A Synthesis of the Ecology and Conservation of Pseudoplatystoma Catfishes in the Neotropics
Abstract
:1. Introduction
2. What Species Are in the Genus, and Where Are They Distributed?
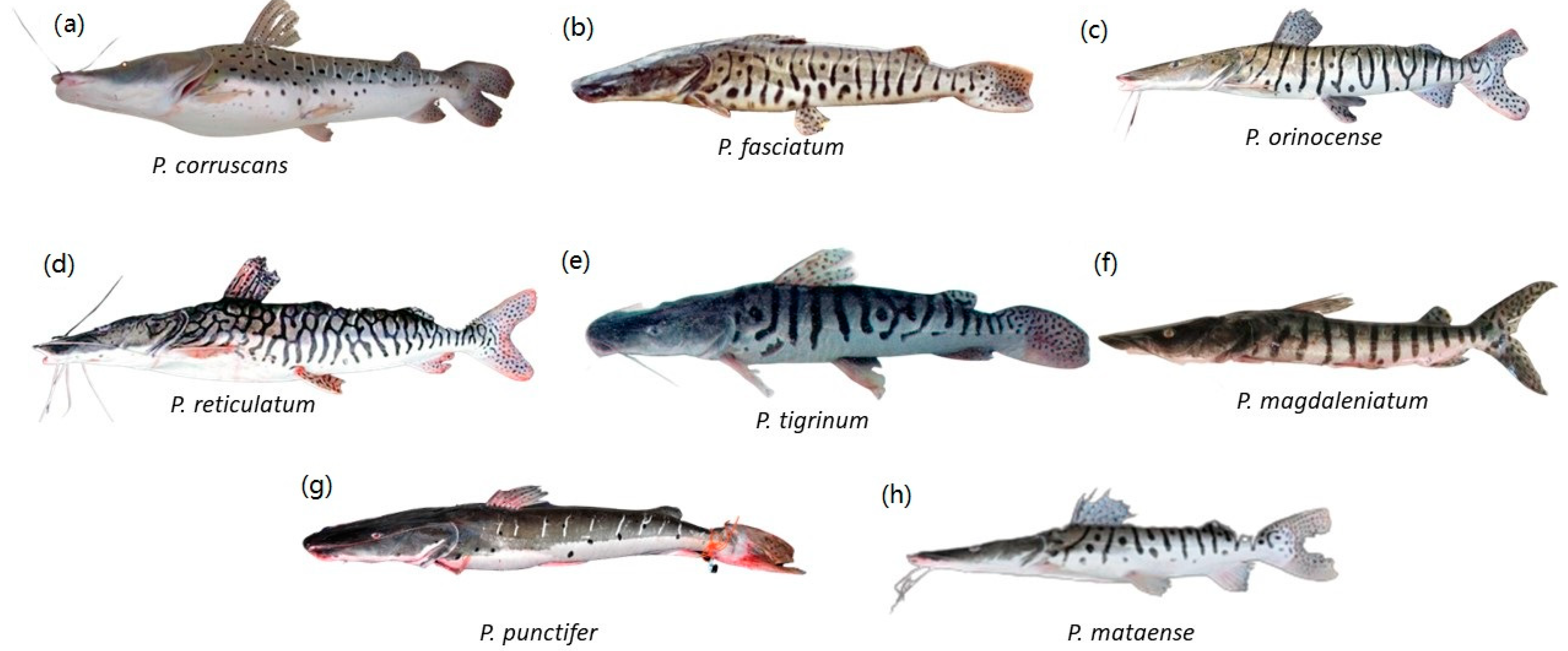
2.1. Natural History
2.2. Systematics and Taxonomy
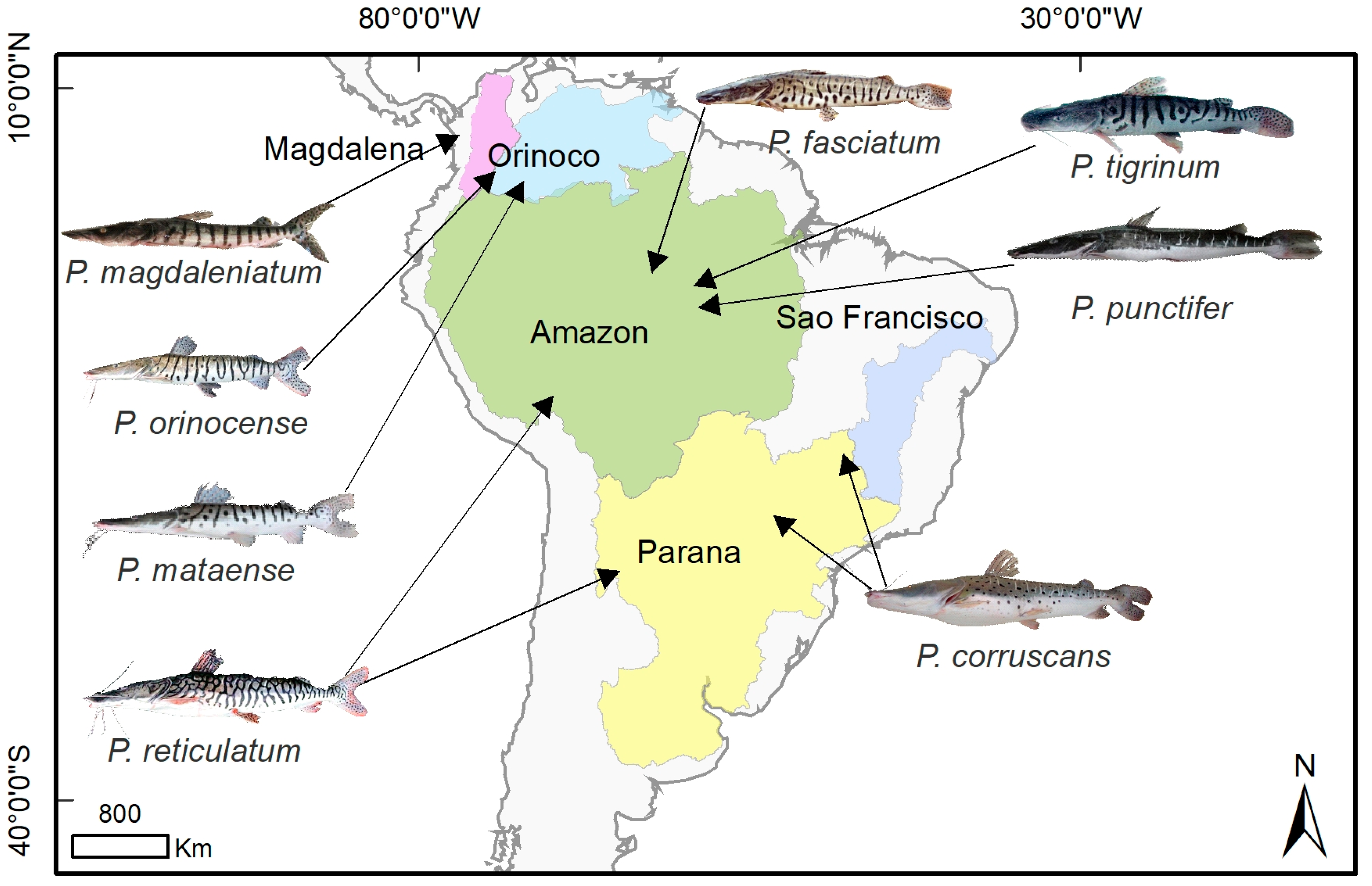
2.3. Distributions
3. What Are the Life Histories and Ecologies of Pseudoplatystoma Species?
3.1. Movement Ecology
3.2. Reproduction
| Species | Size at First Maturation (cm) | Spawning Season | Spawning Type | Oocyte Diameter (mm) | Ref. |
|---|---|---|---|---|---|
| P. corruscans | 62.92 | Rising water | Total | 0.9 | [32,48] |
| P. reticulatum | 57.84 | Rising water | Total | - | [48] |
| P. tigrinum | 69.4 | Rising water | Total | - | [45,53] |
| P. fasciatum | 53 | Rising water | Total | 0.64 | [31,53] |
| P.magdaleniatum | 82 | Rising water | - | 0.9 | [47] |
| P. tigrinum | 69.4 | High water | - | - | [42] |
| P. fasciatum | 53 | Rising water | - | 0.64 | [42] |
3.3. Growth
4. What Is Known about the Biomass Production and Population Dynamics of Pseudoplatystoma Species?
5. What Is the Geographic Distribution of Genetic Variation within Pseudoplatystoma Species?
6. What Are the Threats to the Conservation of These Species?
