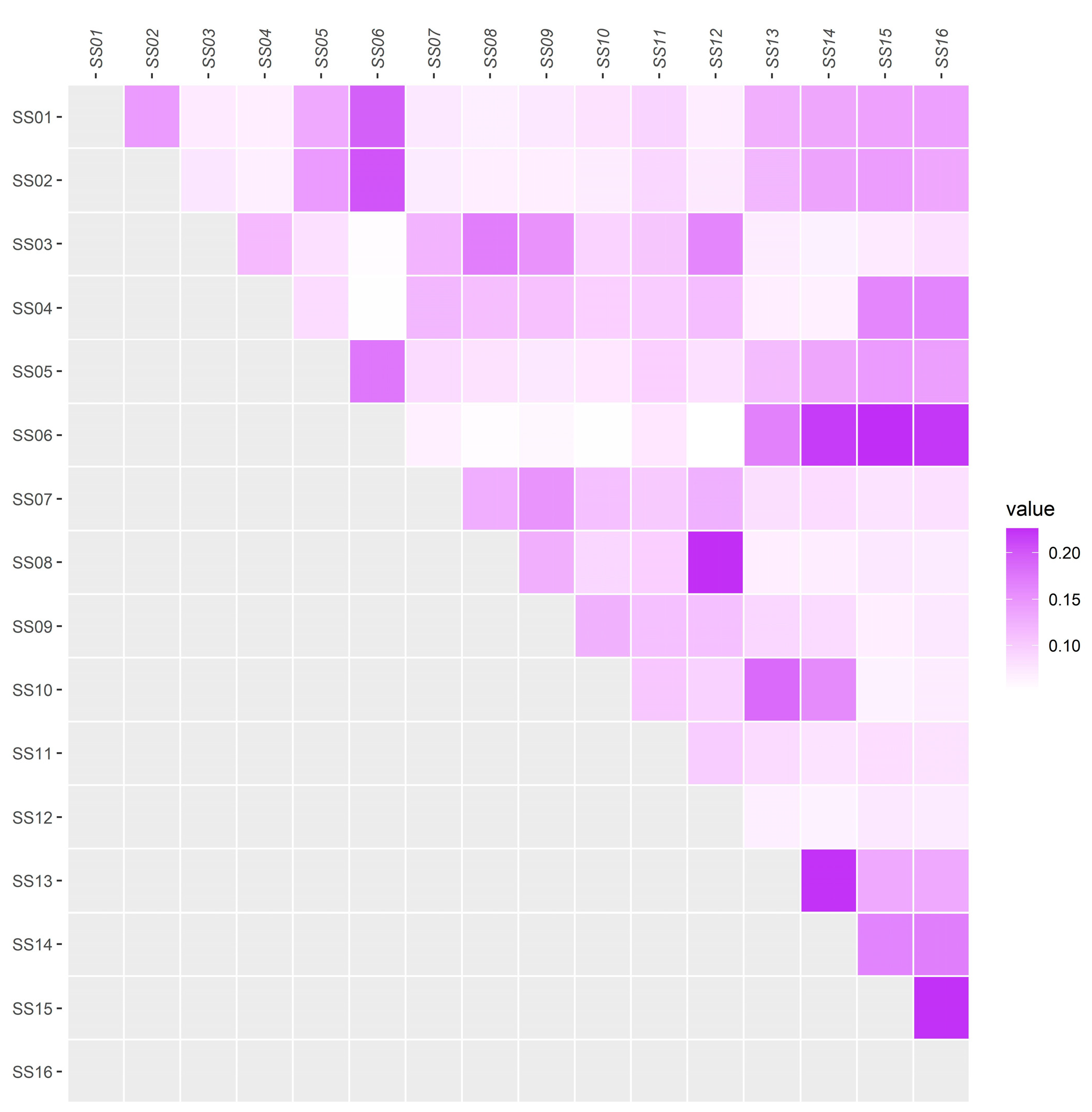
3.2. Analysis of the Genomic Structure of the Population and Individual Kinship
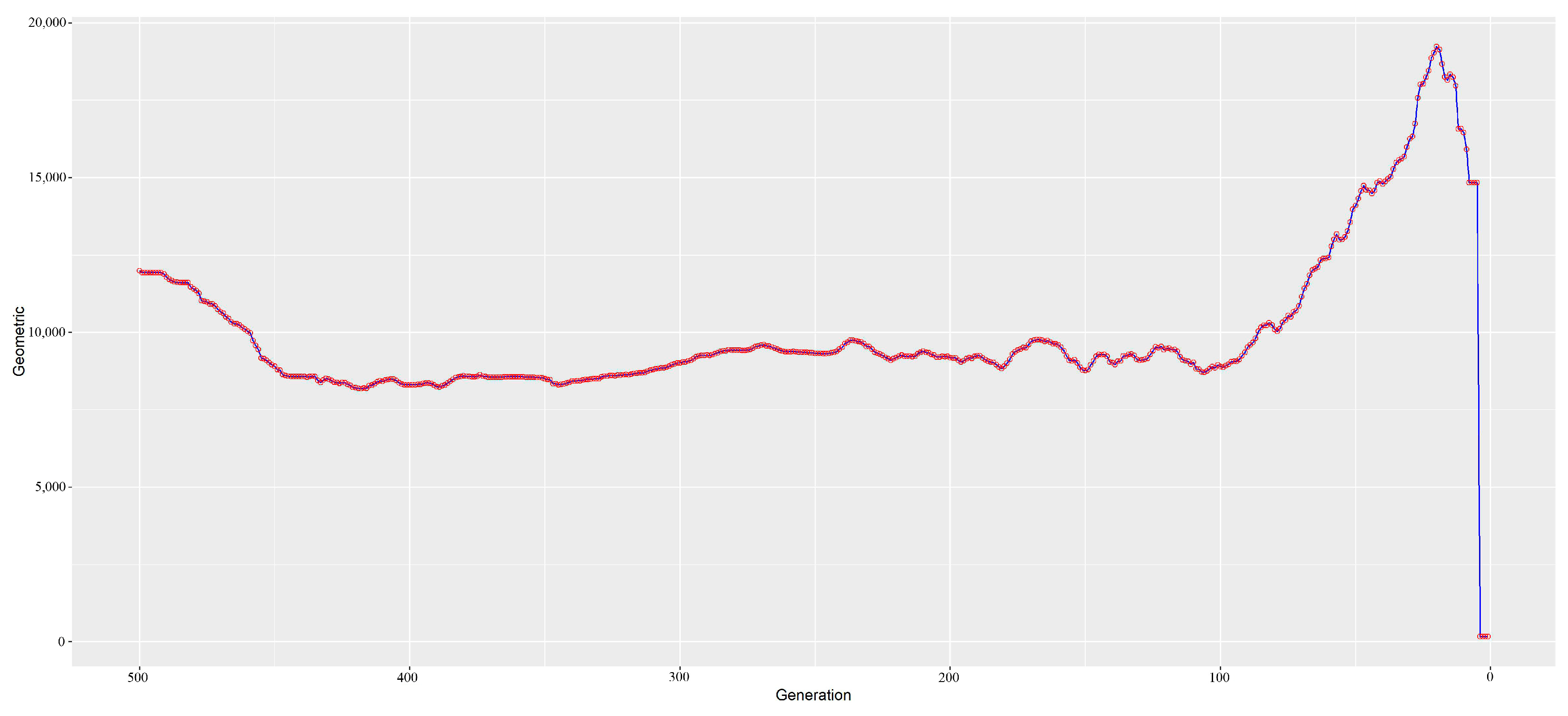
3.3. Estimation of Ancestral and Contemporary Effective Population Sizes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Bekbergenova, V. Current Biological Data on the Ship Sturgeon Acipenser Nudiventris Lovetsky; 1828 (Review); Springer International Publishing: Cham, Switzerland, 2023. [Google Scholar]
- Orlov, A.; Rabazanov, N.; Barkhalov, R. The Endangered Fringebarbel Sturgeon, Acipenser Nudiventris; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Hu, G.; Ji, F.; Zheng, P.; Jia, D.; Lv, W.; Wang, N.; Zhang, Y. Technical report for controlled propagation of the critically endangered ship sturgeon (Acipenser nudiventris) in the Ili River. J. Fish. Sci. China 2021, 28, 403–410. [Google Scholar]
- Pueppke, S.G.; Zhang, Q.; Nurtazin, S.T. Irrigation in the Ili River Basin of Central Asia: From Ditches to Dams and Diversion. Water 2018, 10, 1650. [Google Scholar] [CrossRef]
- Sun, D. Sturgeon Culture in China; China Agriculture Press: Beijing, China, 2015. [Google Scholar]
- Graham, N.A.; Pueppke, S.G.; Uderbayev, T. The Current Status and Future of Central Asia’s Fish and Fisheries: Confronting a Wicked Problem. Water 2017, 9, 701. [Google Scholar] [CrossRef]
- Chen, T.; Chen, X.; Zhang, S.; Zhu, J.; Tang, B.; Wang, A.; Dong, L.; Zhang, Z.; Yu, C.; Sun, Y.; et al. The Genome Sequence Archive Family: Toward Explosive Data Growth and Diverse Data Types. Genom. Proteom. Bioinform. 2021, 19, 578–583. [Google Scholar] [CrossRef] [PubMed]
