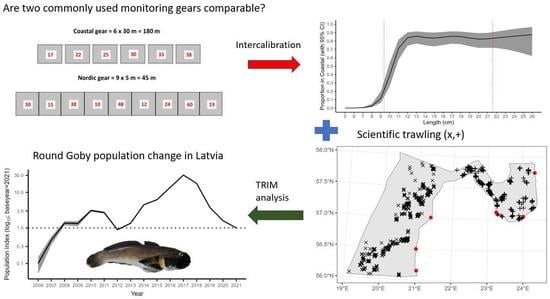
The Population Development of the Invasive Round Goby Neogobius melanostomus in Latvian Waters of the Baltic Sea
Abstract
1. Introduction
2. Materials and Methods
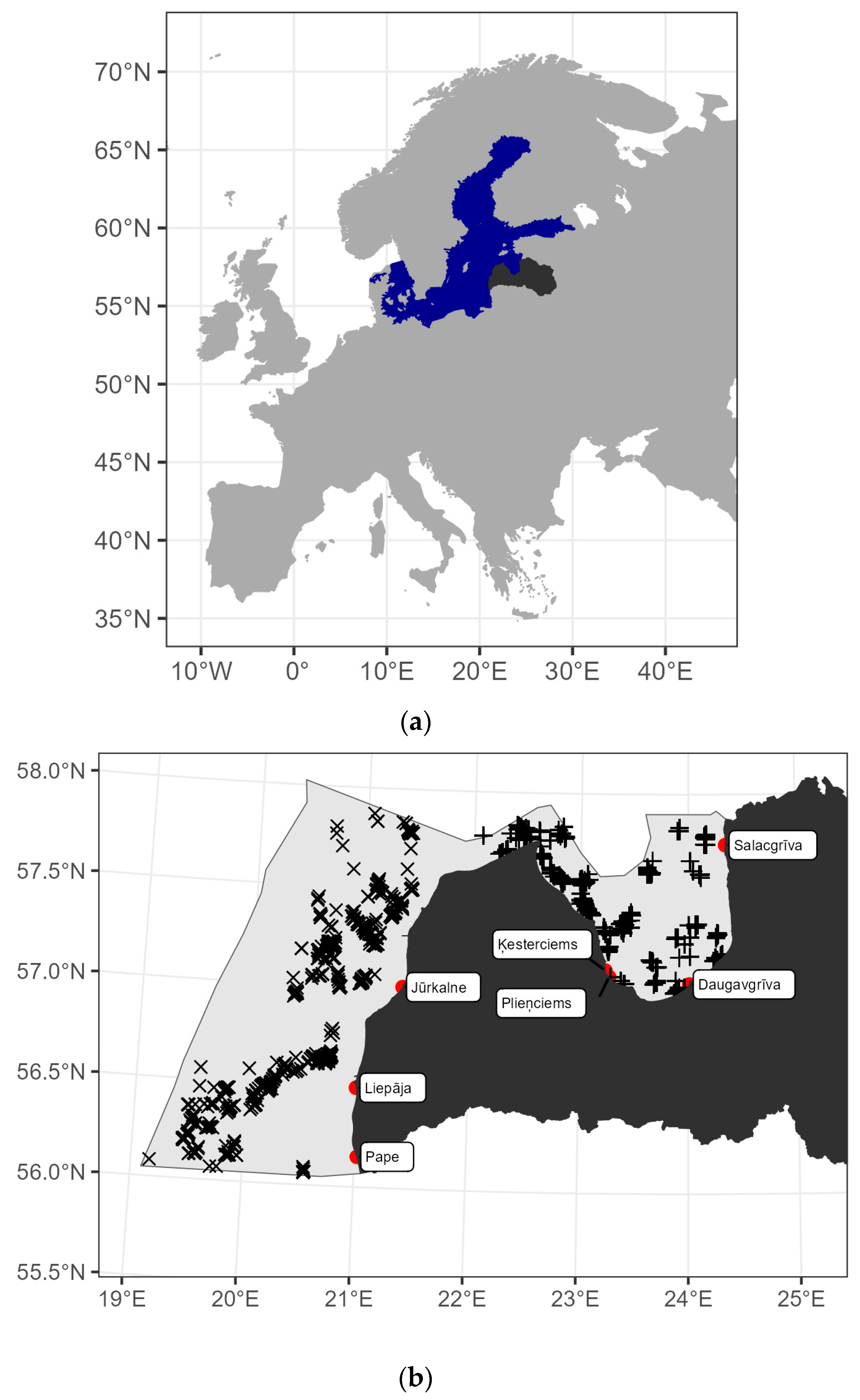
2.1. Study Area and Methods
2.2. Fish Monitoring Methods
2.2.1. Latvian National Coastal Fish Monitoring (CFM)
2.2.2. Latvian National Benthic Trawl Surveys in the Gulf of Riga (GORDEM)
2.2.3. Baltic International Benthic Trawl Survey (BITS)
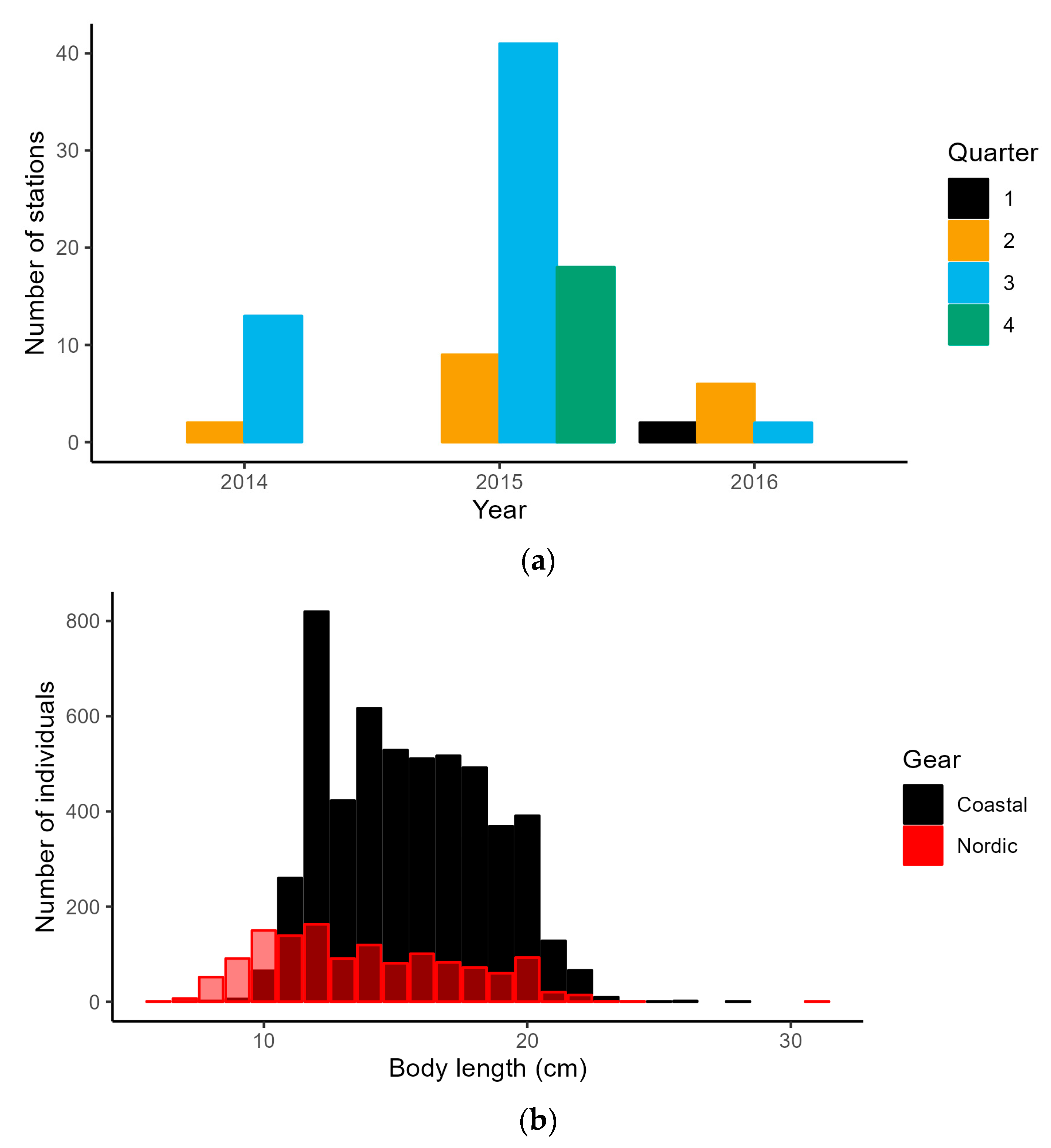
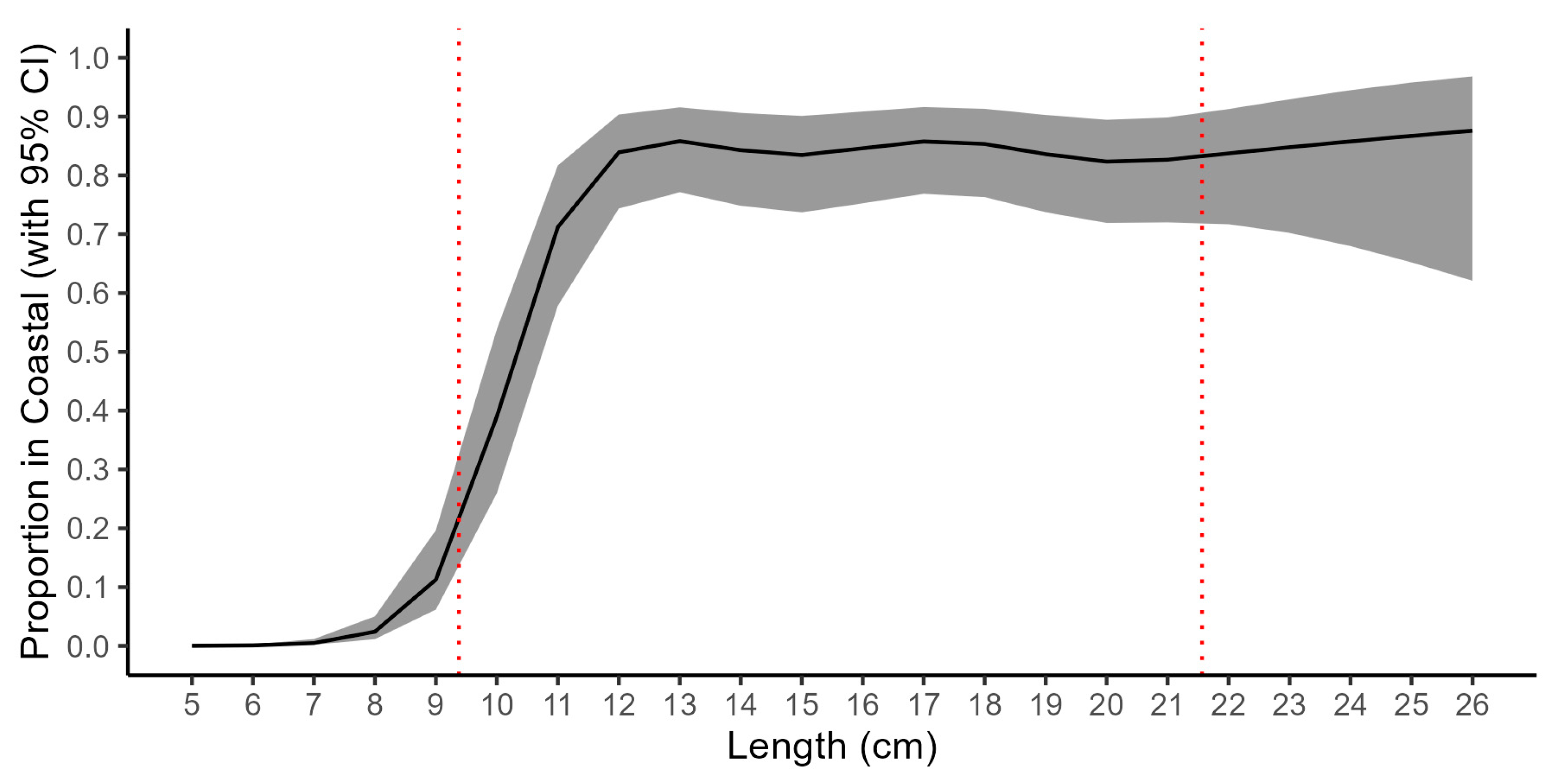
2.3. CFM Gear Calibration
2.4. Population Change Analysis
