Potential of Otolith Microchemistry to Distinguish Nursery Areas of Salmon within River Simojoki
Abstract
1. Introduction
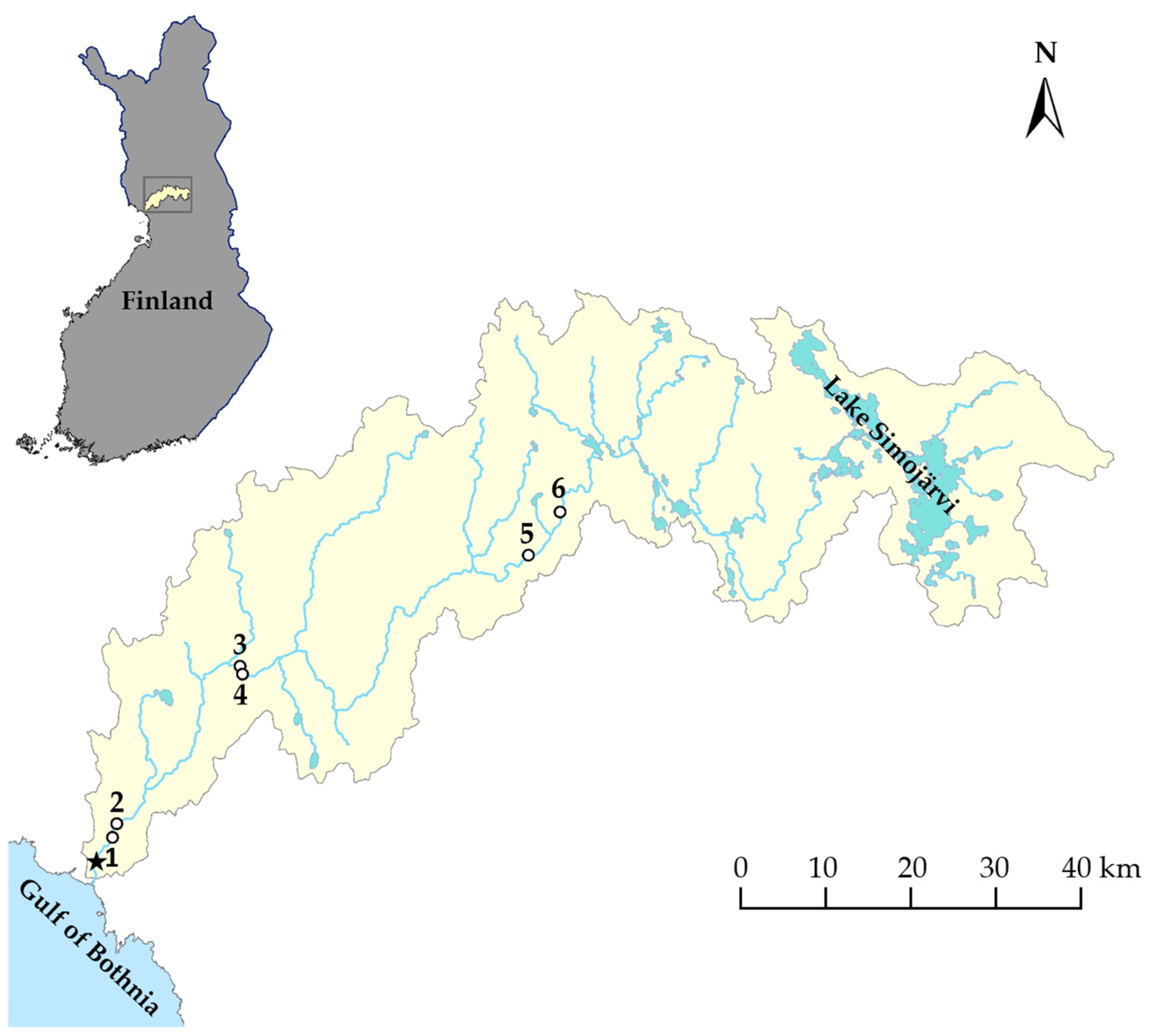
2. Materials and Methods
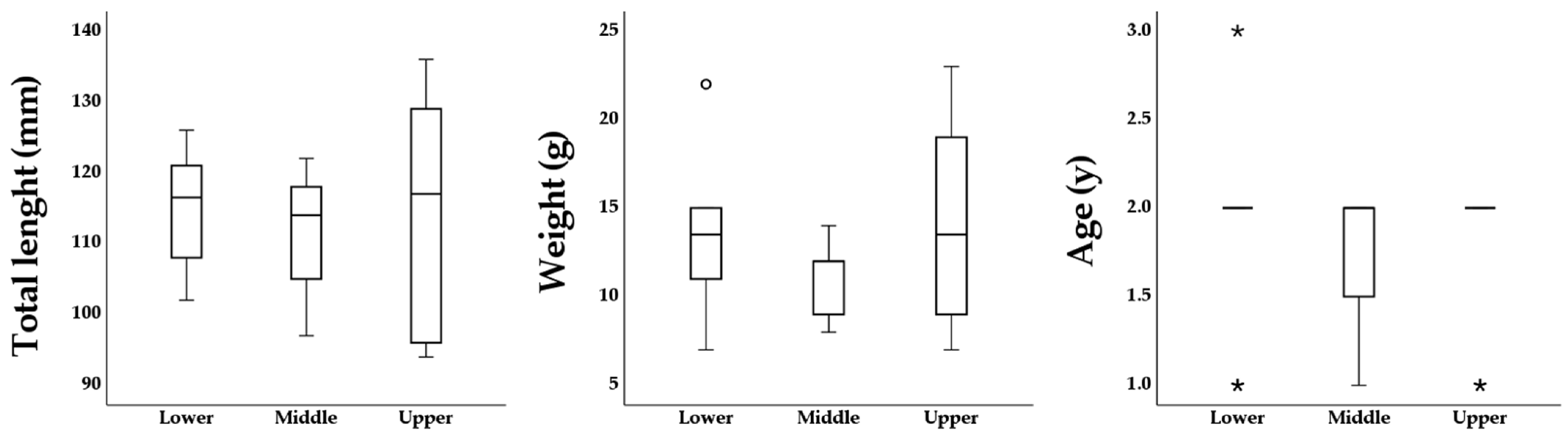
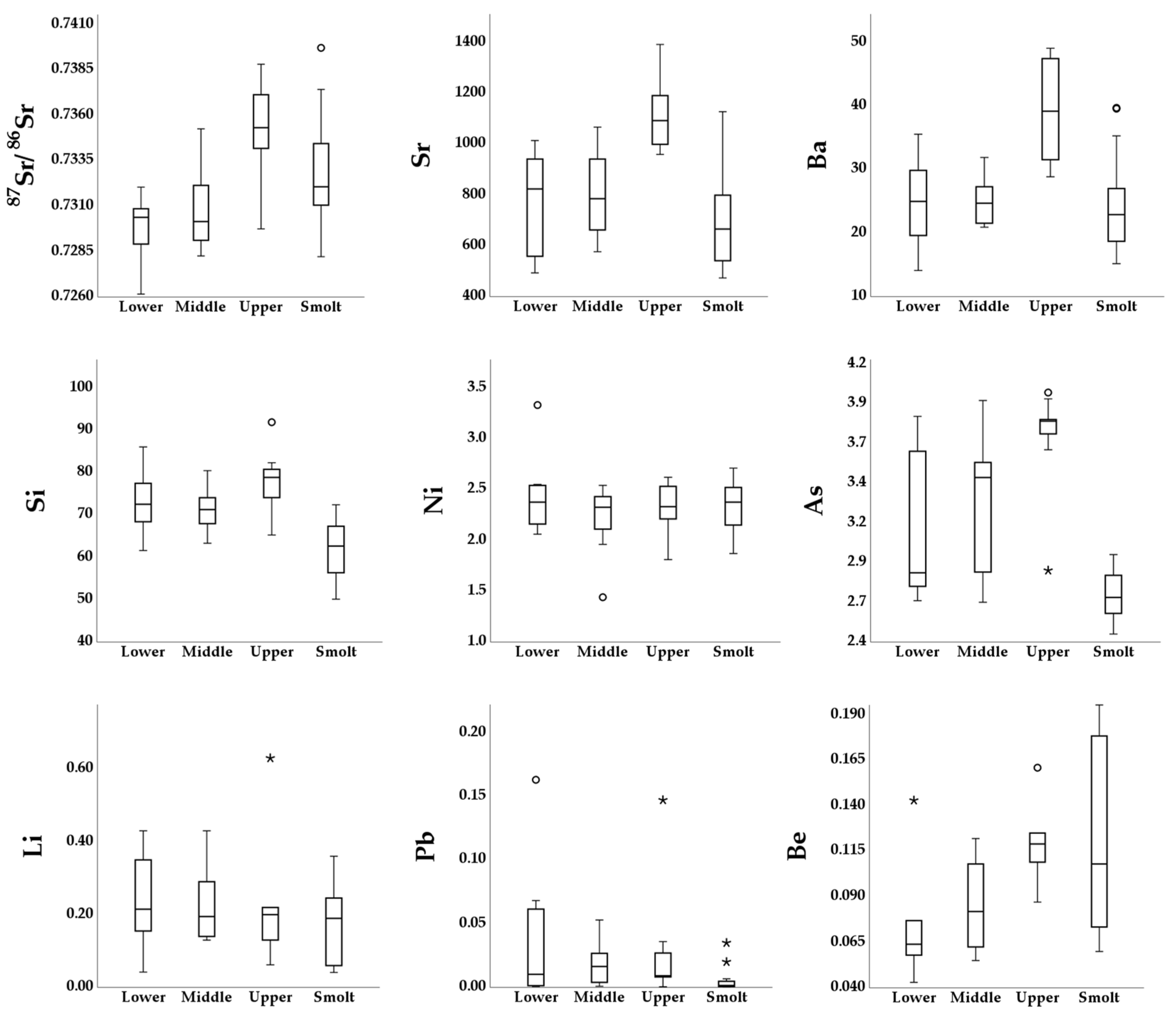
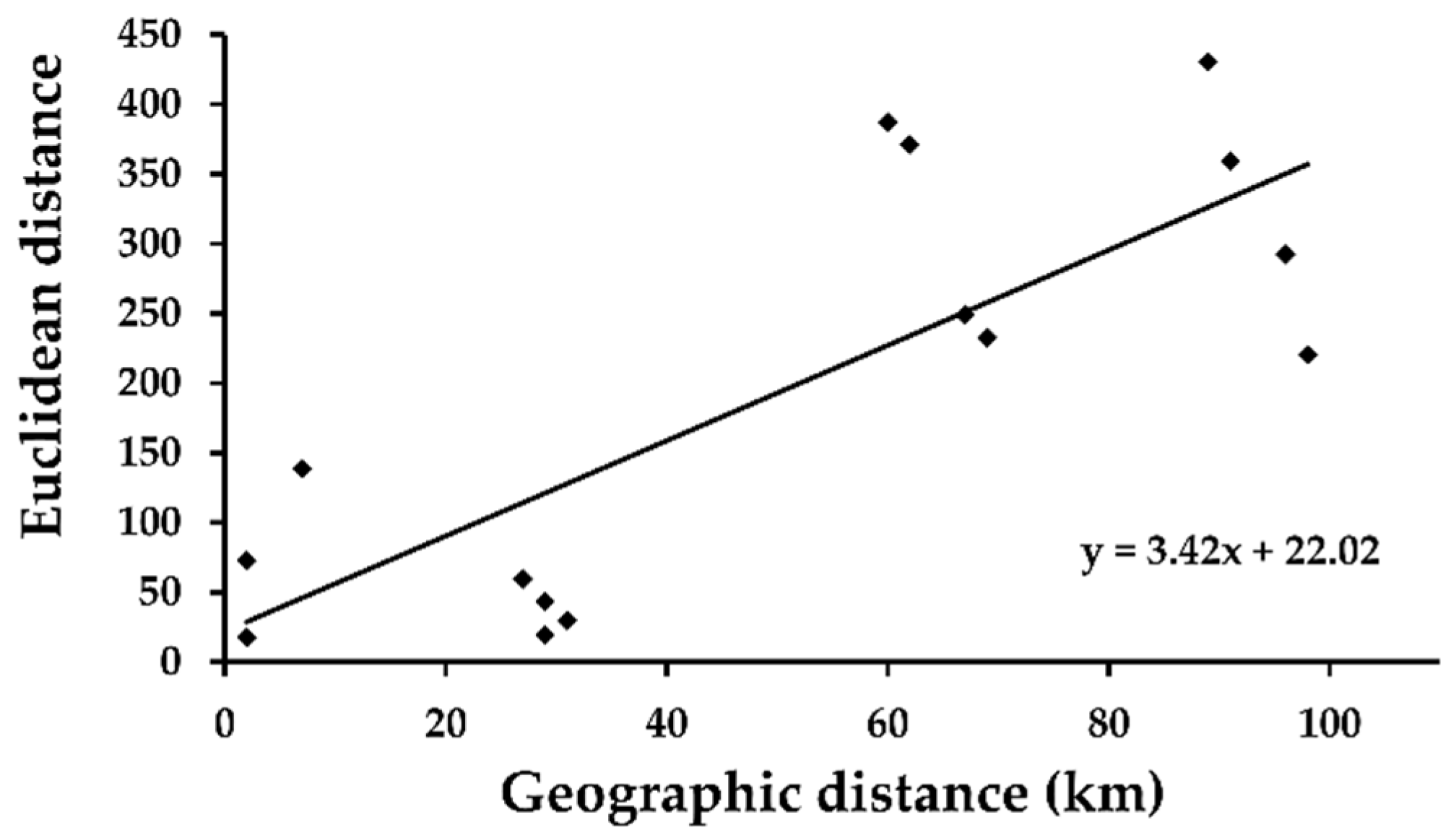
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| ID | Sampled Rapid | Nursery Area | Date | Length (mm) | Weight (g) | Age (Y) | |
|---|---|---|---|---|---|---|---|
| Parr | SJ.P 73 | 1 | lower | 7.8 | 126 | 22 | 3 |
| SJ.P 74 | 1 | lower | 7.8 | 102 | 7 | 2 | |
| SJ.P 75 | 1 | lower | 7.8 | 113 | 11 | 2 | |
| SJ.P 286 | 2 | lower | 16.8 | 121 | 15 | 2 | |
| SJ.P 287 | 2 | lower | 16.8 | 108 | 10 | 1 | |
| SJ.P 288 | 2 | lower | 16.8 | 117 | 14 | 2 | |
| SJ.P 289 | 2 | lower | 16.8 | 117 | 13 | 2 | |
| SJ.P 290 | 2 | lower | 16.8 | 122 | 14 | 2 | |
| SJ.P 291 | 2 | lower | 16.8 | 107 | 11 | 1 | |
| SJ.P 292 | 2 | lower | 16.8 | 116 | 15 | 2 | |
| SJ.P 246 | 3 | middle | 15.8 | 113 | 12 | 2 | |
| SJ.P 247 | 3 | middle | 15.8 | 122 | 14 | 2 | |
| SJ.P 248 | 3 | middle | 15.8 | 115 | 12 | 2 | |
| SJ.P 249 | 3 | middle | 15.8 | 105 | 9 | 2 | |
| SJ.P 250 | 3 | middle | 15.8 | 97 | 8 | 1 | |
| SJ.P 188 | 4 | middle | 14.8 | 118 | 13 | * | |
| SJ.P 189 | 4 | middle | 14.8 | 107 | 9 | 1 | |
| SJ.P 190 | 4 | middle | 14.8 | 117 | 12 | 2 | |
| SJ.P 191 | 4 | middle | 14.8 | 118 | 12 | * | |
| SJ.P 192 | 4 | middle | 14.8 | 102 | 11 | 2 | |
| SJ.P 116 | 5 | upper | 8.8 | 129 | 19 | 2 | |
| SJ.P 117 | 5 | upper | 8.8 | 96 | 9 | 2 | |
| SJ.P 118 | 5 | upper | 8.8 | 94 | 7 | 1 | |
| SJ.P 119 | 5 | upper | 8.8 | 117 | 14 | 2 | |
| SJ.P 120 | 5 | upper | 8.8 | 95 | 7 | 1 | |
| SJ.P 106 | 6 | upper | 8.8 | 136 | 22 | 2 | |
| SJ.P 107 | 6 | upper | 8.8 | 136 | 23 | 2 | |
| SJ.P 108 | 6 | upper | 8.8 | 113 | 13 | 2 | |
| SJ.P 109 | 6 | upper | 8.8 | 120 | 14 | * | |
| SJ.P 110 | 6 | upper | 8.8 | 117 | 13 | 2 | |
| Smolt | SJ.S 83 | 24.5 | 121 | 12 | 3 | ||
| SJ.S 90 | 24.5 | 131 | 16 | 3 | |||
| SJ.S 91 | 24.5 | 128 | 15 | 3 | |||
| SJ.S 92 | 24.5 | 130 | 15 | 3 | |||
| SJ.S 93 | 24.5 | 150 | 21 | 3 | |||
| SJ.S 94 | 24.5 | 145 | 21 | 4 | |||
| SJ.S 95 | 24.5 | 142 | 21 | 3 | |||
