DUSP2 Deletion Inhibits Macrophage Migration by Inhibiting ERK Activation in Zebrafish
Abstract
1. Introduction
2. Materials and Methods
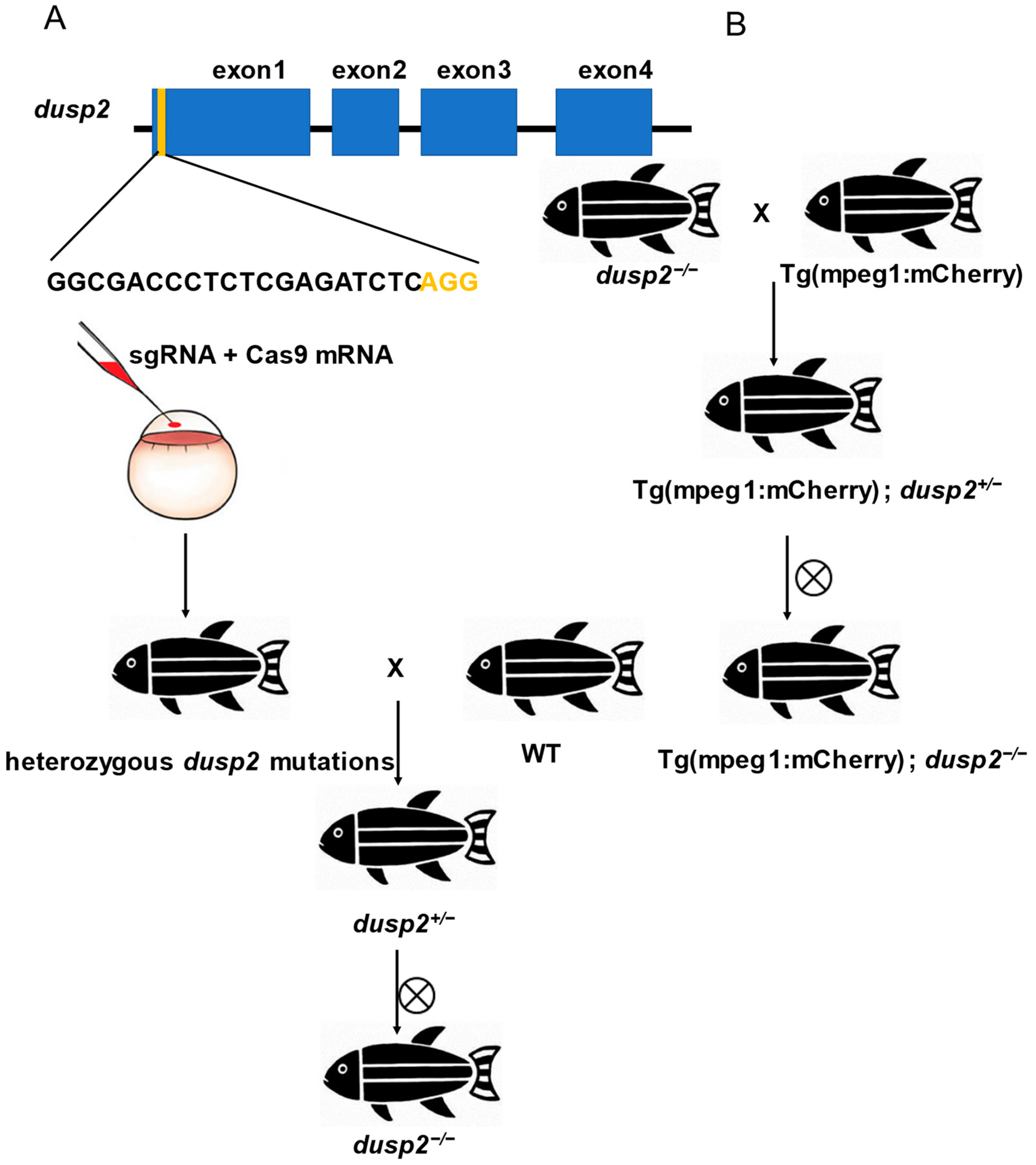
2.1. Zebrafish Lines
2.2. Tail Fin Injury
2.3. In Vivo Imaging
2.4. Drug Treatment
2.5. Quantitative Real-Time PCR
2.6. Western Blot
2.7. Statistical Analysis
3. Results
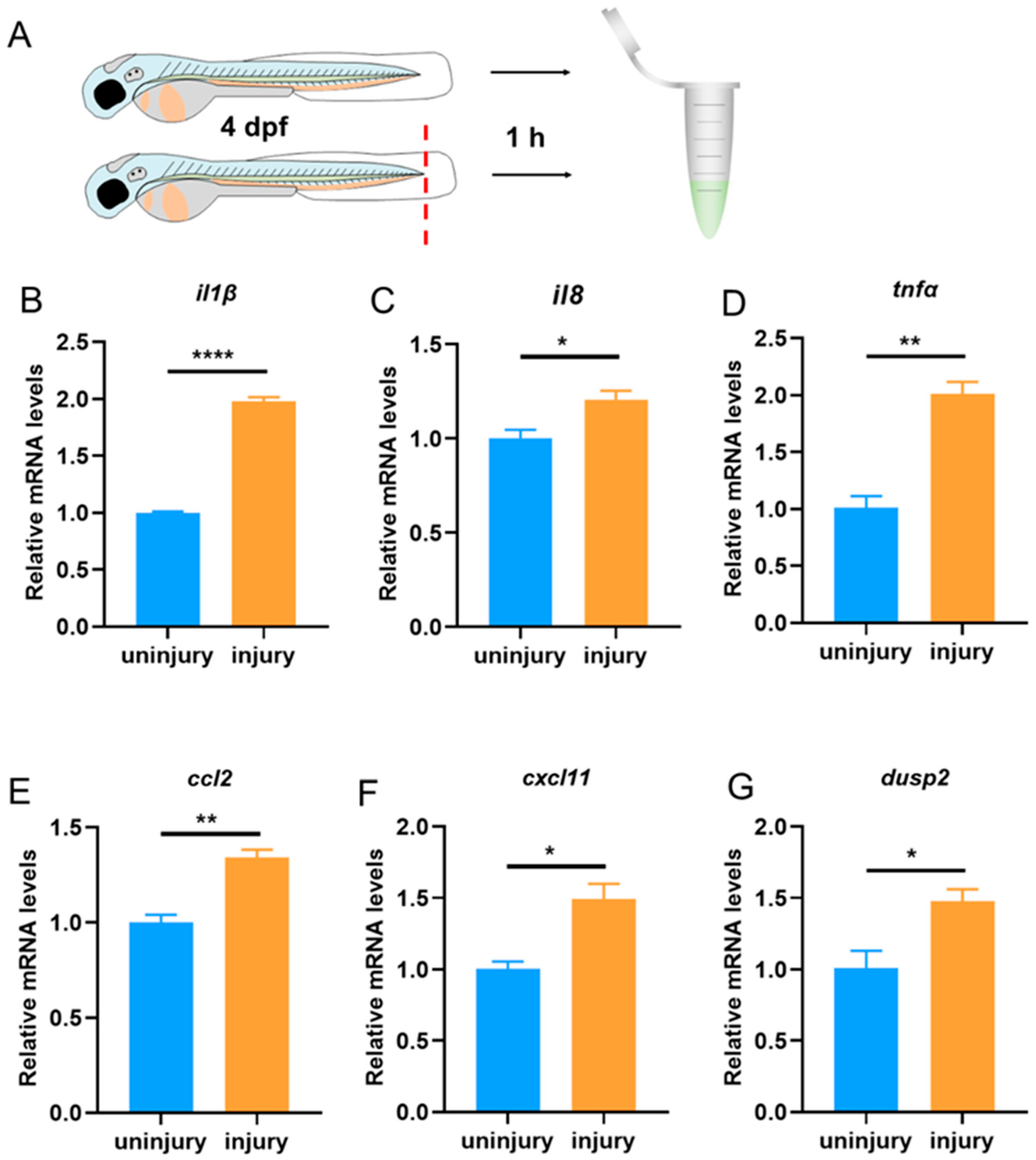
3.1. The Expression of dusp2 Was Significantly Up-Regulated in Acute Inflammation Induced by Tail Fin Injury
3.2. DUSP2 Deletion Significantly Decreased the Expression of Pro-Inflammatory Cytokines and Chemokines after Tail Fin Injury
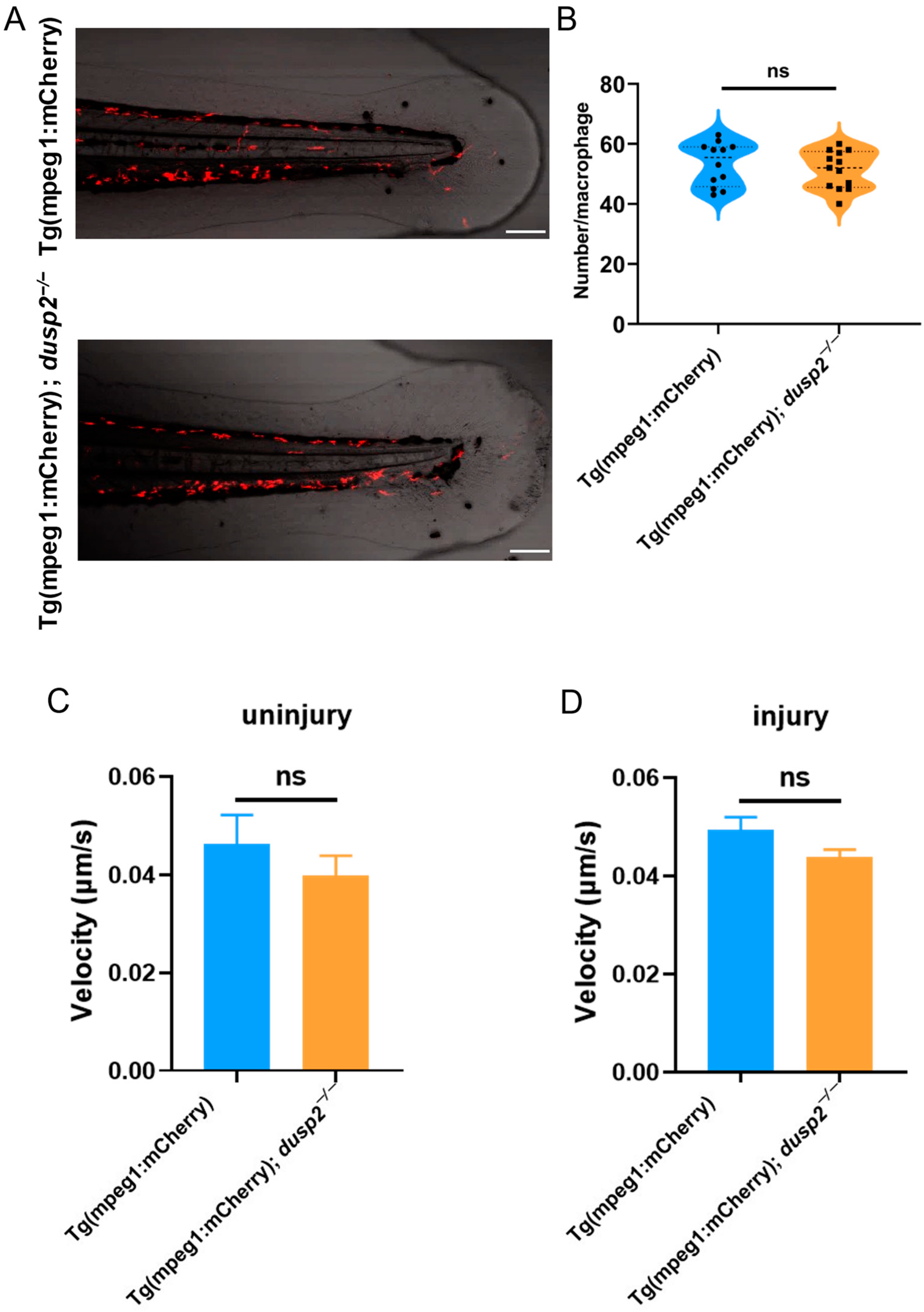
3.3. DUSP2 Deletion Inhibited the Migration of Macrophages to the Injury Site
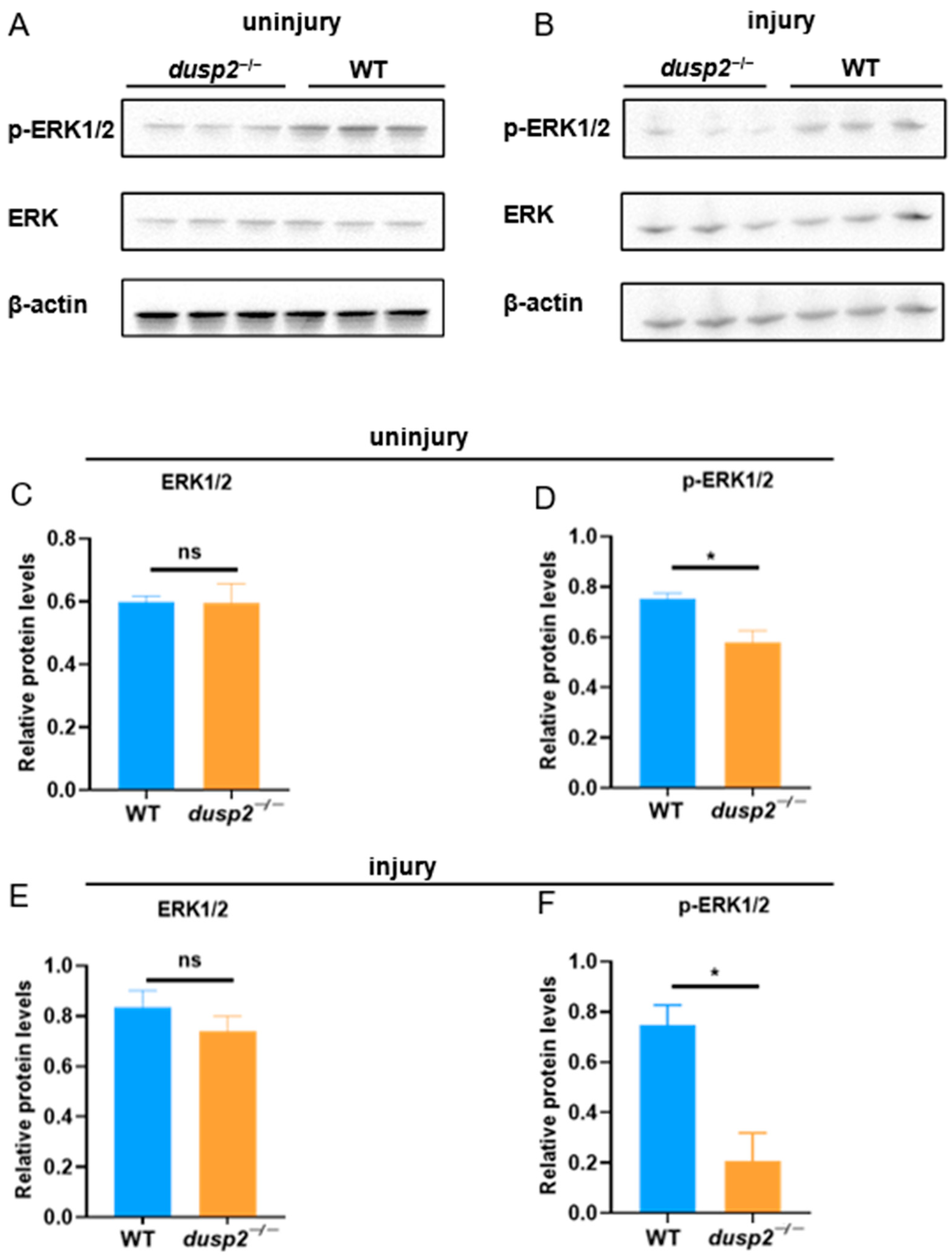
3.4. DUSP2 Deletion Inhibited ERK Protein Phosphorylation
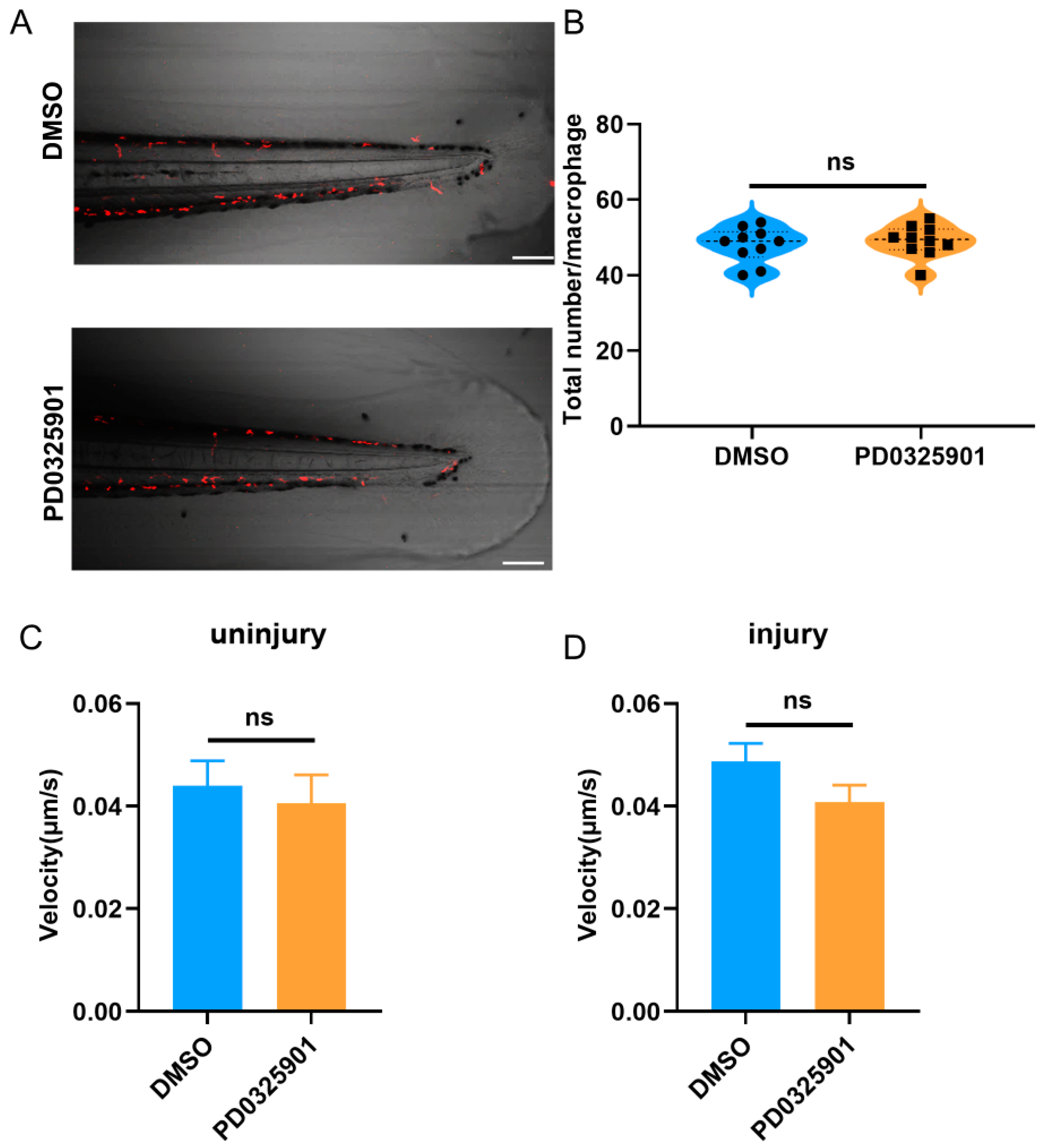
3.5. ERK Inhibitor Inhibited the Migration of Macrophages
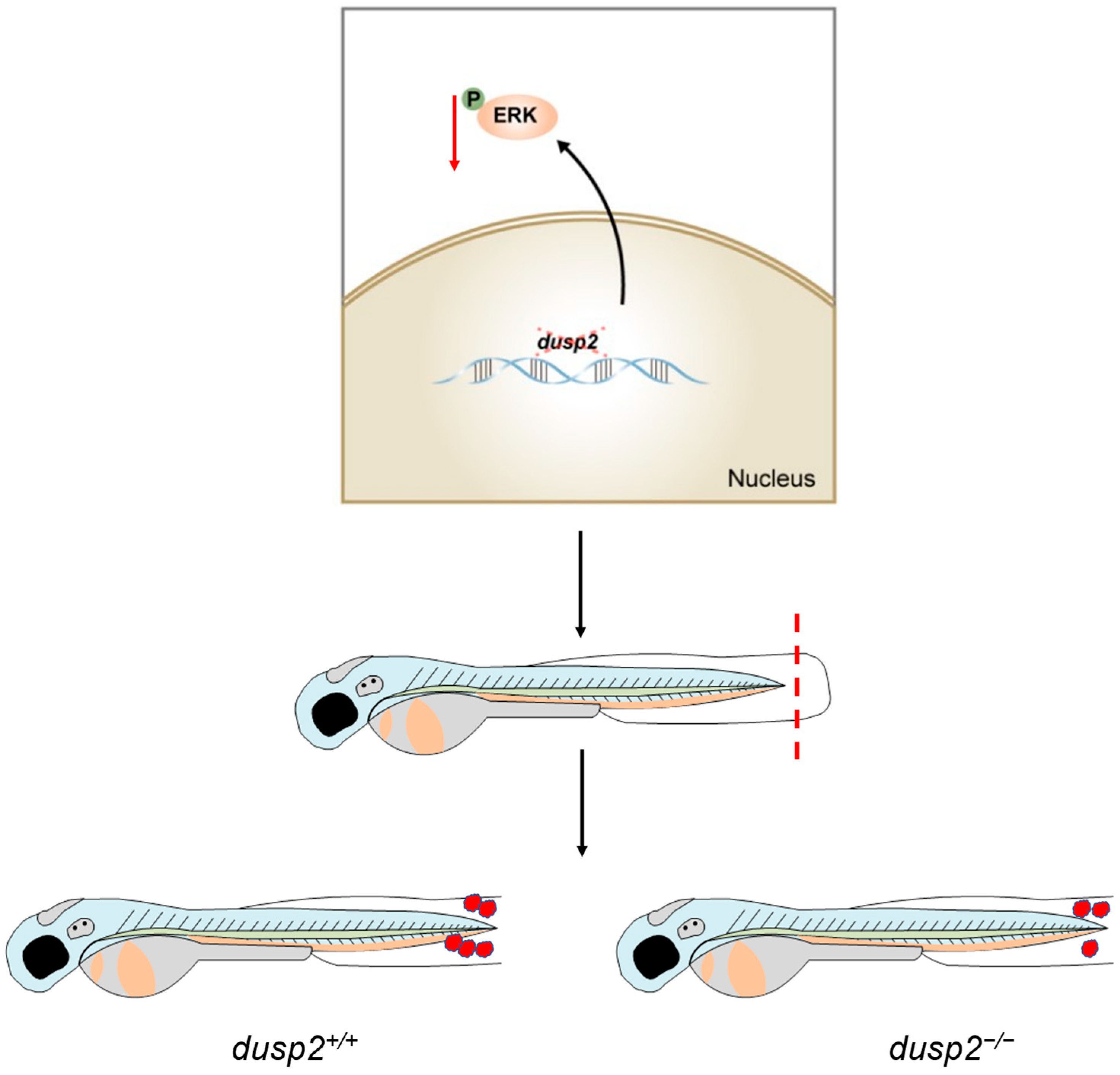
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Trepat, X.; Chen, Z.; Jacobson, K. Cell migration. Compr. Physiol. 2012, 2, 2369–2392. [Google Scholar] [CrossRef]
- Sheu, K.M.; Hoffmann, A. Functional Hallmarks of Healthy Macrophage Responses: Their Regulatory Basis and Disease Relevance. Annu. Rev. Immunol. 2022, 40, 295–321. [Google Scholar] [CrossRef] [PubMed]
- Navegantes, K.C.; de Souza Gomes, R.; Pereira, P.A.T.; Czaikoski, P.G.; Azevedo, C.H.M.; Monteiro, M.C. Immune modulation of some autoimmune diseases: The critical role of macrophages and neutrophils in the innate and adaptive immunity. J. Transl. Med. 2017, 15, 36. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.J.; Liu, J.X.; Liu, M.N.; Yao, Y.D.; Liu, Z.Q.; Liu, L.; He, H.H.; Zhou, H. Macrophage 3D migration: A potential therapeutic target for inflammation and deleterious progression in diseases. Pharmacol. Res. 2021, 167, 105563. [Google Scholar] [CrossRef]