2.4.1. Coastal Fish Monitoring Data Preprocessing
2.4.2. BITS and GORDEM Data Preprocessing
2.4.3. Standardization across Surveys
2.4.4. Population Model
2.4.5. Trend Combination
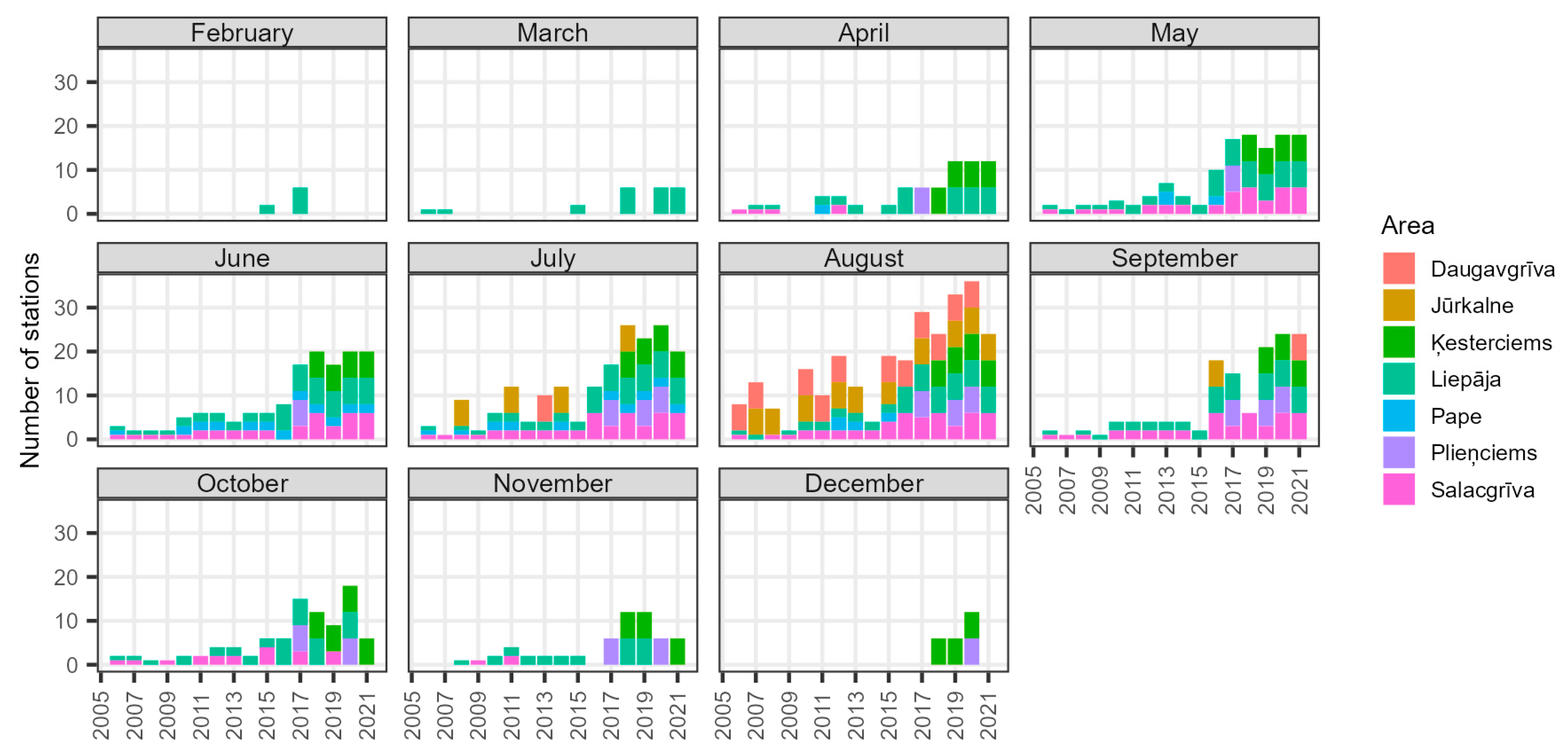
2.4.6. Monthly Catch Rates
2.5. Commercial Fisheries Data
3. Results
3.1. Coastal Fish Monitoring Gear Calibration
3.2. Population Development of the RG
3.2.1. The Trend in the CFM
3.2.2. The Trend in the Gulf of Riga from GORDEM
3.2.3. The Trend in Open Sea from BITS
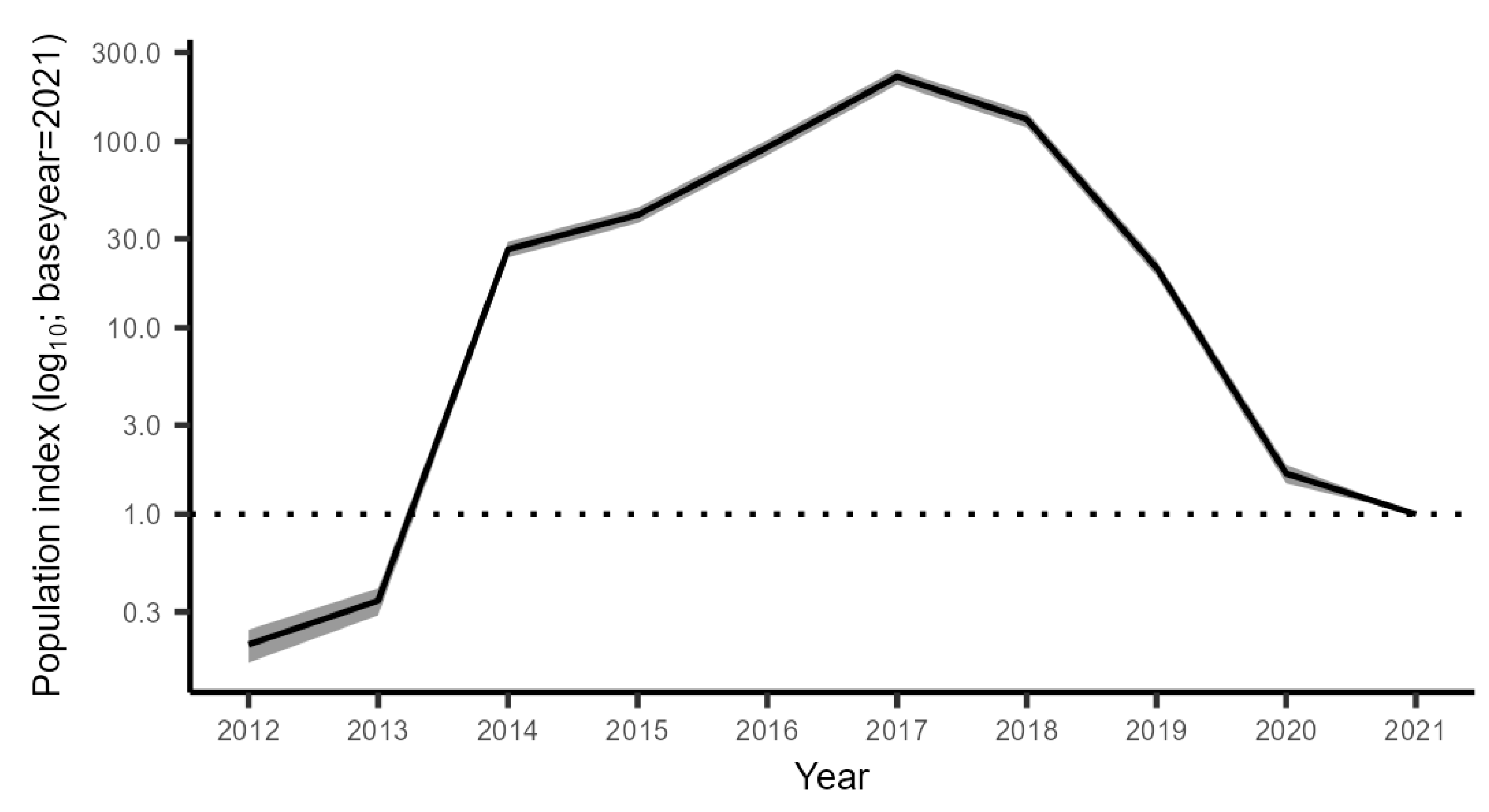
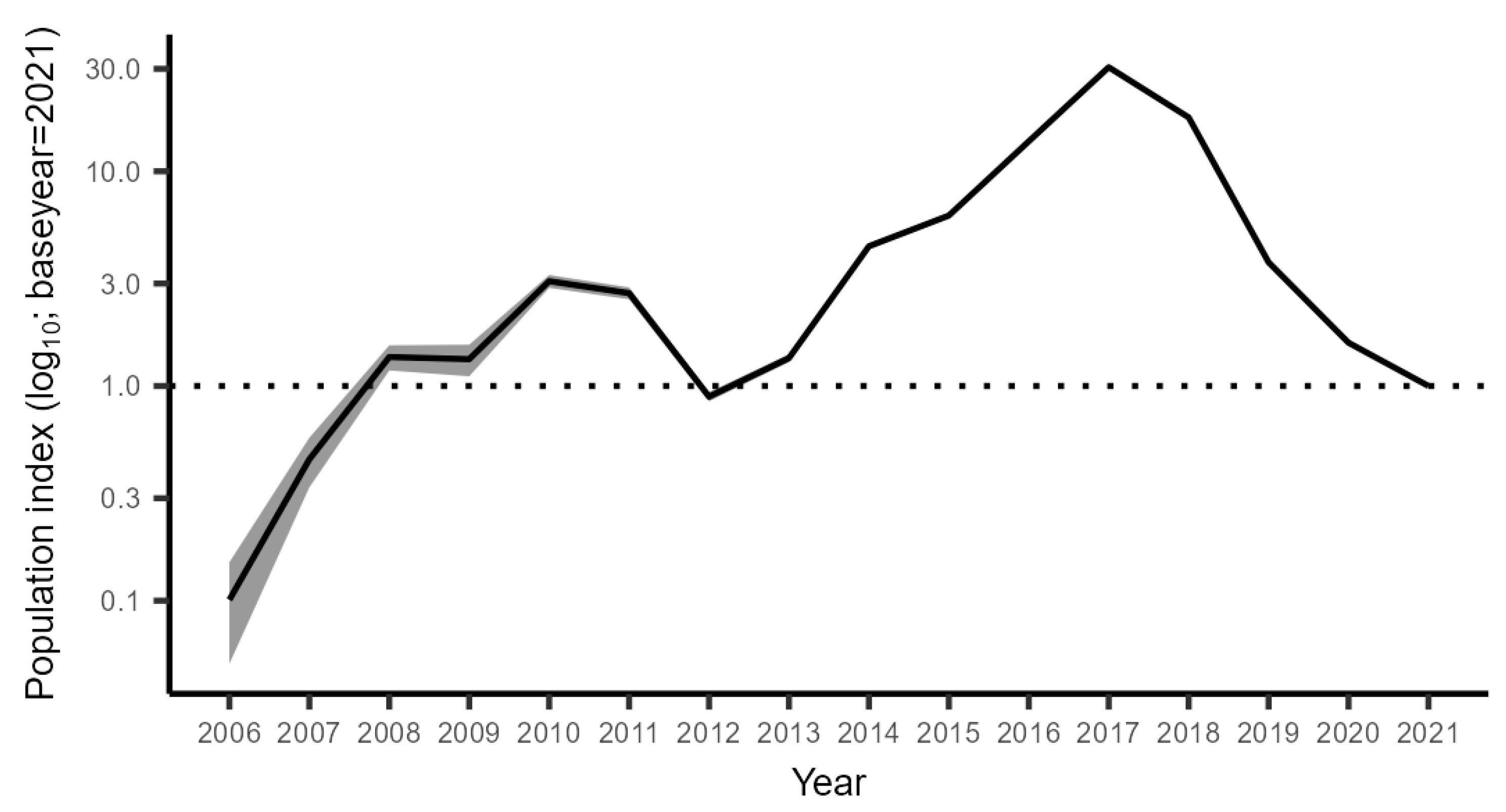
3.2.4. Combined Trend across All Monitoring Data