| SJ.S 96 | 24.5 | 147 | 22 | 3 | |||
| SJ.S 97 | 24.5 | 144 | 20 | 3 | |||
| SJ.S 99 | 24.5 | 148 | 21 | 3 | |||
| SJ.S 312 | 31.5 | 125 | 15 | 3 | |||
| SJ.S 319 | 31.5 | 124 | 15 | 3 | |||
| SJ.S 320 | 31.5 | 122 | 14 | 3 | |||
| SJ.S 321 | 31.5 | 131 | 17 | 3 | |||
| SJ.S 322 | 31.5 | 132 | 15 | 3 | |||
| SJ.S 323 | 31.5 | 134 | 18 | 3 | |||
| SJ.S 324 | 31.5 | 134 | 15 | 3 | |||
| SJ.S 325 | 31.5 | 134 | 17 | 3 | |||
| SJ.S 326 | 31.5 | 127 | 13 | 3 | |||
| SJ.S 328 | 31.5 | 116 | 13 | 3 | |||
| SJ.S 474 | 10.6 | 129 | 18 | 3 | |||
| SJ.S 477 | 10.6 | 123 | 15 | 2 | |||
| SJ.S 479 | 10.6 | 162 | * | 4 | |||
| SJ.S 480 | 10.6 | 128 | * | 3 | |||
| SJ.S 481 | 10.6 | 128 | * | 3 | |||
| SJ.S 482 | 10.6 | 118 | * | 2 | |||
| SJ.S 483 | 10.6 | 124 | * | 2 | |||
| SJ.S 484 | 10.6 | 129 | * | 3 | |||
| SJ.S 485 | 10.6 | 152 | 27 | 2 | |||
| SJ.S 486 | 10.6 | 142 | 20 | 4 |
| ID | Nursery Area | 87Sr/86Sr | Li | Si | Ni | Sr | Ba | Pb | Be | As | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Parr | SJ.P 73 | lower | 0.73088 | 0.25 | 86.0 | 3.33 | 713 | 27.4 | 0.163 | 0.064 | 3.7 |
| SJ.P 74 | lower | 0.72616 | 0.39 | 83.8 | 2.48 | 862 | 35.5 | BDL | 0.043 | 3.8 | |
| SJ.P 75 | lower | 0.73059 | 0.43 | 75.2 | 2.46 | 899 | 29.9 | 0.0181 | BDL | 3.6 | |
| SJ.P 286 | lower | 0.73083 | 0.04 | 77.5 | 2.55 | 954 | 22.6 | 0.055 | 0.077 | 2.8 | |
| SJ.P 287 | lower | 0.73018 | 0.15 | 68.4 | 2.54 | 941 | 28.2 | 0.068 | 0.058 | 2.8 | |
| SJ.P 288 | lower | 0.72963 | 0.31 | 61.6 | 2.06 | 784 | 20.5 | 0.00128 | BDL | 2.7 | |
| SJ.P 289 | lower | 0.73205 | BDL | 70.4 | 2.16 | 558 | 19.6 | 0.00246 | 0.143 | 2.8 | |
| SJ.P 290 | lower | 0.73086 | 0.16 | 74.6 | 2.29 | 493 | 14.5 | BDL | BDL | 2.8 | |
| SJ.P 291 | lower | 0.72891 | BDL | 69.1 | 2.22 | 1014 | 31.6 | 0.00072 | BDL | 3.1 | |
| SJ.P 292 | lower | 0.7264 | 0.18 | 67.9 | 2.16 | 531 | 14.1 | 0.00131 | BDL | 2.7 | |
| SJ.P 246 | middle | 0.73154 | BDL | 73.2 | 2.33 | 662 | 24.0 | 0.00388 | BDL | 3.5 | |
| SJ.P 247 | middle | 0.73526 | 0.22 | 66.4 | 1.44 | 804 | 21.3 | 0.0207 | BDL | 2.8 | |
| SJ.P 248 | middle | 0.72948 | BDL | 67.9 | 2.54 | 875 | 25.4 | 0.0122 | 0.07 | 2.9 | |
| SJ.P 249 | middle | 0.73478 | 0.29 | 63.3 | 2.11 | 766 | 21.7 | 0.0219 | BDL | 2.8 | |