- Lang, R.; Hammer, M.; Mages, J. DUSP meet immunology: Dual specificity MAPK phosphatases in control of the inflammatory response. J. Immunol. 2006, 177, 7497–7504. [Google Scholar] [CrossRef]
- Wu, M.H.; Lin, S.C.; Hsiao, K.Y.; Tsai, S.J. Hypoxia-inhibited dual-specificity phosphatase-2 expression in endometriotic cells regulates cyclooxygenase-2 expression. J. Pathol. 2011, 225, 390–400. [Google Scholar] [CrossRef]
- Loesch, M.; Chen, G. The p38 MAPK stress pathway as a tumor suppressor or more? Front. Biosci. 2008, 13, 3581–3593. [Google Scholar] [CrossRef]
- Ji, R.R.; Gereau, R.W.t.; Malcangio, M.; Strichartz, G.R. MAP kinase and pain. Brain Res. Rev. 2009, 60, 135–148. [Google Scholar] [CrossRef]
- Jin, Z.; Sheng, H.; Wang, S.; Wang, Y.; Cheng, Y. Network pharmacology study to reveal active compounds of Qinggan Yin formula against pulmonary inflammation by inhibiting MAPK activation. J. Ethnopharmacol. 2022, 296, 115513. [Google Scholar] [CrossRef]
- Kciuk, M.; Gielecińska, A.; Budzinska, A.; Mojzych, M.; Kontek, R. Metastasis and MAPK Pathways. Int. J. Mol. Sci. 2022, 23, 3847. [Google Scholar] [CrossRef] [PubMed]
- Jeffrey, K.L.; Brummer, T.; Rolph, M.S.; Liu, S.M.; Callejas, N.A.; Grumont, R.J.; Gillieron, C.; Mackay, F.; Grey, S.; Camps, M.; et al. Positive regulation of immune cell function and inflammatory responses by phosphatase PAC-1. Nat. Immunol. 2006, 7, 274–283. [Google Scholar] [CrossRef]
- Dan, L.; Liu, L.; Sun, Y.; Song, J.; Yin, Q.; Zhang, G.; Qi, F.; Hu, Z.; Yang, Z.; Zhou, Z.; et al. The phosphatase PAC1 acts as a T cell suppressor and attenuates host antitumor immunity. Nat. Immunol. 2020, 21, 287–297. [Google Scholar] [CrossRef]