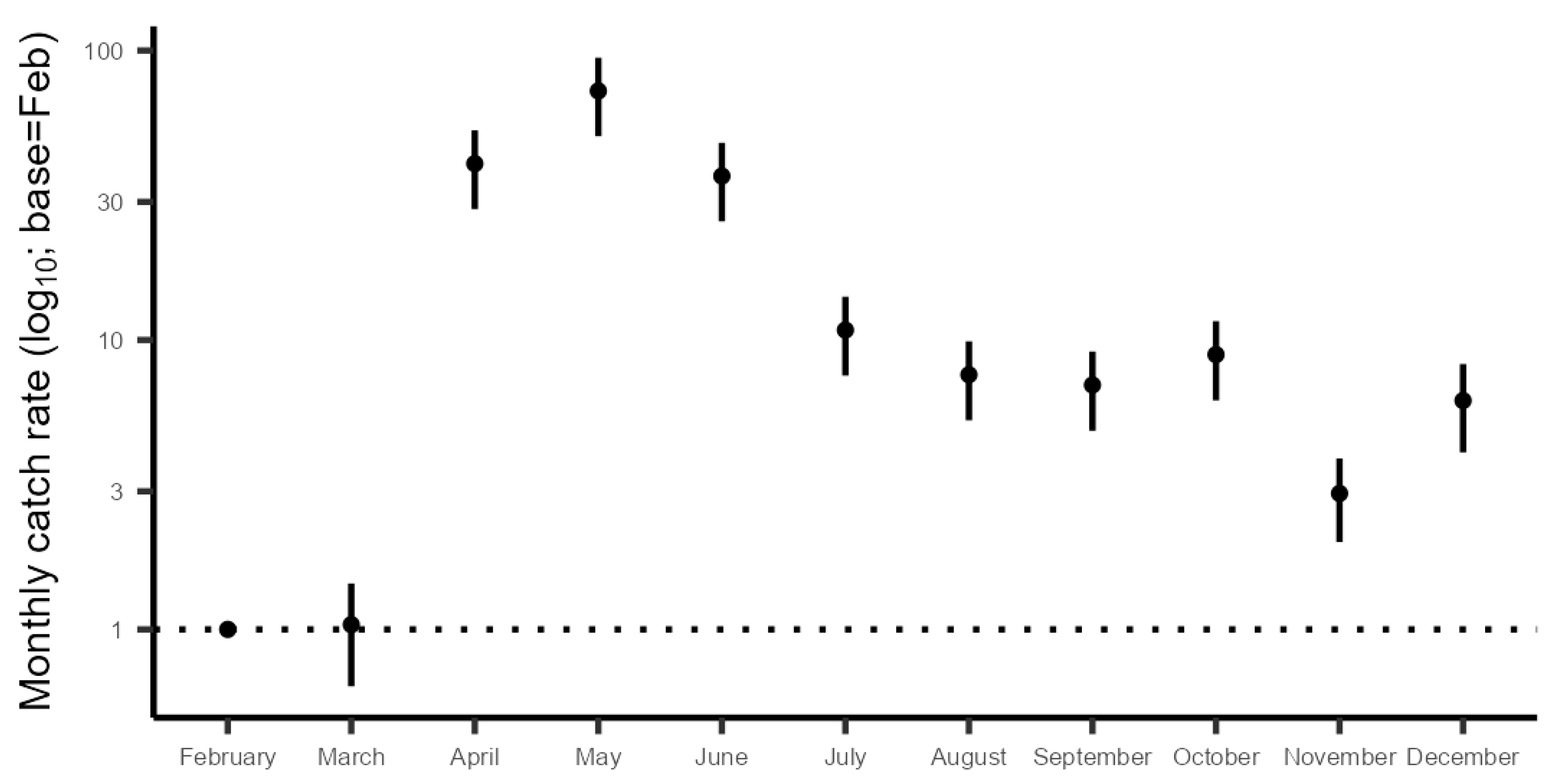
3.2.5. Monthly Catch Rates
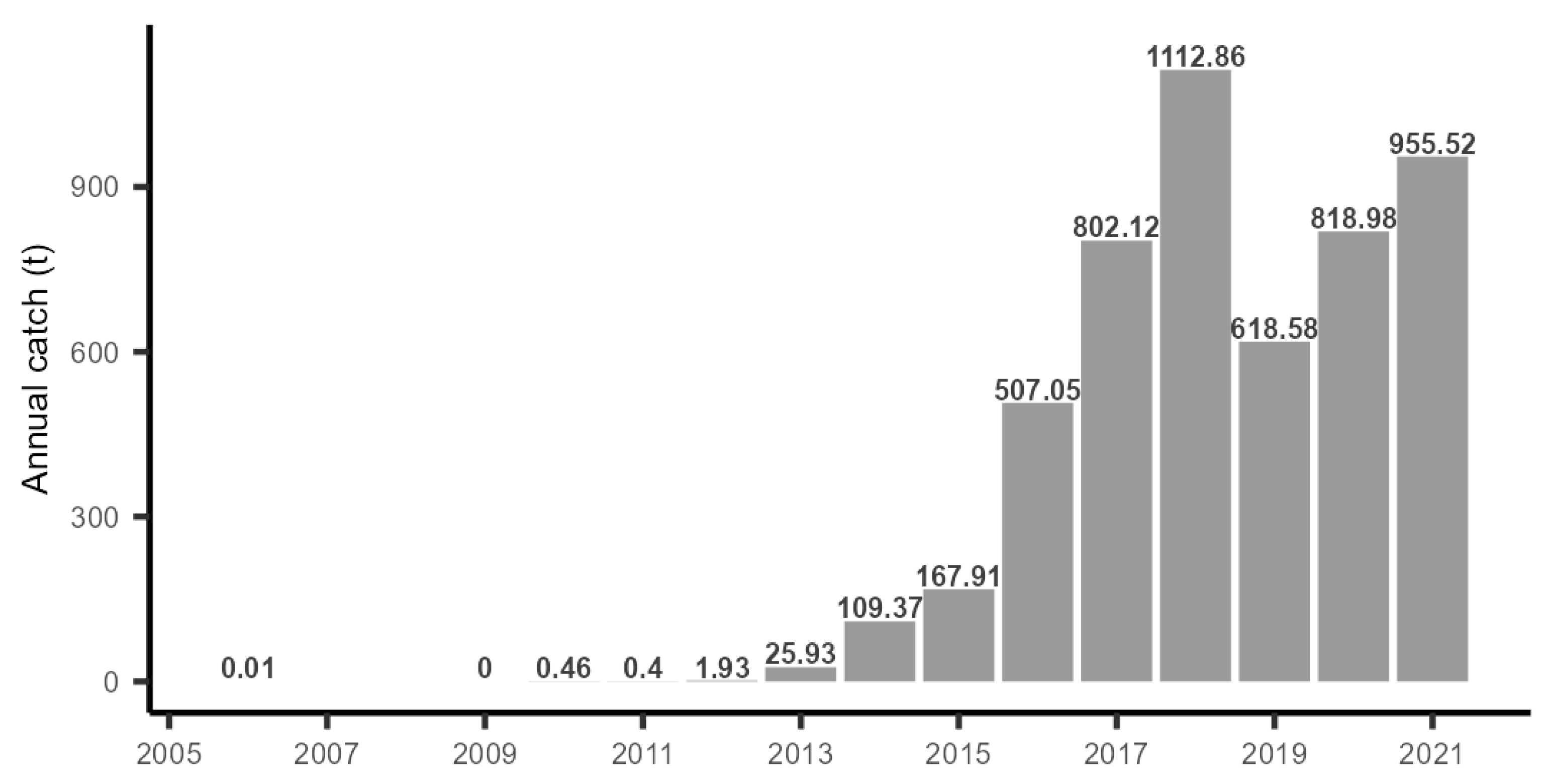
3.3. Catch Records in the Coastal Fishery
4. Discussion
4.1. Intercalibration
4.2. Population Development
4.3. Suggestions for Future Monitoring Programs on RG
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

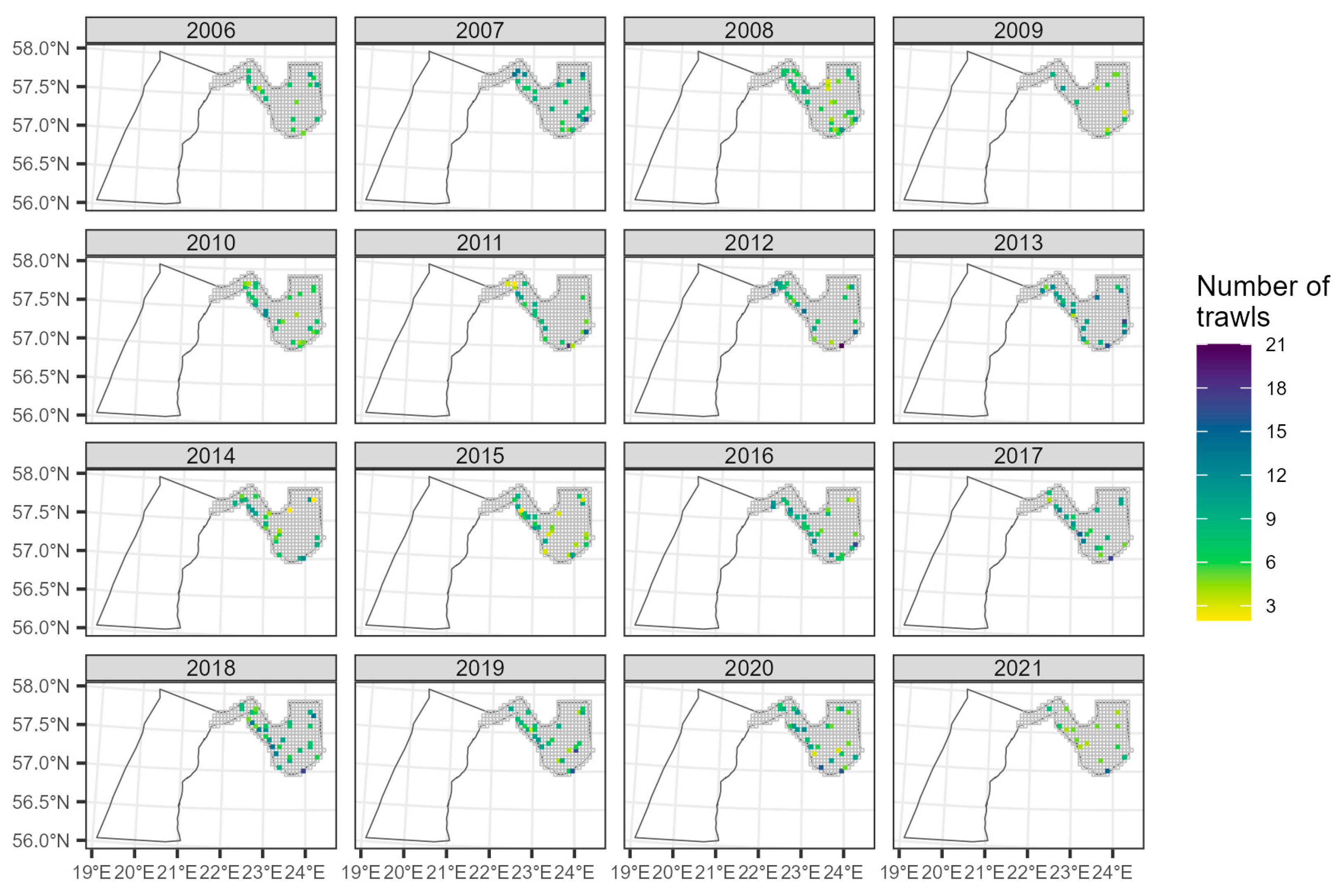
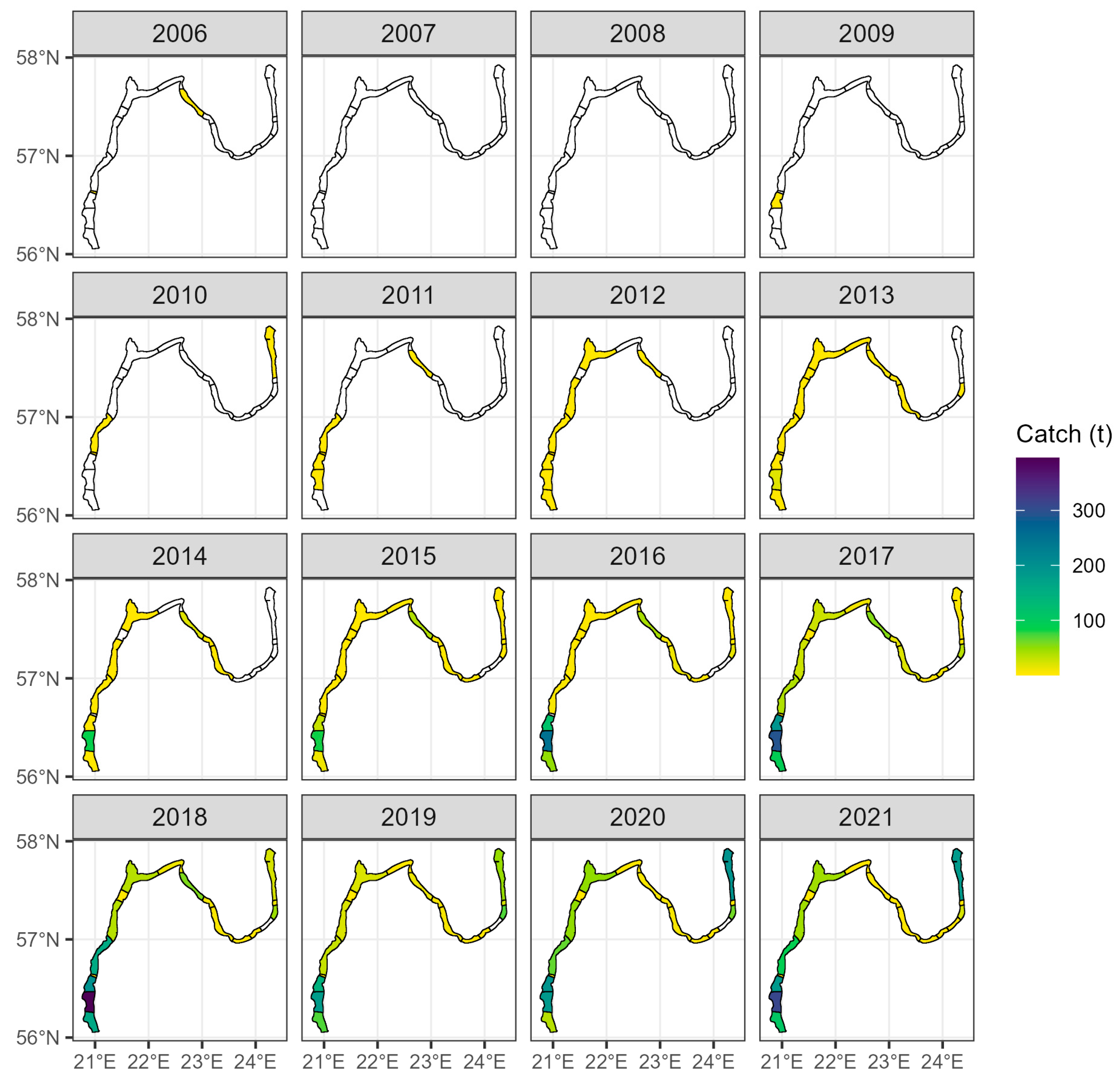
Appendix B
| Length (cm) | Proportion in “Coastal” | Proportion in “Coastal” (95%CI) | Multiplicator for “Nordic” (to Obtain Values at “Coastal”) | Multiplicator (95%CI) | ||
|---|---|---|---|---|---|---|