| SJ.P 250 | middle | 0.72826 | BDL | 70.5 | 1.96 | 959 | 27.3 | 0.053 | 0.094 | 2.7 | |
| SJ.P 188 | middle | 0.73082 | 0.17 | 80.4 | 2.3 | 576 | 20.9 | 0.00086 | 0.055 | 3.9 | |
| SJ.P 189 | middle | 0.72924 | 0.43 | 74.1 | 2.32 | 941 | 31.9 | 0.0064 | 0.122 | 3.7 | |
| SJ.P 190 | middle | 0.72874 | 0.14 | 76.5 | 2.43 | 657 | 21.5 | 0.0454 | BDL | 3.4 | |
| SJ.P 191 | middle | 0.72912 | BDL | 72.0 | 2.34 | 1066 | 29.4 | 0.00177 | BDL | 3.4 | |
| SJ.P 192 | middle | 0.73215 | 0.13 | 69.1 | 2.48 | 743 | 25.7 | 0.0267 | BDL | 3.5 | |
| SJ.P 116 | upper | 0.7369 | 0.22 | 80.8 | 2.34 | 1153 | 41.8 | 0.147 | 0.161 | 3.6 | |
| SJ.P 117 | upper | 0.73496 | BDL | 65.3 | 2.34 | 1392 | 47.4 | 0.036 | BDL | 2.9 | |
| SJ.P 118 | upper | 0.73418 | BDL | 76.3 | 2.53 | 1191 | 33.4 | 0.0085 | 0.119 | 3.8 | |
| SJ.P 119 | upper | 0.73378 | BDL | 80.3 | 2.3 | 1081 | 28.8 | 0.00156 | 0.109 | 3.8 | |
| SJ.P 120 | upper | 0.72975 | 0.13 | 73.3 | 2.6 | 1103 | 40.4 | BDL | BDL | 3.7 | |
| SJ.P 106 | upper | 0.73569 | 0.63 | 91.9 | 2.03 | 999 | 49.1 | 0.00049 | 0.087 | 3.9 | |
| SJ.P 107 | upper | 0.73714 | BDL | 82.3 | 2.62 | 1283 | 47.8 | 0.0091 | 0.125 | 4.0 | |
| SJ.P 108 | upper | 0.73715 | 0.06 | 78.2 | 1.81 | 1001 | 31.5 | 0.027 | BDL | 3.8 | |
| SJ.P 109 | upper | 0.73882 | 0.20 | 79.5 | 2.21 | 984 | 30.9 | 0.0097 | BDL | 3.8 | |
| SJ.P 110 | upper | 0.73463 | BDL | 74.1 | 2.32 | 959 | 38.0 | 0.0082 | BDL | 3.8 | |
| Smolt | SJ.S 83 | 0.72477 | 0.04 | 67.1 | 2.18 | 541 | 18.7 | 0.00232 | BDL | 2.7 | |
| SJ.S 90 | 0.73204 | 0.26 | 70.3 | 2.17 | 514 | 18.2 | 0.00062 | BDL | 2.9 | ||
| SJ.S 91 | 0.73166 | 0.20 | 66.7 | 2.56 | 555 | 17.0 | 0.00065 | BDL | 2.6 | ||
| SJ.S 92 | 0.73173 | 0.16 | 70.5 | 2.01 | 889 | 26.0 | 0.00069 | BDL | 2.5 | ||
| SJ.S 93 | 0.73495 | 0.14 | 72.4 | 2.68 | 948 | 35.3 | 0.035 | 0.09 | 2.7 | ||
| SJ.S 94 | 0.73353 | 0.26 | 71.4 | 2.38 | 531 | 15.2 | BDL | BDL | 2.7 | ||
| SJ.S 95 | 0.7317 | 0.04 | 68.0 | 2.25 | 505 | 18.7 | 0.00084 | BDL | 2.6 | ||
| SJ.S 96 | 0.73231 | BDL | 67.8 | 2.51 | 503 | 18.7 | 0.0049 | 0.22 | 2.5 | ||
| SJ.S 97 | 0.72985 | BDL | 68.1 | 2.45 | 799 | 24.8 | 0.0067 | BDL | 2.9 | ||