7. Conclusions
8. Future Directions: Knowledge Gaps
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arthington, A.H.; Dulvy, N.K.; Gladstone, W.; Winfield, I.J. Fish Conservation in Freshwater and Marine Realms: Status, Threats and Management. Aquat. Conserv. 2016, 26, 838–857. [Google Scholar] [CrossRef] [Green Version]
- Pelayo-Villamil, P.; Astor Guisande, C.; Vari, R.P.; Manjarr Es-Hern Andez, A.; Garc Ia-Rosell, E.; Gonz Alez-Dacosta, J.; Urgen Heine, J.; Gonz Alez Vilas, L.; Patti, B.; Mar Ia Quinci, E.; et al. Global Diversity Patterns of Freshwater Fishes-Potential Victims of Their Own Success Diversity and Distributions. Divers. Distrib. 2015, 21, 345–356. [Google Scholar] [CrossRef]
- Pinder, A.C.; Britton, J.R.; Harrison, A.J.; Nautiyal, P.; Bower, S.D.; Cooke, S.J.; Lockett, S.; Everard, M.; Katwate, U.; Ranjeet, K.; et al. Mahseer. Fishes of the World: Status, Challenges and Opportunities for Conservation. Rev. Fish Biol. Fish. 2019, 29, 417–452. [Google Scholar] [CrossRef] [Green Version]
- Fricke, R.; Eschmeyer, W.N.; van der Laan, R. Eschmeyer’s Catalog of Fishes: Genera, Species, References. Available online: http://researcharchive.calacademy.org/research/ichthyology/catalog/fishcatmain.asp (accessed on 16 May 2023).
