A Geospatial Approach to Improving Fish Species Detection in Maumee Bay, Lake Erie
Abstract
1. Introduction
2. Methods
2.1. Study Area
2.2. Fish Sampling
2.3. Geospatial Analysis
2.4. Species Accumulation Curve
3. Results
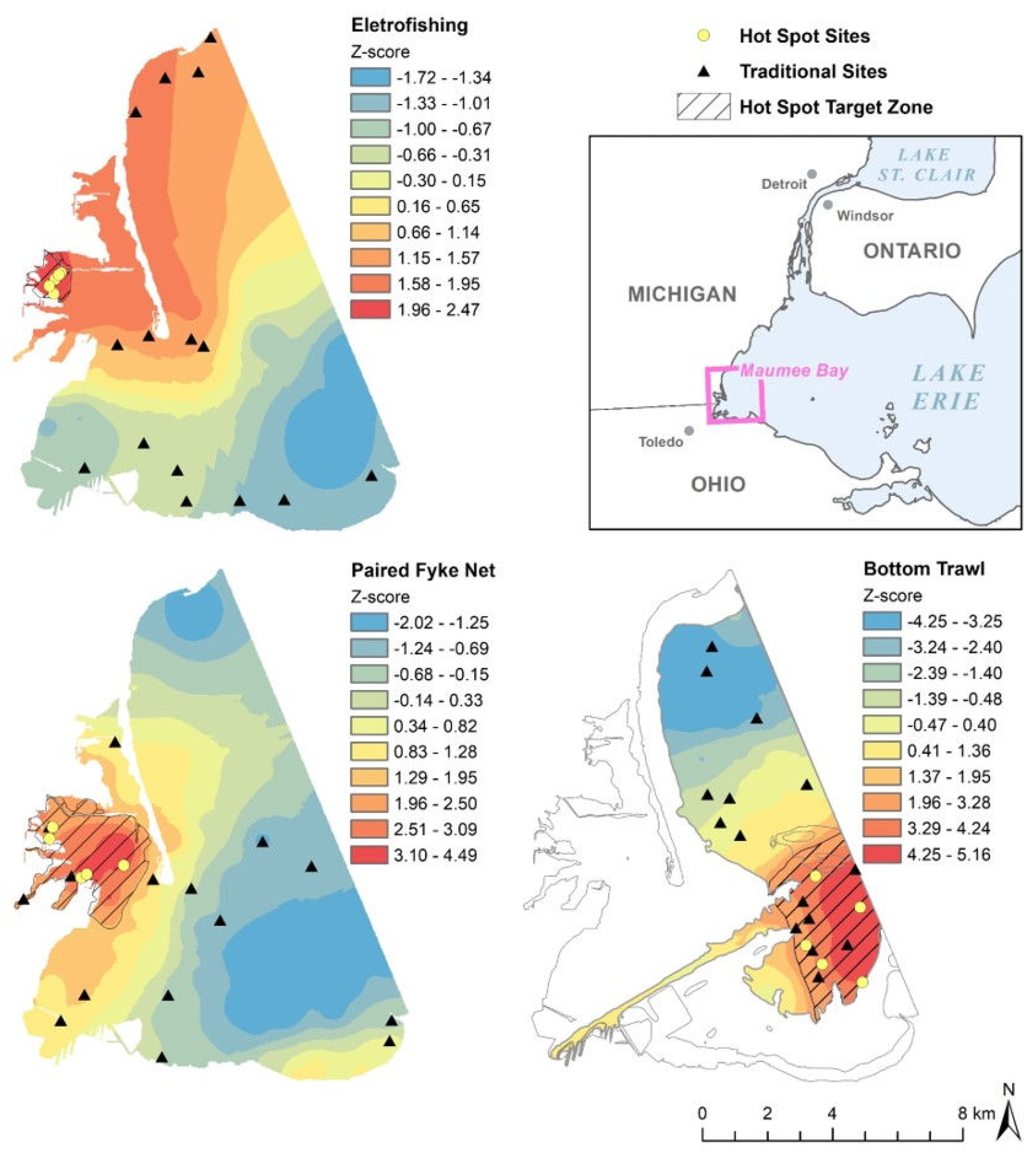
3.1. Hot Spot Determination
3.2. Random vs. Hybrid Sampling
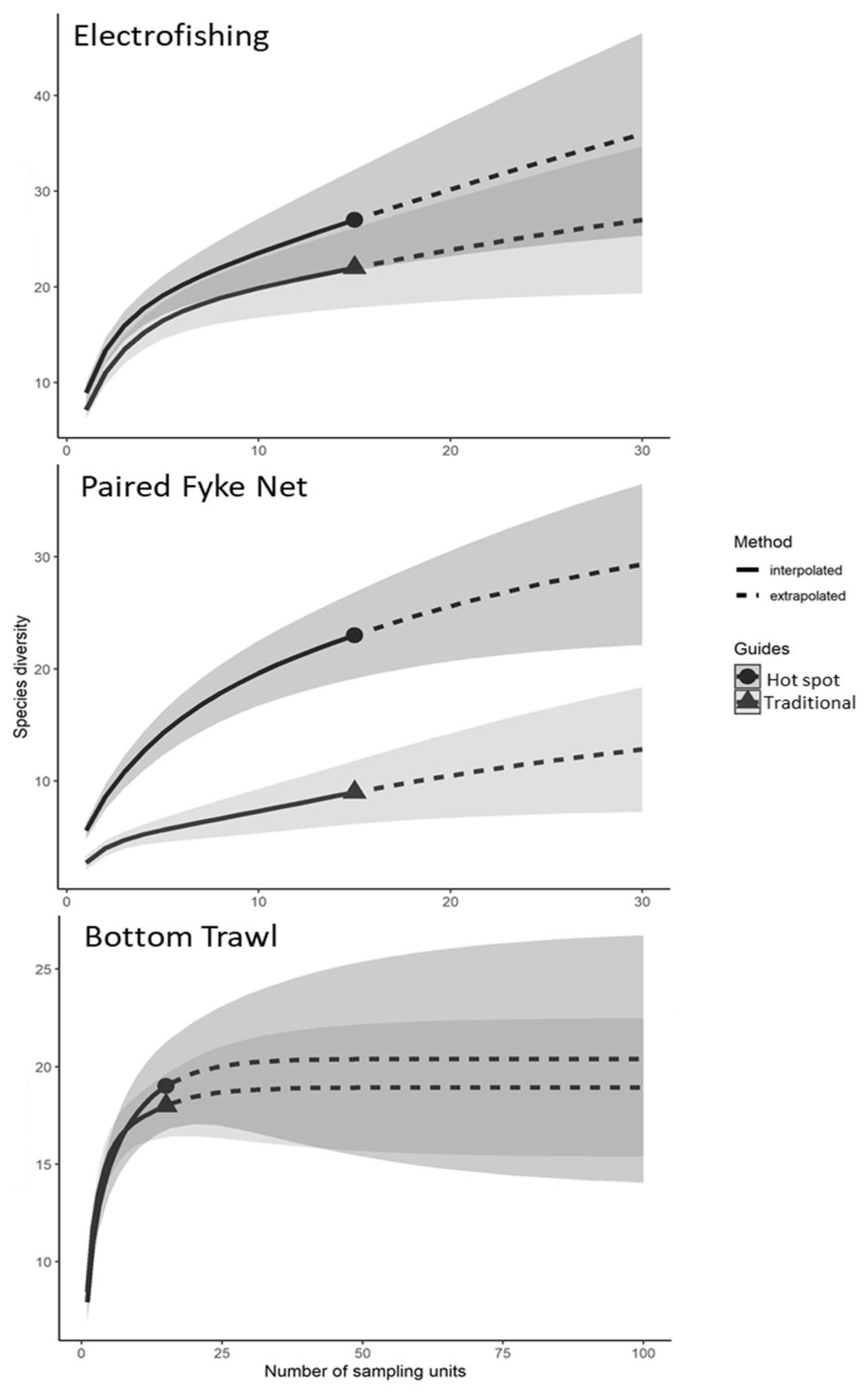
3.3. Species Accumulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cao, Y.; Williams, D.D.; Williams, N.E. How important are rare species in aquatic community ecology and bioassessment? Limnol. Oceanogr. 1998, 43, 1403–1409. [Google Scholar] [CrossRef]
- Jerde, C.L.; Mahon, A.R.; Chadderton, W.L.; Lodge, D.M. “Sight-unseen” detection of rare aquatic species using environmental DNA. Conserv. Lett. 2011, 4, 150–157. [Google Scholar] [CrossRef]
- Harvey, R.G.; Mazzotti, F.J. The Invasion Curve: A Tool for Understanding Invasive Species Management in South Florida. IFAS Publication Number; WEC347. University of Florida: Gainesville, FL, USA, 2014. Available online: https://edis.ifas.ufl.edu/publication/UW392 (accessed on 18 December 2022).