| SJ.S 99 | 0.73255 | 0.17 | 51.5 | 2.52 | 593 | 26.2 | 0.00072 | BDL | 2.6 | ||
| SJ.S 312 | 0.73388 | 0.06 | 62.1 | 1.95 | 574 | 25.0 | 0.0012 | 0.06 | 2.6 | ||
| SJ.S 319 | 0.73651 | 0.33 | 67.3 | 2.06 | 750 | 21.9 | 0.00045 | 0.078 | 2.7 | ||
| SJ.S 320 | 0.73201 | 0.36 | 66.8 | 2.19 | 553 | 15.9 | 0.0015 | BDL | 2.6 | ||
| SJ.S 321 | 0.73533 | 0.30 | 64.6 | 2.15 | 662 | 25.4 | BDL | 0.108 | 2.6 | ||
| SJ.S 322 | 0.735 | BDL | 60.3 | 2.37 | 779 | 25.0 | BDL | 0.068 | 2.5 | ||
| SJ.S 323 | 0.72852 | 0.06 | 63.1 | 2.13 | 508 | 16.3 | BDL | 0.069 | 2.7 | ||
| SJ.S 324 | 0.73445 | BDL | 57.9 | 2.17 | 681 | 29.9 | 0.00158 | 0.218 | 2.5 | ||
| SJ.S 325 | 0.73133 | 0.23 | 60.2 | 2.08 | 558 | 22.7 | 0.005 | BDL | 2.6 | ||
| SJ.S 326 | 0.7321 | 0.05 | 63.4 | 1.87 | 528 | 21.3 | BDL | BDL | 2.5 | ||
| SJ.S 328 | 0.73106 | 0.23 | 53.3 | 2.71 | 473 | 20.3 | 0.00064 | BDL | 2.7 | ||
| SJ.S 474 | 0.73292 | BDL | 56.4 | 2.61 | 756 | 23.1 | 0.00103 | 0.144 | 3.0 | ||
| SJ.S 477 | 0.72822 | BDL | 56.9 | 2.27 | 545 | 18.6 | 0.02 | 0.213 | 2.9 | ||
| SJ.S 479 | 0.73972 | 0.12 | 59.4 | 2.39 | 1002 | 39.6 | 0.0065 | BDL | 2.8 | ||
| SJ.S 480 | 0.7292 | 0.21 | 55.8 | 2.52 | 820 | 19.0 | 0.0016 | BDL | 2.8 | ||
| SJ.S 481 | 0.73074 | BDL | 55.2 | 1.96 | 831 | 27.0 | BDL | BDL | 2.8 | ||
| SJ.S 482 | 0.73111 | 0.06 | 55.0 | 2.67 | 725 | 21.9 | 0.0047 | BDL | 2.7 | ||
| SJ.S 483 | 0.73527 | BDL | 56.8 | 2.62 | 669 | 27.0 | 0.0016 | BDL | 2.9 | ||
| SJ.S 484 | 0.7306 | BDL | 65.2 | 2.5 | 929 | 34.9 | 0.00061 | 0.114 | 2.9 | ||
| SJ.S 485 | 0.73743 | 0.22 | 50.1 | 2.55 | 1127 | 39.7 | 0.00142 | BDL | 2.8 | ||
| SJ.S 486 | 0.73348 | 0.18 | 50.6 | 2.39 | 698 | 34.2 | BDL | BDL | 2.5 | ||
| LOD ± SD | 0.035 ± 0.006 | 0.345 ± 0.036 | 0.0591 ± 0.0035 | 0.003021 ± 0.000601 | 0.000626 ± 0.000090 | 0.000306 ± 0.000080 | 0.0214 ± 0.0060 | 0.023 ± 0.002 | |||
| LOQ ± SD | 0.104 ± 0.019 | 1.034 ± 0.107 | 0.1772 ± 0.0104 | 0.009063 ± 0.001802 | 0.001879 ± 0.000271 | 0.000917 ± 0.000240 | 0.0641 ± 0.0180 | 0.068 ± 0.007 |
References
- HELCOM. Red List Species Information Sheets (SIS) Fish. Available online: https://helcom.fi/wp-content/uploads/2019/08/HELCOM-RedList-All-SIS_Fish.pdf (accessed on 19 November 2018).