- CNCB-NGDC Members and Partners. Database Resources of the National Genomics Data Center, China National Center for Bioinformation in 2022. Nucleic Acids Res. 2021, 50, D27–D38. [Google Scholar]
- Du, K.; Stock, M.; Kneitz, S.; Klopp, C.; Woltering, J.M.; Adolfi, M.C.; Feron, R.; Prokopov, D.; Makunin, A.; Kichigin, I.; et al. The sterlet sturgeon genome sequence and the mechanisms of segmental rediploidization. Nat. Ecol. Evol. 2020, 4, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 1754–1760. [Google Scholar] [CrossRef] [PubMed]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve years of SAMtools and BCFtools. Gigascience 2021, 10, giab008. [Google Scholar] [CrossRef] [PubMed]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef] [PubMed]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.; Daly, M.J.; et al. PLINK: A tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.C.; Chow, C.C.; Tellier, L.C.; Vattikuti, S.; Purcell, S.M.; Lee, J.J. Second-generation PLINK: Rising to the challenge of larger and richer datasets. Gigascience 2015, 4, 7. [Google Scholar] [CrossRef]
- Zhang, C.; Dong, S.-S.; Xu, J.-Y.; He, W.-M.; Yang, T.-L. PopLDdecay: A fast and effective tool for linkage disequilibrium decay analysis based on variant call format files. Bioinformatics 2018, 35, 1786–1788. [Google Scholar] [CrossRef]
- Hanghøj, K.; Moltke, I.; Andersen, P.A.; Manica, A.; Korneliussen, T.S. Fast and accurate relatedness estimation from high-throughput sequencing data in the presence of inbreeding. GigaScience 2019, 8, giz034. [Google Scholar] [CrossRef] [PubMed]