- Lu, D.; Liu, L.; Ji, X.; Gao, Y.; Chen, X.; Liu, Y.; Liu, Y.; Zhao, X.; Li, Y.; Li, Y.; et al. The phosphatase DUSP2 controls the activity of the transcription activator STAT3 and regulates TH17 differentiation. Nat. Immunol. 2015, 16, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Ellett, F.; Pase, L.; Hayman, J.W.; Andrianopoulos, A.; Lieschke, G.J. mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood 2011, 117, e49–e56. [Google Scholar] [CrossRef] [PubMed]
- Shao, G.J.; Wang, X.L.; Wei, M.L.; Ren, D.L.; Hu, B. DUSP2 deletion with CRISPR/Cas9 promotes Mauthner cell axonal regeneration at the early stage of zebrafish. Neural Regen. Res. 2023, 18, 577–581. [Google Scholar] [CrossRef] [PubMed]
- Stockhammer, O.W.; Zakrzewska, A.; Hegedûs, Z.; Spaink, H.P.; Meijer, A.H. Transcriptome profiling and functional analyses of the zebrafish embryonic innate immune response to Salmonella infection. J. Immunol. 2009, 182, 5641–5653. [Google Scholar] [CrossRef]
- Brugman, S. The zebrafish as a model to study intestinal inflammation. Dev. Comp. Immunol. 2016, 64, 82–92. [Google Scholar] [CrossRef]
- Novoa, B.; Figueras, A. Zebrafish: Model for the study of inflammation and the innate immune response to infectious diseases. Adv. Exp. Med. Biol. 2012, 946, 253–275. [Google Scholar] [CrossRef]
- Jiang, T.; Gong, Y.; Zhang, W.; Qiu, J.; Zheng, X.; Li, Z.; Yang, G.; Hong, Z. PD0325901, an ERK inhibitor, attenuates RANKL-induced osteoclast formation and mitigates cartilage inflammation by inhibiting the NF-κB and MAPK pathways. Bioorg. Chem. 2022, 132, 106321. [Google Scholar] [CrossRef]
- Nguyen-Chi, M.; Laplace-Builhé, B.; Travnickova, J.; Luz-Crawford, P.; Tejedor, G.; Lutfalla, G.; Kissa, K.; Jorgensen, C.; Djouad, F. TNF signaling and macrophages govern fin regeneration in zebrafish larvae. Cell. Death Dis. 2017, 8, e2979. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Tolmeijer, S.; Oskam, J.M.; Tonkens, T.; Meijer, A.H.; Schaaf, M.J.M. Glucocorticoids inhibit macrophage differentiation towards a pro-inflammatory phenotype upon wounding without affecting their migration. Dis. Model. Mech. 2019, 12, dmm037887. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.L.; Li, Y.J.; Hu, B.B.; Wang, H.; Hu, B. Melatonin regulates the rhythmic migration of neutrophils in live zebrafish. J. Pineal Res. 2015, 58, 452–460. [Google Scholar] [CrossRef]
- Li, L.; Yan, B.; Shi, Y.Q.; Zhang, W.Q.; Wen, Z.L. Live imaging reveals differing roles of macrophages and neutrophils during zebrafish tail fin regeneration. J. Biol. Chem. 2012, 287, 25353–25360. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, L.; Sun, S.; Tang, L.S.; He, S.M.; Chen, A.Q.; Yao, L.N.; Ren, D.L. Cordycepin inhibits inflammatory responses through suppression of ERK activation in zebrafish. Dev. Comp. Immunol. 2021, 124, 104178. [Google Scholar] [CrossRef]
- van den Bos, R.; Cromwijk, S.; Tschigg, K.; Althuizen, J.; Zethof, J.; Whelan, R.; Flik, G.; Schaaf, M. Early Life Glucocorticoid Exposure Modulates Immune Function in Zebrafish (Danio rerio) Larvae. Front. Immunol. 2020, 11, 727. [Google Scholar] [CrossRef]
- Bohaud, C.; Johansen, M.D.; Jorgensen, C.; Ipseiz, N.; Kremer, L.; Djouad, F. The Role of Macrophages During Zebrafish Injury and Tissue Regeneration Under Infectious and Non-Infectious Conditions. Front. Immunol. 2021, 12, 707824. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Whelan, E.C.; Guan, X.; Deng, B.; Wang, S.; Sun, J.; Avarbock, M.R.; Wu, X.; Brinster, R.L. FGF9 promotes mouse spermatogonial stem cell proliferation mediated by p38 MAPK signalling. Cell. Prolif. 2021, 54, e12933. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Zhao, S.; Tan, Y.; Pan, S.; An, W.; Chen, Q.; Wang, X.; Xu, H. The SKA3-DUSP2 Axis Promotes Gastric Cancer Tumorigenesis and Epithelial-Mesenchymal Transition by Activating the MAPK/ERK Pathway. Front. Pharmacol. 2022, 13, 777612. [Google Scholar] [CrossRef] [PubMed]