- Karlsson, L.; Karlström, Ö. The Baltic salmon (Salmo salar L.): Its history, present situation and future. Dana 1994, 10, 61–85. [Google Scholar]
- McCormick, S.D.; Hansen, L.P.; Quinn, T.P.; Saunders, R.L. Movement, migration, and smolting of Atlantic salmon (Salmo salar) life history. Can. J. Fish. Aquat. Sci. 1998, 55, 48–58. [Google Scholar] [CrossRef]
- Linnansaari, T.; Romakkaniemi, A.; Vähä, V.; Perä, I.; Cunjak, R.; Graham, B. Pysyvien isotooppien käyttö Tornionjoen eri vesistönosien lohen poikastuotannon arvioinnissa. [The Use of Stable Isotopes in the Assessment of Salmon Fry Production in Different Parts of the Tornionjoki Water Body]. Available online: https://tlinnans.ext.unb.ca/SIATornio_Linnansaarietal.pdf (accessed on 29 May 2023).
- Jutila, E.; Jokikokko, E.; Julkunen, M. The smolt run and postsmolt survival of Atlantic salmon, Salmo salar L., in relation to early summer water temperatures in the northern Baltic Sea. Ecol. Freshw. Fish. 2005, 14, 69–78. [Google Scholar] [CrossRef]
- Klemetsen, A.; Amundsen, P.-A.; Dempson, J.B.; Jonsson, B.; Jonsson, N.; O’Connell, M.F.; Mortensen, E. Atlantic salmon Salmo salar L., brown trout Salmo trutta L. and Arctic charr Salvelinus alpinus (L.): A review of aspects of their life histories. Ecol. Freshw. Fish. 2003, 12, 1–59. [Google Scholar] [CrossRef]
- ICES. Baltic Salmon and Trout assessment working group (WGBAST). ICES Sci. Rep. 2021, 3, 331. [Google Scholar] [CrossRef]
- Romakkaniemi, A.; Perä, I.; Karlsson, L.; Jutila, E.; Carlsson, O.; Pakarinen, T. Development of wild Atlantic salmon stocks in the rivers of the northern Baltic Sea in response to management measures. ICES J. Mar. Sci. 2003, 60, 329–342. [Google Scholar] [CrossRef]
- HELCOM. Salmon and Sea Trout Populations and Rivers in the Baltic Sea—HELCOM assessment of salmon (Salmo salar) and sea trout (Salmo trutta) populations and habitats in rivers flowing to the Baltic Sea. Balt. Sea Environ. Proc. 2011, 126A, 82. [Google Scholar]
- HELCOM. Abundance of Salmon Spawners and Smolt. HELCOM Core Indicator Report. Available online: www.helcom.fi/wp-content/uploads/2019/08/Abundance-of-salmon-spawners-and-smolt-HELCOM-core-indicator-2018.pdf (accessed on 8 August 2022).