- Dudgeon, D.; Arthington, A.H.; Gessner, M.O.; Kawabata, Z.I.; Knowler, D.J.; Lévêque, C.; Naiman, R.J.; Prieur-Richard, A.H.; Soto, D.; Stiassny, M.L.J.; et al. Freshwater Biodiversity: Importance, Threats, Status and Conservation Challenges. Biol. Rev. Camb. Philos. Soc. 2006, 81, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Cooke, S.J.; Paukert, C.; Hogan, Z. Endangered River Fish: Factors Hindering Conservation and Restoration. Endanger. Species Res. 2012, 17, 179–191. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, C.; Foresti, A.F.; Hilsdorf, A.A.W.S. Genetics of Neotropical Fish: From Chromosomes to Populations. Fish. Physiol. Biochem. 2009, 35, 81–100. [Google Scholar] [CrossRef]
- Ahmed, S.F.; Kumar, P.S.; Kabir, M.; Zuhara, F.T.; Mehjabin, A.; Tasannum, N.; Hoang, A.T.; Kabir, Z.; Mofijur, M. Threats, Challenges and Sustainable Conservation Strategies for Freshwater Biodiversity. Environ. Res. 2022, 214, 113808. [Google Scholar] [CrossRef]
- Nelson, J.S. Fishes of the World, 3rd ed.; J. Wiley and Sons: New York, NY, USA, 1994; ISBN 0471547131. [Google Scholar]
- Lundberg, J.G.; Pérez, M.H.S.; Dahdul, W.M.; Aguilera, O.A. The Amazonian Neogene Fish Fauna. In Amazonia, Landscape and Species Evolution: A Look into the Past; Wiley-Blackwell: Hoboken, NJ, USA, 2010; pp. 281–301. [Google Scholar] [CrossRef]
- Nuñez, J.; Dugué, R.; Corcuy Arana, N.; Duponchelle, F.; Renno, J.F.; Raynaud, T.; Hubert, N.; Legendre, M. Induced Breeding and Larval Rearing of Surubí, Pseudoplatystoma fasciatum (Linnaeus, 1766), from the Bolivian Amazon. Aquac. Res. 2008, 39, 764–776. [Google Scholar] [CrossRef]
- Dantas, H.L.; Arcanjo, M.; Neto, S.; Kelly, K.; Oliveira, C.; Severi, W.; Mendonça Diniz, F.; Raquel, M.; Coimbra, M.; Moura, M.R. Genetic Diversity of Captive and Wild Threatened Catfish Pseudoplatystoma corruscans in the São Francisco River. Rev. Fish. Sci. 2013, 21, 237–246. [Google Scholar] [CrossRef]
- Mello, P.H.; Venturieri, R.L.L.; Honji, R.M.; Moreira, R.G. Threatened Fishes of the World: Pseudoplatystoma corruscans (Agassiz, 1829) (Siluriformes: Pimelodidae). Environ. Biol. Fish. 2009, 85, 359–360. [Google Scholar] [CrossRef]
- Armas, M.; Ortega, H.; García-Vasquez, A.; García-Dávila, C.; Vargas, G.; Nuñez, J.; Renno, J.-F.; Duponchelle, F. Age Validation and Contrasted Growth Performances of Pseudoplatystoma punctifer (Siluriformes: Pimelodidae) in Two River Systems of the Western Amazon. Neotrop. Ichthyol. 2022, 20, e210099. [Google Scholar] [CrossRef]
- Santos, R.E.; Pinto-Coelho, R.M.; Fonseca, R.; Simões, N.R.; Zanchi, F.B. The Decline of Fisheries on the Madeira River, Brazil: The High Cost of the Hydroelectric Dams in the Amazon Basin. Fish. Manag. Ecol. 2018, 25, 380–391. [Google Scholar] [CrossRef]
- Agostinho, A.A.; Pelicice, F.M.; Petry, A.C.; Gomes, L.C.; Júlio, H.F. Fish Diversity in the Upper Paraná River Basin: Habitats, Fisheries, Management and Conservation. Aquat. Ecosyst. Health Manag. 2007, 10, 174–186. [Google Scholar] [CrossRef]
- Peluso, L.M.; Mateus, L.; Penha, J.; Bailly, D.; Cassemiro, F.; Suárez, Y.; Fantin-Cruz, I.; Kashiwaqui, E.; Lemes, P. Climate Change Negative Effects on the Neotropical Fishery Resources May Be Exacerbated by Hydroelectric Dams. Sci. Total Environ. 2022, 828, 154485. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Machado, A.S.; Farias, I.P.; Hrbek, T.; Lizarazo, M.D.E.; Alves-Gomes, J.A.; Formiga, K.; Da Silva Batista, J. Genetic Differentiation and Gene Flow of the Amazonian Catfish Pseudoplatystoma punctifer across the Madeira River Rapids Prior to the Construction of Hydroelectric Dams. Hydrobiologia 2022, 849, 29–46. [Google Scholar] [CrossRef]
- Mojica, J.; Valderrama, M.; Jimenez-Segura, L.; Alonso, J.C. Pseudoplatystoma magdaleniatum. IUCN Red List Threatened Species 2016, e.T58439165A61474168. [Google Scholar] [CrossRef]
- Brambilla, L.; Toledo, M.J.; Ibarra, D.A. First Fossil Record of Pseudoplatystoma corruscans (Siluriformes, Pimelodidae) from the Late Pleistocene, Santa Fe, Argentina. J. S. Am. Earth Sci. 2021, 105, 102987. [Google Scholar] [CrossRef]