- Dong, N.; Li, X.; Xue, C.; Zhang, L.; Wang, C.; Xu, X.; Shan, A. Astragalus polysaccharides alleviates LPS-induced inflammation via the NF-κB/MAPK signaling pathway. J. Cell. Physiol. 2020, 235, 5525–5540. [Google Scholar] [CrossRef]
- Lancaster, G.I.; Kraakman, M.J.; Kammoun, H.L.; Langley, K.G.; Estevez, E.; Banerjee, A.; Grumont, R.J.; Febbraio, M.A.; Gerondakis, S. The dual-specificity phosphatase 2 (DUSP2) does not regulate obesity-associated inflammation or insulin resistance in mice. PLoS ONE 2014, 9, e111524. [Google Scholar] [CrossRef]
- Sun, Y.; Liu, W.Z.; Liu, T.; Feng, X.; Yang, N.; Zhou, H.F. Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. Res. 2015, 35, 600–604. [Google Scholar] [CrossRef]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed]
- Decean, H.P.; Brie, I.C.; Tatomir, C.B.; Perde-Schrepler, M.; Fischer-Fodor, E.; Virag, P. Targeting MAPK (p38, ERK, JNK) and inflammatory CK (GDF-15, GM-CSF) in UVB-Activated Human Skin Cells with Vitis vinifera Seed Extract. J. Environ. Pathol. Toxicol. Oncol. 2018, 37, 261–272. [Google Scholar] [CrossRef]
- Carmo, A.A.; Costa, B.R.; Vago, J.P.; de Oliveira, L.C.; Tavares, L.P.; Nogueira, C.R.; Ribeiro, A.L.; Garcia, C.C.; Barbosa, A.S.; Brasil, B.S.; et al. Plasmin induces in vivo monocyte recruitment through protease-activated receptor-1-, MEK/ERK-, and CCR2-mediated signaling. J. Immunol. 2014, 193, 3654–3663. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Kim, S.; Oh, Y.; Moniruzzaman, M.; Lee, G.; Cho, J. Effect of CCL2 on BV2 microglial cell migration: Involvement of probable signaling pathways. Cytokine 2016, 81, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Samson, S.C.; Khan, A.M.; Mendoza, M.C. ERK signaling for cell migration and invasion. Front. Mol. Biosci. 2022, 9, 998475. [Google Scholar] [CrossRef]