- Jutila, E. From the River to the Open Sea—A Critical Life Phase of Young Atlantic Salmon Migrating from the Simojoki River. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 13 June 2008. [Google Scholar]
- Romakkaniemi, A.; Jokikokko, E.; Turunen, J.; Pakarinen, T.; Pulkkinen, H. Toimenpideohjelma Simojoen lohikannan elvyttämiseksi. [Action Programme for the Recovery of the River Simojoki Salmon Population]. Maa-Ja Metsätalousministeriö 2020, 12, 52. [Google Scholar]
- WATERINFO.fi. Available online: wwwi2.ymparisto.fi/i2/64/q6400410y/wqfi.html (accessed on 4 April 2023).
- Jokikokko, E.; Mäntyniemi, S. The survival of stocked Atlantic salmon smolts during sea run and the timing of migration in the river Simojoki, northern Finland. Aquaculture 2003, 219, 431–444. [Google Scholar] [CrossRef]
- Jokikokko, E.; Jutila, E. The effects of stocking with salmon parr, Salmo salar, on the smolt production in the River Simojoki, Northern Finland. In Stocking and Introduction of Fish; Cowx, I.G., Ed.; Blackwell Science Ltd.: Oxford, UK, 1998; pp. 35–45. [Google Scholar]
- Jutila, E.; Jokikokko, E.; Julkunen, M. Management of Atlantic salmon in the Simojoki river, northern Gulf of Bothnia: Effects of stocking and fishing regulation. Fish. Res. 2003, 64, 5–17. [Google Scholar] [CrossRef]
- Jokikokko, E. Atlantic Salmon (Salmo salar L.) Stocking in the Simojoki River as a Management Practice. Ph.D. Thesis, University of Oulu, Oulu, Finland, 24 November 2006. [Google Scholar]
- Jokikokko, E.; Jutila, E.; Kallio-Nyberg, I. Changes in smolt traits of Atlantic salmon (Salmo salar Linnaeus, 1758) and linkages to parr density and water temperature. J. Appl. Ichthyol. 2016, 32, 832–839. [Google Scholar] [CrossRef]
- Jutila, E.; Jokikokko, E.; Ikonen, E. Post-smolt migration of Atlantic salmon, Salmo salar L., from the Simojoki river to the Baltic Sea. J. Appl. Ichthyol. 2009, 25, 190–194. [Google Scholar] [CrossRef]
- Lilja, J.; Jaala, E.; Jokikokko, E.; Romakkaniemi, A. Simojoen nousulohien kaikuluotaustutkimukset vuosina 2003–2005. [Hydroacoustic assessment of salmon spawning migration into the River Simojoki in 2003–2005]. Kala-Ja Riistarapporteja 2006, 387, 29. [Google Scholar]
- Rasmussen, G.; Aarestrup, K.; Jepsen, N. Mortality of sea trout (Salmo trutta L.) and Atlantic salmon (Salmo salar L.) smolts during seaward migration through rivers and lakes in Denmark. ICES C.M. 1996, T:9, 1–14. [Google Scholar]
- Rohtla, M.; Vetemaa, M.; Svirgsden, R.; Taal, I.; Saks, L.; Kesler, M.; Verliin, A.; Saat, T. Using otolith 87Sr:86Sr as a natal chemical tag in the progeny of anadromous Baltic Sea pike (Esox lucius)—A pilot study. Boreal Environ. Res. 2014, 19, 379–386. [Google Scholar]
- Martin, J.; Bareille, G.; Berail, S.; Pécheyran, C.; Daverat, F.; Bru, N.; Tabouret, H.; Donard, O. Spatial and temporal variations in otolith chemistry and relationships with water chemistry: A useful tool to distinguish Atlantic salmon Salmo salar parr from different natal streams. J. Fish Biol. 2013, 82, 1556–1581. [Google Scholar] [CrossRef] [PubMed]
- Reader, J.M.; Spares, A.D.; Stokesbury, M.J.; Avery, T.; Dadswell, M.J. Elemental fingerprints of otoliths from smolt of Atlantic salmon, Salmo salar Linnaeus, 1758, from three maritime watersheds: Natural tag for stock discrimination. Proc. Nova Scotian Inst. Sci. 2015, 48, 91–123. [Google Scholar] [CrossRef]
- Veinott, G.; Porter, R. Using otolith microchemistry to distinguish Atlantic salmon (Salmo salar) parr from different natal streams. Fish. Res. 2005, 71, 349–355. [Google Scholar] [CrossRef]
- Engstedt, O.; Engkvist, R.; Larsson, P. Elemental fingerprinting in otoliths reveals natal homing of anadromous Baltic Sea pike (Esox lucius L.). Ecol. Freshw. Fish. 2014, 23, 313–321. [Google Scholar] [CrossRef]
- Gahagan, B.I.; Vokoun, J.C.; Whitledge, G.W.; Schultz, E.T. Evaluation of Otolith Microchemistry for Identifying Natal Origin of Anadromous River Herring in Connecticut. Mar. Coast. Fish. 2012, 4, 358–372. [Google Scholar] [CrossRef]
- Barbee, N.; Swearer, S. Characterizing natal source population signatures in the diadromous fish Galaxias maculatus, using embryonic otolith chemistry. Mar. Ecol. Prog. Ser. 2007, 343, 273–282. [Google Scholar] [CrossRef]
- Wells, B.K.; Rieman, B.E.; Clayton, J.L.; Horan, D.L.; Jones, C.M. Relationships between Water, Otolith, and Scale Chemistries of Westslope Cutthroat Trout from the Coeur d’Alene River, Idaho: The Potential Application of Hard-Part Chemistry to Describe Movements in Freshwater. Trans. Am. Fish. Soc. 2003, 132, 409–424. [Google Scholar] [CrossRef]