- Vallone, E.; Ignacio Vezzosi, R.; Luis Cione, A. First Fossil Fish (Teleostei, Siluriformes) from the Late Pleistocene of Santa Fe Province, Argentina Mammal Paleoneurology View Project Cranio-Appendicular Anatomy of American Fossil and Living Deers (Mammalia, Cervidae): Systematic and Phylogenetic Implications of the Most Representative Taxa from the Litoral Region View Project. Alcheringa Austral J. Palaeontol. 2017, 41, 369–377. [Google Scholar] [CrossRef]
- Hilsdorf, A.W.S.; Hallerman, E.M. Genetic Resources of Freshwater Neotropical Fishes. In Genetic Resources of Neotropical Fishes; Springer International Publishing: Cham, Switzerland, 2017; pp. 119–210. [Google Scholar]
- Cassemiro, F.A.S.; Albert, J.S.; Antonelli, A.; Menegotto, A.; Wüest, R.O.; Cerezer, F.; Coelho, M.T.P.; Reis, R.E.; Tan, M.; Tagliacollo, V.; et al. Landscape Dynamics and Diversification of the Megadiverse South American Freshwater Fish Fauna. Proc. Natl. Acad. Sci. USA 2023, 120, e2211974120. [Google Scholar] [CrossRef]
- Géry, J. The Fresh-Water Fishes of South. Am. Biogeogr. Ecol. S. Am. 1969, 2, 828–848. [Google Scholar]
- Hubert, N.; Renno, J.F. Historical Biogeography of South American Freshwater Fishes. J. Biogeogr. 2006, 33, 1414–1436. [Google Scholar] [CrossRef]
- Buitrago-Suárez, U.A.; Burr, B.M. Taxonomy of the Catfish Genus Pseudoplatystoma Bleeker (Siluriformes: Pimelodidae) with Recognition of Eight Species. Zootaxa 2007, 1512, 1–38. [Google Scholar] [CrossRef] [Green Version]
- Torrico, J.P.; Hubert, N.; Desmarais, E.; Duponchelle, F.; Nuñez Rodriguez, J.; Montoya-Burgos, J.; Garcia Davila, C.; Carvajal-Vallejos, F.M.; Grajales, A.A.; Bonhomme, F.; et al. Molecular Phylogeny of the Genus Pseudoplatystoma (Bleeker, 1862): Biogeographic and Evolutionary Implications. Mol. Phylogenet. Evol. 2009, 51, 588–594. [Google Scholar] [CrossRef]
- Carvalho-Costa, L.F.; Piorski, N.M.; Willis, S.C.; Galetti, P.M.; Ortí, G. Molecular Systematics of the Neotropical Shovelnose Catfish Genus Pseudoplatystoma Bleeker 1862 Based on Nuclear and MtDNA Markers. Mol. Phylogenet. Evol. 2011, 59, 177–194. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.G.; Sullivan, J.P.; Hardman, M. Phylogenetics of the South American Catfish Family Pimelodidae (Teleostei: Siluriformes) Using Nuclear and Mitochondrial Gene Sequences. Proc. Acad. Nat. Sci. Phila. 2011, 161, 153–189. [Google Scholar] [CrossRef]
- García-Dávila, C.; Duponchelle, F.; Castro-Ruiz, D.; Villacorta, J.; Quérouil, S.; Chota-Macuyama, W.; Nú, J.; Römer, U.; Carvajal-Vallejos, F.; Renno, J.-F.; et al. Molecular Identification of a Cryptic Species in the Amazonian Predatory Catfish Genus Pseudoplatystoma (Bleeker, 1962) from Peru. Genetica 2013, 141, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Loubens, G.; Panfili, J. Biologie de Pseudoplatystoma fasciatum et P. tigrinum (Teleostei: Pimelodidae) Dans Le Bassin Du Mamoré (Amazonie Bolivienne). Ichthyol. Explor. Freshw. 2000, 11, 13–34. [Google Scholar]
- Carolsfeld, J.; Harvey, B.; Ross, C.; Baer, A. (Eds.) Migratory Fishes of South. America; World Fisheries Trust: Victoria, BC, USA, 2003; ISBN 9780444536433. [Google Scholar]
- Barthem, R.; Goulding, M. Os Bagres Balizadores: Ecologia, Migração e Conservação de Peixes Amazônicos; Tefé: Sociedade Civil Mamirauá; CNPq: Tefe, Brazil, 1997. [Google Scholar]
- Diaz-Sarmineto, J.A.; Alvarez-León, R. Migratory Fishes of the Colombian Amazon. In Migratory Fishes of South America Chapter 7; Carolsfeld, J., Harvey, B., Ross, C., Baer, A., Eds.; World Fisheries Trust: Victoria, BC, USA, 2003; pp. 303–344. [Google Scholar]
- Goulding, M.; Lowe-McConnell, R.H. (Eds.) The Fishes and the Forest; University of California Press: Berkeley, CA, USA, 1980. [Google Scholar]
- Araujo-Lima, C.A.R.M.; Oliveira, E.C. Transport of Larval Fish in the Amazon. J. Fish. Biol. 1998, 53, 297–306. [Google Scholar] [CrossRef]
- Pavlov, S.; Nezdoliy, V.; Urteaga, A.; Sanches, O. Downstream Migration of Juvenile Fishes in the Rivers. J. Ichthyol./Vopr. Ikhtiologii 1995, 35, 227–248. [Google Scholar]
- Barthem, R.B.; Goulding, M.; Leite, R.G.; Cañas, C.; Forsberg, B.; Venticinque, E.; Petry, P.; Ribeiro, M.L.D.B.; Chuctaya, J.; Mercado, A. Goliath Catfish Spawning in the Far Western Amazon Confirmed by the Distribution of Mature Adults, Drifting Larvae and Migrating Juveniles. Sci. Rep. 2017, 7, 41784. [Google Scholar] [CrossRef] [Green Version]