- Santiago, E.; Novo, I.; Pardinas, A.F.; Saura, M.; Wang, J.; Caballero, A. Recent Demographic History Inferred by High-Resolution Analysis of Linkage Disequilibrium. Mol. Biol. Evol. 2020, 37, 3642–3653. [Google Scholar] [CrossRef] [PubMed]
- Chandra, G.; Fopp-Bayat, D. Trends in aquaculture and conservation of sturgeons: A review of molecular and cytogenetic tools. Rev. Aquac. 2021, 13, 119–137. [Google Scholar] [CrossRef]
- Findeis, E.K. Osteology and phylogenetic interrelationships of sturgeons (Acipenseridae). Environ. Biol. Fishes 1997, 48, 73–126. [Google Scholar] [CrossRef]
- Zhang, Y.; Luan, P.; Ren, G.; Hu, G.; Yin, J. Estimating the inbreeding level and genetic relatedness in an isolated population of critically endangered Sichuan taimen (Hucho bleekeri) using genome-wide SNP markers. Ecol. Evol. 2020, 10, 1390–1400. [Google Scholar] [CrossRef] [PubMed]
| Sample | Clean Reads | Clean Base | Q20 (%) | GC (%) |
|---|---|---|---|---|
| SS01 | 160,042,004 | 48,012,601,200 | 97.13 | 39.86 |
| SS02 | 160,082,176 | 48,024,652,800 | 97.23 | 39.92 |
| SS03 | 160,132,305 | 48,039,691,500 | 97.08 | 39.84 |
| SS04 | 160,073,512 | 48,022,053,600 | 97.4 | 40.03 |
| SS05 | 160,007,653 | 48,002,295,900 | 97.27 | 40.01 |
| SS06 | 160,157,713 | 48,047,313,900 | 97.45 | 39.98 |
| SS07 | 160,105,711 | 48,031,713,300 | 97.7 | 40.12 |
| SS08 | 160,105,071 | 48,031,521,300 | 97.61 | 40.16 |
| SS09 | 160,044,445 | 48,013,333,500 | 97.43 | 39.81 |
| SS10 | 128,969,960 | 38,690,988,000 | 97.17 | 40.1 |
| SS11 | 159,114,361 | 47,734,308,300 | 97.33 | 39.59 |
| SS12 | 160,037,844 | 48,011,353,200 | 97.29 | 39.45 |
| SS13 | 145,365,836 | 43,6097,50,800 | 97.43 | 39.77 |