| 5 | 0.0002 | 0.0001 | 0.0006 | 0.0002 | 0.0001 | 0.0006 |
| 6 | 0.0009 | 0.0003 | 0.0027 | 0.0009 | 0.0003 | 0.0027 |
| 7 | 0.0048 | 0.0020 | 0.0116 | 0.0048 | 0.0020 | 0.0118 |
| 8 | 0.0242 | 0.0115 | 0.0503 | 0.0248 | 0.0116 | 0.0529 |
| 9 | 0.1127 | 0.0617 | 0.1968 | 0.1270 | 0.0658 | 0.2451 |
| 10 | 0.3901 | 0.2597 | 0.5385 | 0.6397 | 0.3508 | 1.1666 |
| 11 | 0.7121 | 0.5783 | 0.8169 | 2.4734 | 1.3713 | 4.4612 |
| 12 | 0.8391 | 0.7437 | 0.9036 | 5.2138 | 2.9012 | 9.3699 |
| 13 | 0.8582 | 0.7713 | 0.9156 | 6.0506 | 3.3725 | 10.8554 |
| 14 | 0.8428 | 0.7483 | 0.9063 | 5.3620 | 2.9729 | 9.6712 |
| 15 | 0.8347 | 0.7370 | 0.9010 | 5.0503 | 2.8022 | 9.1022 |