- Zhou, S.L.; Hu, Z.Q.; Zhou, Z.J.; Dai, Z.; Wang, Z.; Cao, Y.; Fan, J.; Huang, X.W.; Zhou, J. miR-28-5p-IL-34-macrophage feedback loop modulates hepatocellular carcinoma metastasis. Hepatology 2016, 63, 1560–1575. [Google Scholar] [CrossRef]
- Hamamura, K.; Nishimura, A.; Chen, A.; Takigawa, S.; Sudo, A.; Yokota, H. Salubrinal acts as a Dusp2 inhibitor and suppresses inflammation in anti-collagen antibody-induced arthritis. Cell. Signal. 2015, 27, 828–835. [Google Scholar] [CrossRef]
- Dong, W.; Li, N.; Pei, X.; Wu, X. Differential expression of DUSP2 in left- and right-sided colon cancer is associated with poor prognosis in colorectal cancer. Oncol. Lett. 2018, 15, 4207–4214. [Google Scholar] [CrossRef]
- Lo Nigro, C.; Vivenza, D.; Denaro, N.; Lattanzio, L.; Fortunato, M.; Crook, T.; Merlano, M.C. DUSP2 methylation is a candidate biomarker of outcome in head and neck cancer. Ann. Transl. Med. 2018, 6, 271. [Google Scholar] [CrossRef] [PubMed]
- Morente, V.; Pérez-Sen, R.; Ortega, F.; Huerta-Cepas, J.; Delicado, E.G.; Miras-Portugal, M.T. Neuroprotection elicited by P2Y13 receptors against genotoxic stress by inducing DUSP2 expression and MAPK signaling recovery. Biochim. Biophys. Acta 2014, 1843, 1886–1898. [Google Scholar] [CrossRef] [PubMed]
- Wiessner, C. The dual specificity phosphatase PAC-1 is transcriptionally induced in the rat brain following transient forebrain ischemia. Brain Res. Mol. Brain Res. 1995, 28, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Talsma, A.D.; Niemi, J.P.; Pachter, J.S.; Zigmond, R.E. The primary macrophage chemokine, CCL2, is not necessary after a peripheral nerve injury for macrophage recruitment and activation or for conditioning lesion enhanced peripheral regeneration. J. Neuroinflamm. 2022, 19, 179. [Google Scholar] [CrossRef]