- Magnuson, J.J.; Benson, B.J.; McLain, A.S. Insights on species richness and turnover from long-term ecological research: Fishes in north temperate lakes. Am. Zool. 1994, 34, 437–451. [Google Scholar] [CrossRef]
- Hoffman, J.C.; Kelly, J.R.; Trebitz, A.S.; Peterson, G.S.; West, C.W. Effort and potential efficiencies for aquatic non-native species early detection. Can. J. Fish. Aquat. Sci. 2011, 68, 2064–2079. [Google Scholar] [CrossRef]
- Trebitz, A.S.; Hoffman, J.C.; Darling, J.A.; Pilgrim, E.M.; Kelly, J.R.; Brown, E.A.; Chadderton, W.L.; Egan, S.P.; Grey, E.K.; Hashsham, S.A.; et al. Early detection monitoring for aquatic non-indigenous species: Optimizing surveillance, incorporating, advanced technologies, and identifying, research needs. J. Environ. Manage. 2017, 202, 299–310. [Google Scholar] [CrossRef]
- MICRA (Mississippi Interstate Cooperative Resource Association). Asian carp threat to the Great Lakes. River Crossings Newsl. Miss. Interstate Coop. Resour. Assoc. 2002, 11, 1–2. [Google Scholar]
- Ricciardi, A. Patterns of invasion in the Laurentian Great Lakes in relation to changes in vector activity. Divers. Distrib. 2006, 12, 425–433. [Google Scholar] [CrossRef]
- Trebitz, A.S.; Kelly, J.R.; Hoffman, J.C.; Peterson, G.S.; West, C.W. Exploiting habitat and gear patterns for efficient detection of rare and non-native benthos and fish in Great Lakes coastal ecosystems. Aquat. Invasions 2009, 4, 651–667. [Google Scholar] [CrossRef]
- USFWS. Strategic framework for the early detection in monitoring of non-native fishes and select benthic macroinvertebrates in the Great Lakes. In Great Lakes Comprehensive Aquatic Invasive Species Early Detection Monitoring Plan; U. S. Fish and Wildlife Service: Bloomington, MN, USA, 2014. [Google Scholar]
- Harris, B.S.; Smith, B.J.; Hayer, C.A. Development and implementation of an adaptive management approach for monitoring non-indigenous fishes in lower Green Bay, Lake Michigan. J. Great Lakes Res. 2018, 44, 960–969. [Google Scholar] [CrossRef]
- Tucker, A.J.; Chadderton, W.L.; Annis, G.; Davidson, A.D.; Hoffman, J.; Bossenbroek, J.; Hensler, S.; Hoff, M.; Jensen, E.; Kashian, D.; et al. A framework for aquatic species surveillance site selection and prioritization in the US waters of the Laurentian Great Lakes. Manag. Biol. Invasions 2020, 11, 607–632. [Google Scholar] [CrossRef]
- USFWS. Lake Erie Implementation Plan for the Early Detection of Non-Native Fishes and Select Benthic Macroinvertebrates; U. S. Fish and Wildlife Service, Alpena Fish and Wildlife Conservation Office, Alpena, Michigan and Lower Great Lakes Fish and Wildlife Conservation Office: Basom, NY, USA, 2016.