- Hahn, L.; Martins, E.G.; Nunes, L.D.; da Câmara, L.F.; Machado, L.S.; Garrone-Neto, D. Biotelemetry Reveals Migratory Behaviour of Large Catfish in the Xingu River, Eastern Amazon. Sci. Rep. 2019, 9, 8464. [Google Scholar] [CrossRef] [Green Version]
- Godinho, A.L.; Kynard, B.; Godinho, H.P. Migration and Spawning of Female Surubim (Pseudoplatystoma corruscans, Pimelodidae) in the São Francisco River, Brazil. Environ. Biol. Fish. 2007, 80, 421–433. [Google Scholar] [CrossRef]
- Avigliano, E.; Pouilly, M.; Bouchez, J.; Domanico, A.; Sánchez, S.; Llamazares Vegh, S.; Clavijo, C.; Scarabotti, P.; Facetti, J.F.; Caffetti, J.D.; et al. Strontium Isotopes (87Sr/86Sr) Reveal the Life History of Freshwater Migratory Fishes in the La Plata Basin. River Res. Appl. 2020, 36, 1985–2000. [Google Scholar] [CrossRef]
- Mariac, C.; Renno, J.-F.; Garcia-Davila, C.; Vigouroux, Y.; Mejia, E.; Angulo, C.; Ruiz, D.C.; Estivals, G.; Nolorbe, C.; Vasquez, A.G.; et al. Species-Level Ichthyoplankton Dynamics for 97 Fishes in Two Major River Basins of the Amazon Using Quantitative Metabarcoding. Mol. Ecol. 2021, 31, 1627–1648. [Google Scholar] [CrossRef] [PubMed]
- Crepaldi, D.V.; Faria, P.M.C.; Teixeira, E.D.A.; Ribeiro, L.P.; Augusto, Â.; Costa, P.; Chemim De Melo, D.; Paula, A.; Cintra, R.; Prado, S.D.A.; et al. Estructura Histologica de los ovarios de Pseudoplatystoma corruscans (Agassiz, 1829) Pimelodidae, Siluriformes. Boletim do Instituto de Pesca 1996, 23, 203–212. [Google Scholar]
- Brito, M.F.G.; Bazzoli, N. Reproduction of the Surubim Catfish (Pisces, Pimelodidae) in the São Francisco River, Pirapora Region, Minas Gerais, Brazil. Arq. Bras. Med. Vet. Zootec. 2003, 55, 624–633. [Google Scholar] [CrossRef]
- Pérez, A.; Castillo, O.; Barbarino, A.; Fabré, N. Aspectos Reproductivos Del Bagre Rayado Pseudoplatystoma tigrinum (Siluriformes, Pimelodidae) En La Cuenca Del Río Apure, Venezuela. Zootecnia Trop. 2012, 30, 251–262. [Google Scholar]
- Jiménez-Segura, L.F.; Palacio, J.; López, R. Caracteristicas Biologicas del Blanquillo Sorobim cuspicaudas Littmann, Burr y Nass, 2000 y Bagre rayado Pseudoplatystoma magdaleniatum Buitrago-Suarez y Burr 2007 (Siluriformes: Pimelodidae) Relacionadas con su Reproducction en la Cuenca Media del Rio Magdalena, Colombia. Actual. Biol. 2009, 31, 53–66. [Google Scholar]
- Barzotto, E.; Oliveira, M.; Mateus, L. Reproductive Biology of Pseudoplatystoma corruscans (Spix and Agassiz, 1829) and Pseudoplatystoma reticulatum (Eigenmann and Eigenmann, 1889), Two Species of Fisheries Importance in the Cuiabá River Basin, Brazil. J. Appl. Ichthyol. 2017, 33, 29–36. [Google Scholar] [CrossRef]
- Rizzo, E.; Godinho, H.P. Superfície de Ovos de Peixes Characiformes e Siluriformes. In Águas, Peixes e Pescadores do São Francisco das Minas Gerais; Godinho, H.P., Godinho, A.L., Eds.; PUC Minas: Belo Horizonte, Brazil, 2003; pp. 115–132. [Google Scholar]
- Reid, J. La Biología de Los Bagres Rayados Pseudoplatystoma fasciatum y P. tigrinum En La Cuenca Del Río Apure, Venezuela. Revista Unellez de Ciencia y Tecnología 1983, 1.1, 13–41. [Google Scholar]
- Resende, A.; Kawakami, E.; Catella, C.; Lima, F.; Shirley Da, N.; Palmeira, S.; Aparecida, R.; Pereira, C.; De, M.; Lima, S. Biologia Do Curimbatá (Prochilodus lineatus), Pintado (Pseudoplatystoma corruscans) e Cachara (Pseudoplatystoma fasciatum) Na Bacia Hidrográfica Do Rio Miranda. Infoteca.Cnptia.Embrapa.br, 1995; 75p, (EMBRAPA-CPAP. Boletim de Pesquisa, 02). [Google Scholar]
- Padilla Pérez, P.P.; Alcántara Bocanegra, F.; Orbe, R.I. Reproducción Inducida de La Doncella Pseudoplatystoma fasciatum y Desarrollo Embrionario-Larval. Folia amazónica 2001, 12, 141–154. [Google Scholar] [CrossRef] [Green Version]
- Garcia, V.A.; Montreuil, V. Rodriguez R Talla de Primera Maduracion y Epoca de Desove de La “Doncella”, Pseudoplatystoma fasciatum (Linnaeus), y Del “Tigre Zungaro”, Pseudoplatystoma tigrinum (Valenciennes), En La Amazonia Peruana. Boletin del Museo Para Emilio Goeldi Séria Zoologia 2001, 17, 3–13. [Google Scholar]