- Xie, Y.; Meijer, A.H.; Schaaf, M.J.M. Modeling Inflammation in Zebrafish for the Development of Anti-inflammatory Drugs. Front. Cell Dev. Biol. 2020, 8, 620984. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, S.; Borau, C.; García-Aznar, J.M.; Gonzalo-Asensio, J. A microfluidic-based analysis of 3D macrophage migration after stimulation by Mycobacterium, Salmonella and Escherichia. BMC Microbiol. 2022, 22, 211. [Google Scholar] [CrossRef]
- Shiraishi, A.; Uruno, T.; Sanematsu, F.; Ushijima, M.; Sakata, D.; Hara, T.; Fukui, Y. DOCK8 Protein Regulates Macrophage Migration through Cdc42 Protein Activation and LRAP35a Protein Interaction. J. Biol. Chem. 2017, 292, 2191–2202. [Google Scholar] [CrossRef]
- Wang, Y.; Su, J.; Yuan, B.; Fu, D.; Niu, Y.; Yue, D. The role of C1QBP in CSF-1-dependent PKCζ activation and macrophage migration. Exp. Cell. Res. 2018, 362, 11–16. [Google Scholar] [CrossRef]
- Gui, P.; Labrousse, A.; Van Goethem, E.; Besson, A.; Maridonneau-Parini, I.; Le Cabec, V. Rho/ROCK pathway inhibition by the CDK inhibitor p27(kip1) participates in the onset of macrophage 3D-mesenchymal migration. J. Cell Sci. 2014, 127 Pt 18, 4009–4023. [Google Scholar] [CrossRef]
- Zhang, L.; Xia, X.; Zhang, M.; Wang, Y.; Xing, G.; Yin, X.; Song, L.; He, F.; Zhang, L. Integrated analysis of genomics and proteomics reveals that CKIP-1 is a novel macrophage migration regulator. Biochem. Biophys. Res. Commun. 2013, 436, 382–387. [Google Scholar] [CrossRef]
- Gao, W.J.; Liu, J.X.; Xie, Y.; Luo, P.; Liu, Z.Q.; Liu, L.; Zhou, H. Suppression of macrophage migration by down-regulating Src/FAK/P130Cas activation contributed to the anti-inflammatory activity of sinomenine. Pharmacol. Res. 2021, 167, 105513. [Google Scholar] [CrossRef] [PubMed]