| 16 | 0.8460 | 0.7525 | 0.9085 | 5.4953 | 3.0405 | 9.9319 |
| 17 | 0.8576 | 0.7687 | 0.9161 | 6.0249 | 3.3236 | 10.9215 |
| 18 | 0.8534 | 0.7630 | 0.9132 | 5.8203 | 3.2201 | 10.5201 |
| 19 | 0.8361 | 0.7373 | 0.9027 | 5.1028 | 2.8069 | 9.2769 |
| 20 | 0.8234 | 0.7191 | 0.8946 | 4.6618 | 2.5599 | 8.4897 |
| 21 | 0.8267 | 0.7200 | 0.8985 | 4.7711 | 2.5718 | 8.8514 |
| 22 | 0.8374 | 0.7170 | 0.9128 | 5.1491 | 2.5334 | 10.4654 |
| 23 | 0.8478 | 0.7022 | 0.9294 | 5.5724 | 2.3583 | 13.1670 |
| 24 | 0.8578 | 0.6797 | 0.9449 | 6.0304 | 2.1223 | 17.1355 |
| 25 | 0.8671 | 0.6521 | 0.9579 | 6.5261 | 1.8741 | 22.7255 |
| 26 | 0.8760 | 0.6208 | 0.9682 | 7.0626 | 1.6368 | 30.4736 |
References
- Sapota, M.R. The Round Goby (Neogobius melanostomus) in the Gulf of Gdańsk—A Species Introduction into the Baltic Sea. Hydrobiologia 2004, 514, 219–224. [Google Scholar] [CrossRef]
- Kuczyński, J. Babka krągła N. melanostomus (Pallas, 1811)—Emigrant z Basenu Pontokaspijskiego w Zatoce Gdańskiej. Bull. Sea Fish. Inst. 1995, 135, 68–71. [Google Scholar]
- Sciences, A. Abundance and Distribution of Round Goby. Available online: https://helcom.fi/wp-content/uploads/2020/06/BSEFS-Abundance-and-distribution-of-round-goby.pdf (accessed on 18 May 2023).