- Núñez, J.; Castro, D.; Fernández, C.; Dugué, R.; Chu-Koo, F.; Duponchelle, F.; García, C.; Renno, J.F. Hatching Rate and Larval Growth Variations in Pseudoplatystoma punctifer: Maternal and Paternal Effects. Aquac. Res. 2011, 42, 764–775. [Google Scholar] [CrossRef]
- Andrade, F.F.; Lima, A.F.; Assumpção, L.; Makrakis, S.; Kasai, R.I.D.; Makrakis, M.C. Characterization of the Early Development of Pseudoplatystoma reticulatum Eigenmann & Eigenmann, 1889 (Siluriformes: Pimelodidae) from the Paraguay River Basin. Neotrop. Ichthyol. 2016, 14. [Google Scholar] [CrossRef] [Green Version]
- Erard, C.; Keith, P.; Le Bail, P.Y.; Planquette, P. Atlas Des Poissons d’eau Douce de Guyane. Revue d’Écologie (La Terre et La Vie) 2002, 57, 185–187. [Google Scholar]
- Pérez, A.; Fabré, N.N. Life-History Characteristics of Pseudoplatystoma Metaense (Teleostei: Siluriformes: Pimelodidae) from the Northwestern Orinoco River Basin. Neotrop. Ichthyol. 2018, 16, 160140. [Google Scholar] [CrossRef] [Green Version]
- Cutrim, L.; Batista, V.d.S. Determinação de Idade e Crescimento Do Mapará (Hypophthalmus marginatus) Na Amazônia Central. Acta Amazon. 2005, 35, 85–92. [Google Scholar] [CrossRef]
- Hauser, M.; Doria, C.R.C.; Melo, L.R.C.; Santos, A.R.; Ayala, D.M.; Nogueira, L.D.; Amadio, S.; Fabré, N.; Torrente-Vilara, G.; García-Vásquez, Á.; et al. Age and Growth of the Amazonian Migratory Catfish Brachyplatystoma russeauxii in the Madeira River Basin before the Construction of Dams. Neotrop. Ichthyol. 2018, 16, 1127. [Google Scholar] [CrossRef] [Green Version]
- Arantes, C.C.; Castello, L.; Stewart, D.J.; Cetra, M.; Queiroz, H.L. Population Density, Growth and Reproduction of Arapaima in an Amazonian River-Floodplain. Ecol. Freshw. Fish. 2010, 19, 455–465. [Google Scholar] [CrossRef]
- González, S.Á.R.; Mendoza, J.; Arocha, F.; Márquez, A. Edad y Crecimiento Del Bagre Rayado Pseudoplatystoma orinocoense Del Orinoco Medio En Venezuela. Zootec. Trop. 2010, 28, 283–293. [Google Scholar]
- Pérez, A.; Fabré, N.N. Seasonal Growth and Life History of the Catfish Calophysus macropterus (Lichtenstein, 1819) (Siluriformes: Pimelodidae) from the Amazon Floodplain. J. Appl. Ichthyol. 2009, 25, 343–349. [Google Scholar] [CrossRef]
- Henderson, P.A. The Growth of Tropical Fishes. Fish. Physiol. 2005, 21, 85–100. [Google Scholar] [CrossRef]
- Lagler, K.F.; Kapetsky, J.M.; Stewart, D.J. The Fisheries of the Kafue River Flats, Zambia, in Relation to the Kafue Gorge Dam; University of Michigan, School of Natural Resources: Ann Arbor, MI, USA, 1971. [Google Scholar]
- Welcomme, R.L. River Fisheries; Food and Agriculture Organization of the United Nations: Rome, Italy, 1985; ISBN 9251022992. [Google Scholar]
- Winemiller, K.O. Patterns of Variation in Life History among South. American Fishes in Seasonal Environments. Oecologia 1989, 81, 225–241. [Google Scholar] [CrossRef] [PubMed]
- Winemiller, K.O.; Rose, K.A. Patterns of Life-History Diversification in North American Fishes: Implications for Population Regulation. Canad J. Fish. Aquat. Sci. 1992, 49, 2196–2218. [Google Scholar] [CrossRef]
- Doria, C.R.C.; Duponchelle, F.; Lima, M.A.L.; Garcia, A.; Carvajal-Vallejos, F.M.; Méndez, C.C.; Catarino, M.F.; Freitas, C.E.D.C.; Vega, B.; Miranda-Chumacero, G.; et al. Review of Fisheries Resource Use and Status in the Madeira River Basin (Brazil, Bolivia, and Peru) Before Hydroelectric Dam Completion. Rev. Fish. Sci. Aquacult 2018, 26, 494–514. [Google Scholar] [CrossRef]
- Ruffino, M.L.; Isaac, V.J. Dinamica Populacional de Surubim-Tigre, Pseudoplatystoma. Acta Amazon. 1999, 29, 463–476. [Google Scholar] [CrossRef]
- Godinho, H.P.; Miranda, M.O.T.; Godinho, A.L.; Santos, J.E. Pesca e Biologia Do Surubim Pseudoplatystoma corruscans No Rio São Francisco. In Colecão Meio Ambiente. Série de Estudos: Pesca; Miranda, M.O.T., Ed.; Instituto Brasileiro do Meio Ambiente e dos Recursos Naturais Renováveis: Belo Horizonte, Brazil, 1997; Volume 19, pp. 27–42. [Google Scholar]
- Fullerton, A.H.; Burnett, K.M.; Steel, E.A.; Flitcroft, R.L.; Pess, G.R.; Feist, B.E.; Torgersen, C.E.; Miller, D.J.; Sanderson, B.L. Hydrological Connectivity for Riverine Fish: Measurement Challenges and Research Opportunities. Freshw. Biol. 2010, 55, 2215–2237. [Google Scholar] [CrossRef] [Green Version]