| Primer Name | Primer Sequence (5′–3′) |
|---|---|
| il1β-qRT-PCR | Forward: GTACTCAAGGAGATCAGCGG Reverse: CTCGGTGTCTTTCCTGTCCA |
| il6-qRT-PCR | Forward: TTTGAAGGGGTCAGGATCAG Reverse: TCATCACGCTGGAGAAGTTG |
| il8-qRT-PCR | Forward: CACTTAGGCAAAATGACCAGCA Reverse: AGACCTCTCAAGCTCATTCCTTC |
| tnfα-qRT-PCR | Forward: GCGCTTTTCTGAATCCTACG Reverse: TGCCCAGTCTGTCTCCTTC |
| dusp2-qRT-PCR | Forward: ATCGGCGACCCTCTCGAGATCTC Reverse: GACACCACGGAGCTCTTGGACCT |
| cxcl11-qRT-PCR | Forward: GGCACAGTGAAGAGCTCCAT Reverse: TGAGCTTGTTTGGGCAGTGT |
| ccl2-qRT-PCR | Forward: TCTGCACTAACCCGACTGAGA Reverse: CATCTTAGGCGCTGTCACCAG |
| β-actin-qRT-PCR | Forward: TCCGGTATGTGCAAAGCCGG Reverse: CCACATCTGCTGGAAGGTGG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.-J.; Wang, X.-L.; Shi, L.-Y.; Wang, Z.-Y.; Zhao, Z.-A.; Ge, S.-C.; Hu, B. DUSP2 Deletion Inhibits Macrophage Migration by Inhibiting ERK Activation in Zebrafish. Fishes 2023, 8, 310. https://doi.org/10.3390/fishes8060310
Li Y-J, Wang X-L, Shi L-Y, Wang Z-Y, Zhao Z-A, Ge S-C, Hu B. DUSP2 Deletion Inhibits Macrophage Migration by Inhibiting ERK Activation in Zebrafish. Fishes. 2023; 8(6):310. https://doi.org/10.3390/fishes8060310
Chicago/Turabian StyleLi, Yu-Jiao, Xin-Liang Wang, Ling-Yu Shi, Zong-Yi Wang, Zi-Ang Zhao, Shu-Chao Ge, and Bing Hu. 2023. "DUSP2 Deletion Inhibits Macrophage Migration by Inhibiting ERK Activation in Zebrafish" Fishes 8, no. 6: 310. https://doi.org/10.3390/fishes8060310
APA StyleLi, Y.-J., Wang, X.-L., Shi, L.-Y., Wang, Z.-Y., Zhao, Z.-A., Ge, S.-C., & Hu, B. (2023). DUSP2 Deletion Inhibits Macrophage Migration by Inhibiting ERK Activation in Zebrafish. Fishes, 8(6), 310. https://doi.org/10.3390/fishes8060310