- ICES. Ices Scientific Reports Rapports Scientifiques Du Ciem Ices Workshop on Stickleback and Round Goby in the Baltic Sea (Wkstargate); ICES: Copenhagen, Denmark, 2022. [Google Scholar] [CrossRef]
- Ojaveer, E. Ecosystems and Living Resources of the Baltic Sea; Springer: Cham, Switzerland, 2017; ISBN 9783319530093. [Google Scholar]
- Holmes, M.; Kotta, J.; Persson, A.; Sahlin, U. Marine Protected Areas Modulate Habitat Suitability of the Invasive Round Goby (Neogobius melanostomus) in the Baltic Sea. Estuar. Coast. Shelf Sci. 2019, 229, 106380. [Google Scholar] [CrossRef]
- Saraiva, S.; Markus Meier, H.E.; Andersson, H.; Höglund, A.; Dieterich, C.; Gröger, M.; Hordoir, R.; Eilola, K. Uncertainties in Projections of the Baltic Sea Ecosystem Driven by an Ensemble of Global Climate Models. Front. Earth Sci. 2019, 6, 244. [Google Scholar] [CrossRef]
- Christensen, E.A.F.; Norin, T.; Tabak, I.; Van Deurs, M.; Behrens, J.W. Effects of Temperature on Physiological Performance and Behavioral Thermoregulation in an Invasive Fish, the Round Goby. J. Exp. Biol. 2021, 224, jeb237669. [Google Scholar] [CrossRef] [PubMed]
- Reid, H.B.; Ricciardi, A. Ecological Responses to Elevated Water Temperatures across Invasive Populations of the Round Goby (Neogobius melanostomus) in the Great Lakes Basin. Can. J. Fish. Aquat. Sci. 2022, 79, 277–288. [Google Scholar] [CrossRef]
- Kornis, M.S.; Mercado-Silva, N.; vander Zanden, M.J. Twenty Years of Invasion: A Review of Round Goby Neogobius melanostomus Biology, Spread and Ecological Implications. J. Fish Biol. 2012, 80, 235–285. [Google Scholar] [CrossRef]
- Kotta, J.; Nurkse, K.; Puntila, R.; Ojaveer, H. Shipping and Natural Environmental Conditions Determine the Distribution of the Invasive Non-Indigenous Round Goby Neogobius melanostomus in a Regional Sea. Estuar. Coast. Shelf Sci. 2016, 169, 15–24. [Google Scholar] [CrossRef]
- Azour, F.; van Deurs, M.; Behrens, J.; Carl, H.; Hüssy, K.; Greisen, K.; Ebert, R.; Møller, P.R. Invasion Rate and Population Characteristics of the Round Goby Neogobius melanostomus: Effects of Density and Invasion History. Aquat. Biol. 2015, 24, 41–52. [Google Scholar] [CrossRef]
- Ustups, D.; Bergström, U.; Florin, A.B.; Kruze, E.; Zilniece, D.; Elferts, D.; Knospina, E.; Uzars, D. Diet Overlap between Juvenile Flatfish and the Invasive Round Goby in the Central Baltic Sea. J. Sea Res. 2016, 107, 121–129. [Google Scholar] [CrossRef]
- Nurkse, K.; Kotta, J.; Orav-Kotta, H.; Ojaveer, H. A Successful Non-Native Predator, Round Goby, in the Baltic Sea: Generalist Feeding Strategy, Diverse Diet and High Prey Consumption. Hydrobiologia 2016, 777, 271–281. [Google Scholar] [CrossRef]
- Matern, S.; Herrmann, J.P.; Temming, A. Differences in Diet Compositions and Feeding Strategies of Invasive Round Goby Neogobius melanostomus and Native Black Goby Gobius Niger in the Western Baltic Sea. Aquat. Invasions 2021, 16, 314–328. [Google Scholar] [CrossRef]
- Almqvist, G.; Strandmark, A.K.; Appelberg, M. Has the Invasive Round Goby Caused New Links in Baltic Food Webs? Environ. Biol. Fishes 2010, 89, 79–93. [Google Scholar] [CrossRef]
- Crane, D.P.; Farrell, J.M.; Einhouse, D.W.; Lantry, J.R.; Markham, J.L. Trends in Body Condition of Native Piscivores Following Invasion of Lakes Erie and Ontario by the Round Goby. Freshw. Biol. 2015, 60, 111–124. [Google Scholar] [CrossRef]
- Hempel, M.; Neukamm, R.; Thiel, R. Effects of Introduced Round Goby (Neogobius melanostomus) on Diet Composition and Growth of Zander (Sander lucioperca), a Main Predator in European Brackish Waters. Aquat. Invasions 2016, 11, 167–178. [Google Scholar] [CrossRef]
- Oesterwind, D.; Bock, C.; Förster, A.; Gabel, M.; Henseler, C.; Kotterba, P.; Menge, M.; Myts, D.; Winkler, H.M. Predator and Prey: The Role of the Round Goby Neogobius melanostomus in the Western Baltic. Mar. Biol. Res. 2017, 13, 188–197. [Google Scholar] [CrossRef]
- Rakauskas, V.; Putys, Ž.; Dainys, J.; Lesutiene, J.; Ložys, L.; Arbačiauskas, K. Increasing Population of the Invader Round Goby, Neogobius melanostomus (Actinopterygii: Perciformes: Gobiidae), and Its Trophic Role in the Curonian Lagoon, SE Baltic Sea. Acta Ichthyol. Piscat. 2013, 43, 95–108. [Google Scholar] [CrossRef]
- Jakubas, D. The Response of the Grey Heron to a Rapid Increase of the Round Goby. Waterbirds 2004, 27, 304–307. [Google Scholar] [CrossRef]
- Behrens, J.W.; Ryberg, M.P.; Einberg, H.; Eschbaum, R.; Florin, A.B.; Grygiel, W.; Herrmann, J.P.; Huwer, B.; Hüssy, K.; Knospina, E.; et al. Seasonal Depth Distribution and Thermal Experience of the Non-Indigenous Round Goby Neogobius melanostomus in the Baltic Sea: Implications to Key Trophic Relations. Biol. Invasions 2022, 24, 527–541. [Google Scholar] [CrossRef]
- Karlson, A.M.L.; Almqvist, G.; Skóra, K.E.; Appelberg, M. Indications of Competition between Non-Indigenous Round Goby and Native Flounder in the Baltic Sea. ICES J. Mar. Sci. 2007, 64, 479–486. [Google Scholar] [CrossRef]
- Järv, L.; Kotta, J.; Kotta, I.; Raid, T. Linking the Structure of Benthic Invertebrate Communities and the Diet of Native and Invasive Fish Species in a Brackish Water Ecosystem. Ann. Zool. Fennici 2011, 48, 129–141. [Google Scholar] [CrossRef]
- Funk, S.; Frelat, R.; Möllmann, C.; Temming, A.; Krumme, U. The Forgotten Feeding Ground: Patterns in Seasonal and Depth-Specific Food Intake of Adult Cod Gadus Morhua in the Western Baltic Sea. J. Fish Biol. 2021, 98, 707–722. [Google Scholar] [CrossRef] [PubMed]
- Bowser, J.; Galarowicz, T.; Murry, B.; Johnson, J. Invasive Species Appearance and Climate Change Correspond with Dramatic Regime Shift in Thermal Guild Composition of Lake Huron Beach Fish Assemblages. Fishes 2022, 7, 263. [Google Scholar] [CrossRef]
- Liversage, K.; Nurkse, K.; Kotta, J.; Järv, L. Environmental Heterogeneity Associated with European Perch (Perca fluviatilis) Predation on Invasive Round Goby (Neogobius melanostomus). Mar. Environ. Res. 2017, 132, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Pannekoek, J.; Van Strien, A. TRIM 3 Manual (TRends & Indices for Monitoring Data); Statistics Netherlands: Hague, The Netherlands, 2005; pp. 1–58. [Google Scholar]
- Bernes, C. Change Beneath the Surface: An In-Depth Look at Sweden’s Marine Environment; Royal Swedish Academy of Sciences: Stockholm, Sweden, 2005; ISBN 9162012460, 9789162012465. [Google Scholar]
- Neuman, E.; Sandstrom, O.G.T. Guidelines for Coastal Fish Monitoring. Natl. Board Fish. Inst. Coast. Reserch 1997, 44, 1–19. [Google Scholar]
- ICES Manual for the Baltic International Trawl Surveys (BITS) Version 2.0; ICES: Copenhagen, Denmark, 2017; ISBN 9788774821533.