- Pereira, L.H.G.; Foresti, F.; Oliveira, C. Genetic Structure of the Migratory Catfish Pseudoplatystoma corruscans (Siluriformes: Pimelodidae) Suggests Homing Behaviour. Ecol. Freshw. Fish. 2009, 18, 215–225. [Google Scholar] [CrossRef]
- Telles, M.P.C.; Collevatti, R.G.; Braga, R.S.; Guedes, L.B.S.; Castro, T.G.; Costa, M.C.; Silva-Júnior, N.J.; Barthem, R.B.; Diniz-Filho, J.A.F. Geographical Genetics of Pseudoplatystoma punctifer (Castelnau, 1855) (Siluriformes, Pimelodidae) in the Amazon Basin. Genet. Mol. Res. 2014, 13, 3656–3666. [Google Scholar] [CrossRef]
- Duponchelle, F.; Isaac, V.J.; Doria, C.; Van Damme, P.A.; Herrera-R, G.A.; Anderson, E.P.; Cruz, R.E.A.; Hauser, M.; Hermann, T.W.; Agudelo, E.; et al. Conservation of Migratory Fishes in the Amazon Basin. Aquat. Conserv. 2021, 31, 1087–1105. [Google Scholar] [CrossRef]
- Agostinho, A.A.; Gomes, L.C. Dams and the Fish Fauna of the Neotropical Region: Impacts and Management Related to Diversity and Fisheries. Braz. J. Biol. 2008, 68, 1119–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poff, L.N.; Hart, D.D. How Dams Vary and Why It Matters for the Emerging Science of Dam Removal. Bioscience 2002, 52, 659–668. [Google Scholar] [CrossRef] [Green Version]
- Jackson, D.; Marmulla, G. Dams, Fish and Fisheries. Opportunities, Challenges and Conflict Resolution. FAO Fish. Tech. Paper 2001, 419, 166. [Google Scholar]
- Sato, Y. Reprodução de Peixes Da Bacia Do Rio São Francisco: Indução e Caracterização de Padrões; 179f. Tese (Doutorado); Centro de Ciências Biológicas e da Saúde, Universidade Federal de São Carlos: São Carlos, SP, Brazil, 1999. [Google Scholar]
- Sato, Y.; Godinho, H.P. Migratory Fishes of the São Francisco River. In Migrotory Fish of South America; Carolsfeld, J., Harvey, B., Ross, C., Eds.; World Fisheries Trust: Victoria, BC, USA, 2003; pp. 195–232. [Google Scholar]
- Godinho, H.P.; Miranda, M.O.T.; Godinho, A.L.; Santos, J.E. Pesca e Biologia Do Surubim Pseudoplatystoma Coruscans No Rio São Francisco; Puc Minas: Belo Horizonte, Brazil, 1997. [Google Scholar]
- Godinho, A.L.; Godinho, H.P. Breve Visão Do São Francisco. Belo Horizonte: PUC Minas 468 (2003): 15-23. In Águas, Peixes e Pescadores do São Francisco das Minas Gerais; PUC Minas: Belo Horizonte, Brazil, 2003; Volume 468, pp. 15–23. [Google Scholar]
- Menezes, R.S. Pesca e Piscicultura No Vale Do São Francisco. Boletim da Secretaria da Agricultura Indústria e Comércio do Estado de Pernambuco 1956, 23, 43–105. [Google Scholar]
- Isaac, V.; Ruffino, M.; McGrath, D. In Search of a New Approach to Fisheries Management in the Middle Amazon Region. In Fishery Stock Assessment Models; Alaska Sea Grant, University of Alaska Fairbanks: Fairbanks, AK, USA, 1998; pp. 889–902. [Google Scholar]
- Castello, L.; McGrath, D.G.; Beck, P.S.A. Resource Sustainability in Small-Scale Fisheries in the Lower Amazon Floodplains. Fish. Res. 2011, 110, 356–364. [Google Scholar] [CrossRef]
- Agudelo, E.A.; Acosta-Santos, G.; Gómez, B.D.; Gil, R.E.; Ajiaco-Martínez; Ramírez-Gil, H. Pseudoplatystoma punctifer (Siluriformes, Pimelodidae). In I. Catálogo de los Recursos Pesqueros Continentales de Colombia. Serie Editorial Recursos Hidro-biológicos y Pesqueros continentales de Colombia; Lasso, C.A., Agudelo Córdoba, E., Jiménez-Segura, L.F., Ramírez-Gil, H., Morales-Betancourt, M., Ajiaco-Martínez, R.E., Gutiérrez, F.d.P., Usma Oviedo, J.S., Muñoz Torres, S.E., Sanabria Ochoa, A.I., Eds.; Instituto de Investigación de Recursos Biológicos Alexander von Humboldt (IAvH): Bogotá, Colombia, 2011; Capitulo 7; pp. 509–512. [Google Scholar]
- Araujo-Lima; Carlos, A.; Ruffino, M.L. Migratory Fishes of the Brazilian Amazon. In Migratory Fishes of South America; Carolsfeld, J., Harvey, B., Ross, C., Ross, C., Eds.; World Fisheries Trust: Victoria, BC, USA, 2003; pp. 233–301. [Google Scholar]
- Barthem, R.B. Várzea: Diversity, Development, and Conservation of Amazonia’s Whitewater Floodplains. Adv. Eco Bot. 1999, 13, 7–28. [Google Scholar]
- Lasso, C.A.; Morales-Betancourt, M.A. (Eds.) Catálogo de Los. Recursos Pesqueros Continentales de Colombia; Ministerio de Ambiente, Vivienda y Desarrollo Territorial-Instituto de Investigación de Recursos Biológicos Alexander von Humboldt: Bogota, Colombia, 2011.