- Martin, J.P.; Bareille, G.; Berail, S.; Pécheyran, C.; Gueraud, F.; Lange, F.; Daverat, F.; Bru, N.; Beall, E.P.; Barracou, D.; et al. Persistence of a southern Atlantic salmon population: Diversity of natal origins from otolith elemental and Sr isotopic signatures. Can. J. Fish. Aquat. Sci. 2013, 70, 182–197. [Google Scholar] [CrossRef]
- Shrimpton, J.M.; Warren, K.D.; Todd, N.L.; McRae, C.J.; Glova, G.J.; Telmer, K.; Clarke, A.D. Freshwater movement patterns by juvenile Pacific salmon Oncorhynchus spp. before they migrate to the ocean: Oh the places you’ll go! J. Fish Biol. 2014, 85, 987–1004. [Google Scholar] [CrossRef] [PubMed]
- Bourret, S.L.; Kennedy, B.P.; Caudill, C.C.; Chittaro, P.M. Using otolith chemical and structural analysis to investigate reservoir habitat use by juvenile Chinook salmon Oncorhynchus tshawytscha. J. Fish Biol. 2014, 85, 1507–1525. [Google Scholar] [CrossRef]
- Miyan, K.; Khan, M.A.; Patel, D.K.; Khan, S.; Prasad, S. Otolith fingerprints reveal stock discrimination of Sperata seenghala inhabiting the Gangetic river system. Ichthyol. Res. 2016, 63, 294–301. [Google Scholar] [CrossRef]
- Olley, R.; Young, R.G.; Closs, G.P.; Kristensen, E.A.; Bickel, T.O.; Deans, N.A.; Davey, L.N.; Eggins, S.M. Recruitment sources of brown trout identified by otolith trace element signatures. N. Z. J. Mar. Freshw. Res. 2011, 45, 395–411. [Google Scholar] [CrossRef]
- Turcotte, L.A.; Shrimpton, J.M. Assessment of spawning site fidelity in interior Fraser River coho salmon Oncorhynchus kisutch using otolith microchemistry, in British Columbia, Canada. J. Fish Biol. 2020, 97, 1833–1841. [Google Scholar] [CrossRef]
- Panfili, J.; de Pontual, H.; Troadec, H.; Wright, P. Manual of Fish Sclerochronology; IFREMER-IRD: Brest, France, 2002; p. 464. ISBN 2-84433-067-3. [Google Scholar]
- Campana, S.E. Chemistry and composition of fish otoliths: Pathways, mechanisms and applications. Mar. Ecol. Prog. Ser. 1999, 188, 263–297. [Google Scholar] [CrossRef]
- Hüssy, K.; Limburg, K.E.; de Pontual, H.; Thomas, O.R.; Cook, P.K.; Heimbrand, Y.; Blass, M.; Sturrock, A.M. Trace Element Patterns in Otoliths: The Role of Biomineralization. Rev. Fish. Sci. Aquac. 2020, 29, 445–477. [Google Scholar] [CrossRef]
- Nazir, A.; Khan, M.A. Using otoliths for fish stock discrimination: Status and challenges. Acta. Ichthyol. Piscat. 2021, 51, 199–218. [Google Scholar] [CrossRef]
- Walther, B.D.; Thorrold, S.R. Continental-scale variation in otolith geochemistry of juvenile American shad (Alosa sapidissima). Can. J. Fish. Aquat. Sci. 2008, 65, 2623–2635. [Google Scholar] [CrossRef]
- Löfvendahl, R.; Åberg, G.; Hamilton, P.J. Strontium in rivers of the Baltic Basin. Aquat. Sci. 1990, 52, 315–329. [Google Scholar] [CrossRef]
- Åberg, F.; Wickman, F.E. Variations of 87Sr/86Sr in water from streams discharging into the Bothnian Bay, Baltic Sea. Nord. Hydrol. 1987, 18, 33–42. [Google Scholar] [CrossRef]
- Rohtla, M.; Svirgsden, R.; Verliin, A.; Rumvolt, K.; Matetski, L.; Hommik, K.; Saks, L.; Vetemaa, M. Developing novel means for unravelling population structure, provenance and migration patterns of European whitefish Coregonus lavaretus s.l. in the Baltic Sea. Fish. Res. 2017, 187, 47–57. [Google Scholar] [CrossRef]
- Matetski, L.; Rohtla, M.; Svirgsden, R.; Kesler, M.; Saks, L.; Taal, I.; Hommik, K.; Paiste, P.; Kielman-Schmitt, M.; Kooijman, E.; et al. Variability in stream water chemistry and brown trout (Salmo trutta L.) parr otolith microchemistry on different spatial scales. Ecol. Freshw. Fish. 2022, 31, 438–453. [Google Scholar] [CrossRef]
- Mercier, L.; Darnaude, A.M.; Bruguier, O.; Vasconcelos, R.P.; Cabral, H.N.; Costa, M.J.; Lara, M.R.; Jones, D.L.; Mouillot, D. Selecting statistical models and variable combinations for optimal classification using otolith microchemistry. Ecol. Appl. 2011, 21, 1352–1364. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Díaz-Uriarte, R.; Andrés, S.A. Gene selection and classification of microarray data using random forest. BMC Bioinform. 2006, 73, 3. [Google Scholar] [CrossRef]
- Finnish Environment Institute. OIVA—Ympäristö-ja Paikkatietopalvelu Asiantuntijoille. [OIVA—Environmental and Geographic Information Service for Experts]. Available online: https://wwwp2.ymparisto.fi/scripts/oiva.asp (accessed on 22 February 2011).
- National Land Survey of Finland (Helsinki, Finland). Permission 53/MML/10, 2023.