- Olsson, J.; Bergström, L.; Gårdmark, A. Abiotic Drivers of Coastal Fish Community Change during Four Decades in the Baltic Sea-ICES. J. Mar. Sci. 2012, 69, 961–970. [Google Scholar] [CrossRef]
- Wood, S.N. Generalized Additive Models: An Introduction with R; Chapman and Hall/CRC: Boca Raton, FL, USA, 2006. [Google Scholar]
- Burnham, K.P.; Anderson, D.R. Model Selection and Multimodel Inference: A Practical Information-Theoretic Approach, 2nd ed.; Springer: New York, NY, USA, 2002; ISBN 0387953647. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2022. [Google Scholar]
- Wood, S.; Scheipl, F. Gamm4: Generalized Additive Mixed Models Using “mgcv” and “Lme4”. 2020. Available online: https://cran.r-project.org/web/packages/gamm4/gamm4.pdf (accessed on 10 February 2023).
- Wood, S.N. Fast Stable Restricted Maximum Likelihood and Marginal Likelihood Estimation of Semiparametric Generalized Linear Models. J. R. Stat. Soc. 2011, 73, 3–36. [Google Scholar] [CrossRef]
- Wickham, H.; Averick, M.; Bryan, J.; Chang, W.; McGowan, L.; François, R.; Grolemund, G.; Hayes, A.; Henry, L.; Hester, J.; et al. Welcome to the Tidyverse. J. Open Source Softw. 2019, 4, 1686. [Google Scholar] [CrossRef]
- Sokal, R.R.; Rohlf, F.J.J. Biometry: The Principles and Practice of Statistics in Biological Research, 3rd ed.; W.H. Freeman and Company: New York, NY, USA, 1995; ISBN 9780716724117. [Google Scholar]
- Pannekoek, J.; Bogaart, P.; van der Loo, M. Models and Statistical Methods in Rtrim; CBS Discussion Paper; Statistics Netherlands: Hague, The Netherlands, 2018; pp. 1–34. [Google Scholar]
- Van Strien, A.J.; Pannekoek, J.; Gibbons, D.W. Indexing European Bird Population Trends Using Results of National Monitoring Schemes: A Trial of a New Method. Bird Study 2001, 48, 200–213. [Google Scholar] [CrossRef]
- Bogaart, P.; van der Loo, M.; Pannekoek, J. Rtrim: Trends and Indices for Monitoring Data; Statistics Netherlands: Voorburg, The Netherlands, 2020. [Google Scholar]
- Urban-Malinga, B.; Wodzinowski, T.; Witalis, B.; Zalewski, M.; Radtke, K.; Grygiel, W. Marine Litter on the Seafloor of the Southern Baltic. Mar. Pollut. Bull. 2018, 127, 612–617. [Google Scholar] [CrossRef]
- Rotherham, D.; Gray, C.A.; Broadhurst, M.K.; Johnson, D.D.; Barnes, L.M.; Jones, M.V. Sampling Estuarine Fish Using Multi-Mesh Gill Nets: Effects of Panel Length and Soak and Setting Times. J. Exp. Mar. Bio. Ecol. 2006, 331, 226–239. [Google Scholar] [CrossRef]
- Specziár, A.; Eros, T.; György, Á.I.; Tátrai, I.; Bíró, P. A Comparison between the Benthic Nordic Gillnet and Whole Water Column Gillnet for Characterizing Fish Assemblages in the Shallow Lake Balaton. Ann. Limnol. 2009, 45, 171–180. [Google Scholar] [CrossRef]
- Zuur, A.F.; Ieno, E.N.; Walker, N.J.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Springer: New York, NY, USA, 2009; ISBN 9780387874579. [Google Scholar]
- Olsson, J.; Andersson, M.L.; Bergström, U.; Arlinghaus, R.; Audzijonyte, A.; Berg, S.; Briekmane, L.; Dainys, J.; Ravn, H.D.; Droll, J.; et al. A Pan-Baltic Assessment of Temporal Trends in Coastal Pike Populations. Fish. Res. 2023, 260, 106594. [Google Scholar] [CrossRef]
- Lappalainen, A.; Hyvönen, J.; Söderkultalahti, P.; Heikkinen, J. Estimating Annual Cpue Indices for Perch (Perca fluviatilis) from Monthly Logbook Data of a Gill-Net Fishery in the Bothnian Bay, Baltic Sea. Boreal Environ. Res. 2020, 25, 79–91. [Google Scholar]
- Kortsch, S.; Frelat, R.; Pecuchet, L.; Olivier, P.; Putnis, I.; Bonsdorff, E.; Ojaveer, H.; Jurgensone, I.; Strāķe, S.; Rubene, G.; et al. Disentangling Temporal Food Web Dynamics Facilitates Understanding of Ecosystem Functioning. J. Anim. Ecol. 2021, 90, 1205–1216. [Google Scholar] [CrossRef] [PubMed]
- Andersson, L. Hydrography and Oxygen in the Deep Basins. HELCOM Balt. Sea Environ. Fact Sheets. 2014, Volume 1–6. Available online: https://helcom.fi/wp-content/uploads/2019/08/BSEFS_Hydrography-and-oxygen-in-the-deep-basins_2010.pdf (accessed on 18 May 2023).
- Casini, M.; Hansson, M.; Orio, A.; Limburg, K. Changes in Population Depth Distribution and Oxygen Stratification Are Involved in the Current Low Condition of the Eastern Baltic Sea Cod (Gadus morhua). Biogeosciences 2021, 18, 1321–1331. [Google Scholar] [CrossRef]
- Tiralongo, F.; Messina, G.; Lombardo, B.M. Invasive Species Control: Predation on the Alien Crab Percnon gibbesi (H. Milne Edwards, 1853) (Malacostraca: Percnidae) by the Rock Goby, Gobius paganellus Linnaeus, 1758 (Actinopterygii: Gobiidae). J. Mar. Sci. Eng. 2021, 9, 393. [Google Scholar] [CrossRef]
- Ustups, D. Latvian Fisheries Yearbook; The Latvian Rural Advisory and Training Centre: Ozolnieku pagasts, Latvia, 2021; Volume 25. [Google Scholar]


| Random Effects Used | AICc | marginalR2 | conditionalR2 | ICC |
|---|---|---|---|---|
| Baseline (no random effects) | 1356.268 | 0.582 | - | - |
| 1|Area | 1299.265 | 0.723 | 0.752 | 0.106 |
| 1|Quarter | 1355.696 | 0.703 | 0.709 | 0.020 |
| 1|Station | 1307.899 | 0.723 | 0.749 | 0.092 |
| 1|Area/Station | 1309.917 | 0.722 | 0.752 | 0.107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kruze, E.; Avotins, A.; Rozenfelde, L.; Putnis, I.; Sics, I.; Briekmane, L.; Olsson, J. The Population Development of the Invasive Round Goby Neogobius melanostomus in Latvian Waters of the Baltic Sea. Fishes 2023, 8, 305. https://doi.org/10.3390/fishes8060305
Kruze E, Avotins A, Rozenfelde L, Putnis I, Sics I, Briekmane L, Olsson J. The Population Development of the Invasive Round Goby Neogobius melanostomus in Latvian Waters of the Baltic Sea. Fishes. 2023; 8(6):305. https://doi.org/10.3390/fishes8060305
Chicago/Turabian StyleKruze, Eriks, Andris Avotins, Loreta Rozenfelde, Ivars Putnis, Ivo Sics, Laura Briekmane, and Jens Olsson. 2023. "The Population Development of the Invasive Round Goby Neogobius melanostomus in Latvian Waters of the Baltic Sea" Fishes 8, no. 6: 305. https://doi.org/10.3390/fishes8060305
APA StyleKruze, E., Avotins, A., Rozenfelde, L., Putnis, I., Sics, I., Briekmane, L., & Olsson, J. (2023). The Population Development of the Invasive Round Goby Neogobius melanostomus in Latvian Waters of the Baltic Sea. Fishes, 8(6), 305. https://doi.org/10.3390/fishes8060305