- Valderrama, M.; Zarate, M.; Vera, G.; Moreno, C.; Caraballo, P.; Martinez, J. Determinación de La Talla Media de Madurez y Análisis de La Problemática Con Referencia a Las Tallas Medias de Captura Del Bagre Rayado (Pseudoplatystoma fasciatum) Linnaeus 1766 (Pisces: Pimelodidae), En La Cuenca Del Río Magdalena, Colombia. Trianea. Act. Cient. Tec.-INDERENA 1988, 2, 537–550. [Google Scholar]
| Species | L∞ (cm) | K | Longevity (Years) | Ø | Ref. | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L∞ | L∞m | L∞f | k | km | kf | Longevity | Male | Female | Ø | Øm | Øf | ||
| P. tigrinum | 180 | - | - | 0.29 | - | - | - | - | - | 0.97 | - | - | [56] |
| P. tigrinum | - | 146 | 170 | - | 0.11 | 0.01 | 25–30 | - | - | - | 0.49 | −0.55 | [51] |
| P. tigrinum | - | 119.8 | 131.8 | - | 0.17 | 0.15 | - | - | - | - | 0.63 | 0.59 | [32] |
| P. fasciatum | - | 77 | 103.1 | - | 0.44 | 0.24 | 15 | - | - | - | 0.91 | 0.72 | [32] |
| P. corruscans | 200 | - | - | 0.08 | - | - | 10 | - | - | 0.47 | - | - | [57] |
| P. corruscans | - | 99.2 | 131.8 | 0.51 | 0.34 | - | - | - | - | 1.05 | 0.95 | [58] | |
| P. reticulatum | - | 72.7 | 82.5 | - | 0.44 | 0.33 | - | - | - | - | 0.89 | 0.81 | [59] |
| P. punctifer | - | 99.5 | 97.3 | - | 0.16 | 0.2 | - | - | - | - | 0.54 | 0.63 | [15] |
| P. mataense | - | 108.6 | 119.9 | - | 0.19 | 0.17 | - | 12.37 | 15.7 | - | 0.64 | 0.62 | [60] |
| Concepts | Ecological or Genetic Meaning |
|---|---|
| Analysis of Molecular Variance (AMOVA) | An analytical algorithm that partitions genetic variation into within- and between-population components. |
| Bayesian methods | Methods of statistical inference in which Bayes’ theorem is used to update the probability for a hypothesis as more evidence or information becomes available. Bayesian approaches underlie some clustering and assignment tests and phylogenetic inference algorithms in population genetic analyses. |
| Evolutionary Significant Units (ESU) | A population or a group of populations that merits priority for conservation and separate management because of high genetic and ecological distinctiveness from other such units. |
| FST | The coefficient of departure of genotype frequencies from Hardy-Weinberg equilibrium due to differentiation among subpopulations within a larger population. |
| Fu’s Fs | A population genetics statistic helpful in assessing historical population dynamics. |
| Genetic differentiation | The condition of populations having contrasting genotype frequencies resulting from the dynamic interplay among mutation, gene flow, selection, and random genetic drift over space and time. |
| Management Unit (MU) | A population that is demographically independent from others and hence should be managed separately. |
| Maximum parsimony | In the context of phylogenetic inference, a method for evaluating relationships between lineages in which the evolutionary tree with the fewest common ancestors is regarded as the most likely. |
| Microsatellite | A genomic sequence with repeats of 1–6 nucleotide motifs, e.g., GT, CAC, or GACA. There is variation in the repeat number at a given locus among members of a chromosome pair in an individual, among individuals within a population, and often among populations. Such genetic markers are highly useful for population genetic studies. |
| Mitochondrial DNA COI region | Mitochondrial cytochrome oxidase subunit 3 (COI) gene. This genetic marker is often used for DNA barcoding to distinguish species. |
| Neighbor-likelihood joining | An agglomerative clustering method for the construction of phylogenetic trees. |
| Panmictic population (panmixia) | A collection of fish with the absence of genetic structure (the condition of being well mixed, as in a population where all individuals could interbreed). |
| Phylogenetic tree | A representation of the inferred evolutionary relationships among individuals, populations, or species that shows the branching from a known or hypothesized ancestor to its derived lineages, which outwardly resembles a tree and hence is referred to as this. |
| RST | A metric of genetic differentiation that incorporates the differences in the size of microsatellite alleles |
| Tajima’s D | The difference between two measures of genetic diversity: the mean number of pairwise differences and the number of segregating sites, each scaled so that they are expected to be the same in a neutrally evolving population of constant size. |
| Species | Genetics | Fisheries Status | Growth | Reproduction | Movement Ecology |
|---|---|---|---|---|---|
| P. fasciatum | Microsatellite DNA showed high genetic diversity with 200 km of genetic isolation by distance | No data | Validation, growth curves | Total spawning in the rising and high-water seasons 31, 52, 43 | Adults migrate, juveniles are nonmigratory 31~164 km, bidirectional movement during the dry season and the rising water period 39 |
| P. tigrinum | No data | No data; exploited very close to maximum sustainable 54 | Validation, growth curves | Total spawning in the rising water season 44, 52 | Adults migrate, juveniles are nonmigratory 31 |
| P. punctifer | No data | No data | Validation, growth curves | No data | No data |
| P. reticulatum | No data | No data | No validation data, growth curves | Total spawning in the rising water season 46 | Believed to be migratory |
| P. mataense | No data | No data | Validation, growth curves | No data | Believed to be migratory |
| P. orinocense | No data | No data | No data | No data | Believed to be migratory |
| P. magdaleniatum | No data | No data | No data | Spawning in the rising water season 45 | Believed to be migratory |
| P. corruscans | Mitochondrial COI region, microsatellite DNA analysis to delineate 2 ESUs | No data; strongly overfished 71 | No validation data, growth curves | Total spawning in the rising water season 32, 46 | Partial migration of females; homing migration 40 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, L.A.; Castello, L.; Orth, D.J.; Duponchelle, F.; Hallerman, E.M. A Synthesis of the Ecology and Conservation of Pseudoplatystoma Catfishes in the Neotropics. Fishes 2023, 8, 306. https://doi.org/10.3390/fishes8060306
Pereira LA, Castello L, Orth DJ, Duponchelle F, Hallerman EM. A Synthesis of the Ecology and Conservation of Pseudoplatystoma Catfishes in the Neotropics. Fishes. 2023; 8(6):306. https://doi.org/10.3390/fishes8060306
Chicago/Turabian StylePereira, Luciana A., Leandro Castello, Donald J. Orth, Fabrice Duponchelle, and Eric M. Hallerman. 2023. "A Synthesis of the Ecology and Conservation of Pseudoplatystoma Catfishes in the Neotropics" Fishes 8, no. 6: 306. https://doi.org/10.3390/fishes8060306