- Müller, W.; Shelley, M.; Miller, P.; Broude, S.V. Initial performance metrics of a new custom-designed ArF excimer LA-ICPMS system coupled to a two-volume laser-ablation cell. J. Anal. At. Spectrom. 2009, 24, 209–214. [Google Scholar] [CrossRef]
- Kalish, J.M. Use of Otolith Microchemistry to Distinguish the Progeny of Sympatric Anadromous and Non-anadromous Salmonids. Fish. Bull. 1990, 88, 657–666. [Google Scholar]
- Meekan, M.G.; Dodson, J.J.; Good, S.P.; Ryan, D.A.J. Otolith and fish size relationships, measurement error, and size-selective mortality during the early life of Atlantic salmon. Can. J. Fish. Aquat. Sci. 1998, 55, 1663–1673. [Google Scholar] [CrossRef]
- Rankenburg, K.; Lassiter, J.C.; Brey, G.P. Origin of megacrysts in volcanic rocks of the Cameroon volcanic chain—Constraints on magma genesis and crustal contamination. Contrib. Mineral. Petrol. 2004, 147, 129–144. [Google Scholar] [CrossRef]
- Yang, Z.; Fryer, B.; Longerich, H.P.; Gagnon, J.E.; Samson, I. 785 nm femtosecond laser ablation for improved precision and reduction of interferences in Sr isotope analyses using MC-ICP-MS. J. Anal. At. Spectrom. 2011, 26, 341–351. [Google Scholar] [CrossRef]
- Thirlwall, M. Long-term reproducibility of multicollector Sr and Nd isotope ratio analysis. Chem. Geol. 1991, 94, 85–104. [Google Scholar] [CrossRef]
- Van Achterbergh, E.; Ryan, C.; Jackson, S.; Griffin, W. GLITTER: Data reduction software for LA-ICP-MS. Mineral. Assoc. Can. 2001, 29, 239–243. [Google Scholar]
- Liaw, A.; Wiener, M.C. Classification and regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Geochemical Atlas of Europe. Available online: http://weppi.gtk.fi/publ/foregsatlas/index.php (accessed on 28 August 2022).
- Zimmerman, C.E.; Edwards, G.W.; Perry, K. Maternal origin and migratory history of steelhead and rainbow trout captured in rivers of the Central Valley. Calif. Trans. Am. Fish. Soc. 2009, 138, 280–291. [Google Scholar] [CrossRef]

| Mean Decrease in Gini | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ni | Si | As | 87Sr/86Sr | Sr | Ba | ntree | mtry | OOB% | |
| RF1 | 1.89 | 2.11 | 2.92 | 4.15 | 4.23 | 4.08 | 801 | 2 | 43.3 |
| RF2 | 2.20 | 3.32 | 4.61 | 4.59 | 4.60 | 501 | 2 | 46.7 | |
| RF3 | 3.45 | 5.18 | 5.31 | 5.40 | 1001 | 2 | 36.7 | ||
| RF4 | 6.34 | 6.52 | 6.50 | 501 | 1 | 36.7 | |||
| RF5 | 9.13 | 10.10 | 1501 | 1 | 43.3 | ||||
| Sampled Rapid | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|
| 1 | 72.89 | 19.23 | 29.58 | 359.14 | 220.42 |
| 2 | 59.56 | 43.55 | 430.68 | 292.34 | |
| 3 | 17.64 | 371.17 | 232.96 | ||
| 4 | 387.43 | 248.95 | |||
| 5 | 138.88 |
| Discriminant Function | Eigenvalue | % of Variance | Canonical Correlation | 87Sr/86Sr | Si | Sr | Ba |
|---|---|---|---|---|---|---|---|
| 1 | 3.288 | 98.5 | 0.876 | 0.757 | 0.254 | 0.464 | 0.389 |
| 2 | 0.05 | 1.5 | 0.218 | −0.495 | 0.902 | 0.31 | −0.133 |
| Predicted Nursery Area | |||||
|---|---|---|---|---|---|
| Sampled Nursery Area | Lower | Middle | Upper | % Correctly Classified | |
| DFA | Lower | 3 | 7 | 0 | 30 |
| Middle | 6 | 4 | 0 | 40 | |
| Upper | 0 | 1 | 9 | 90 | |
| RF3 | Lower | 4 | 6 | 0 | 40 |
| Middle | 5 | 5 | 0 | 50 | |
| Upper | 0 | 0 | 10 | 100 | |
| ID | DFA | RF3 |
|---|---|---|
| SJ.S 83 | 1 | 1 |
| SJ.S 90 | 2 | 1 |
| SJ.S 91 | 2 | 1 |
| SJ.S 92 | 2 | 2 |
| SJ.S 93 | 3 | 3 |
| SJ.S 94 | 2 | 1 |
| SJ.S 95 | 2 | 1 |
| SJ.S 96 | 2 | 1 |
| SJ.S 97 | 2 | 2 |
| SJ.S 99 | 2 | 2 |
| SJ.S 312 | 2 | 2 |
| SJ.S 319 | 2 | 2 |
| SJ.S 320 | 2 | 1 |
| SJ.S 321 | 2 | 2 |
| SJ.S 322 | 2 | 2 |
| SJ.S 323 | 1 | 1 |
| SJ.S 324 | 2 | 2 |
| SJ.S 325 | 2 | 1 |
| SJ.S 326 | 2 | 1 |
| SJ.S 328 | 2 | 1 |
| SJ.S 474 | 2 | 2 |
| SJ.S 477 | 1 | 1 |
| SJ.S 479 | 3 | 3 |
| SJ.S 480 | 2 | 1 |
| SJ.S 481 | 2 | 1 |
| SJ.S 482 | 2 | 1 |
| SJ.S 483 | 2 | 2 |
| SJ.S 484 | 2 | 1 |
| SJ.S 485 | 3 | 3 |
| SJ.S 486 | 2 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Finnäs, V.; Jokikokko, E.; Lill, J.-O.; Lahaye, Y.; Hägerstrand, H.; Lindström, K. Potential of Otolith Microchemistry to Distinguish Nursery Areas of Salmon within River Simojoki. Fishes 2023, 8, 332. https://doi.org/10.3390/fishes8060332
Finnäs V, Jokikokko E, Lill J-O, Lahaye Y, Hägerstrand H, Lindström K. Potential of Otolith Microchemistry to Distinguish Nursery Areas of Salmon within River Simojoki. Fishes. 2023; 8(6):332. https://doi.org/10.3390/fishes8060332
Chicago/Turabian StyleFinnäs, Viktor, Erkki Jokikokko, Jan-Olof Lill, Yann Lahaye, Henry Hägerstrand, and Kai Lindström. 2023. "Potential of Otolith Microchemistry to Distinguish Nursery Areas of Salmon within River Simojoki" Fishes 8, no. 6: 332. https://doi.org/10.3390/fishes8060332
APA StyleFinnäs, V., Jokikokko, E., Lill, J.-O., Lahaye, Y., Hägerstrand, H., & Lindström, K. (2023). Potential of Otolith Microchemistry to Distinguish Nursery Areas of Salmon within River Simojoki. Fishes, 8(6), 332. https://doi.org/10.3390/fishes8060332