| SS14 | 160,061,705 | 48,018,511,500 | 97.56 | 39.62 |
| SS15 | 151,665,763 | 45,499,728,900 | 97.45 | 39.59 |
| SS16 | 139,409,107 | 41,822,732,100 | 97.38 | 39.84 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, G.; Chen, F.; Zhang, Y.; Luan, P.; Luo, Z.; Niu, J.; Zheng, P.; Wang, S.; Zhang, T.; Shu, Y.; et al. Estimates of the Effective Population Size and Genetic Structure of the Critically Endangered Ship Sturgeon (Acipenser nudiventris) in the Chinese Section of the Ili River. Fishes 2023, 8, 354. https://doi.org/10.3390/fishes8070354
Hu G, Chen F, Zhang Y, Luan P, Luo Z, Niu J, Zheng P, Wang S, Zhang T, Shu Y, et al. Estimates of the Effective Population Size and Genetic Structure of the Critically Endangered Ship Sturgeon (Acipenser nudiventris) in the Chinese Section of the Ili River. Fishes. 2023; 8(7):354. https://doi.org/10.3390/fishes8070354
Chicago/Turabian StyleHu, Guo, Feng Chen, Ying Zhang, Peixian Luan, Zhiyuan Luo, Jiangong Niu, Peng Zheng, Sai Wang, Tao Zhang, Yongjun Shu, and et al. 2023. "Estimates of the Effective Population Size and Genetic Structure of the Critically Endangered Ship Sturgeon (Acipenser nudiventris) in the Chinese Section of the Ili River" Fishes 8, no. 7: 354. https://doi.org/10.3390/fishes8070354
APA StyleHu, G., Chen, F., Zhang, Y., Luan, P., Luo, Z., Niu, J., Zheng, P., Wang, S., Zhang, T., Shu, Y., & Ji, F. (2023). Estimates of the Effective Population Size and Genetic Structure of the Critically Endangered Ship Sturgeon (Acipenser nudiventris) in the Chinese Section of the Ili River. Fishes, 8(7), 354. https://doi.org/10.3390/fishes8070354