- Soberon, J.; Llorente, J. The use of species accumulation functions for the prediction of species richness. Conserv. Biol. 1993, 3, 480–488. [Google Scholar] [CrossRef]
- USFWS. Early Detection and Monitoring of Non-Native Fishes and Benthic Macroinvertebrates in Lake Erie, 2016; U. S. Fish and Wildlife Service, Alpena Fish and Wildlife Conservation Office, Alpena, Michigan and U. S. Fish and Wildlife Service, Lower Great Lakes Fish and Wildlife Conservation Office: Basom, NY, USA, 2017.
- Ord, J.K.; Getis, A. Local Spatial Autocorrelation Statistics: Distributional Issues and an Application. Geogr. Anal. 1995, 27, 286–306. [Google Scholar] [CrossRef]
- Stohlgren, T.J.; Binkley, D.; Chong, G.W.; Kalkhan, M.A.; Schell, L.D.; Bull, K.A.; Otsuki, Y.; Newman, G.; Bashkin, M.; Son, Y. Exotic plant species invade hot spots of native plant diversity. Ecol. Monogr. 1999, 69, 25–46. [Google Scholar] [CrossRef]
- USFWS. Recommended Sampling Gear Types and Standard Operating Procedures for the Early Detection of Non-Native Fishes and Select Benthic Macroinvertebrates in the Great Lakes; U. S. Fish and Wildlife Service: Bloomington, MN, USA, 2014.
- Hayer, C.-A. Recommended Sampling Gear Types and Standard Operating Procedures for the Early Detection of Non-native Fishes and Select Benthic Macroinvertebrates in the Great Lakes. U.S. Fish and Wildlife Service, Green Bay Fish and Wildlife Conservation Office Report Number: 2017-014; Green Bay Fish and Wildlife Conservation Office: New Franken, WI, USA, 2018.
- Jalali, M.A.; Ierodiaconou, D.; Gorfine, H.; Monk, J.; Rattray, A. Exploring spatiotemporal trends in commercial fishing effort of an Abalone fishing zone: A GIS-based hotspot model. PLoS ONE 2015, 10, e0122995. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Cuervo, A.M.; Aide, T.M. Identifying hotspots of deforestation and reforestation in Colombia (2001-2010): Implications for protected areas. Ecosphere 2013, 4, 1–21. [Google Scholar] [CrossRef]
- Hsieh, T.C.; Ma, K.H.; Chao, A. iNext: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R. RStudio. PBC: Boston, MA, USA, 2020. Available online: http://www.rstudio.com (accessed on 18 December 2022).
- Chao, A.; Colwell, R.K.; Lin, C.-W.; Gotelli, N.J. Sufficient sampling for asymptotic minimum species richness estimators. Ecology 2009, 90, 1125–1133. [Google Scholar] [CrossRef]
- Francis, J.T.; Chiotti, J.A.; Boase, J.C.; Thomas, M.V.; Manny, B.A.; Roseman, E.F. A description of the nearshore fish communities in the Huron-Erie Corridor using multiple gear types. J. Great Lakes Res. 2014, 40, 52–61. [Google Scholar] [CrossRef]
- Hoffman, J.C.; Schloesser, J.; Trebitz, A.S.; Peterson, G.S.; Gutsch, M.; Quinlan, H.; Kelly, J.R. Sampling design for early detection of aquatic invasive species in Great Lakes ports. Fisheries 2016, 41, 27–37. [Google Scholar] [CrossRef]
- Hickley, P.; Chare, S. Fisheries for non-native species in England and Wales: Angling or the environment? Fish. Manage. Ecol. 2004, 11, 203–212. [Google Scholar] [CrossRef]
- Paavola, M.; Olenin, S.; Leppakoski, E. Are invasive species most successful in habitats of low native species richness across European brackish water seas? Estuar. Coast. Shelf. Sci. 2005, 64, 738–750. [Google Scholar] [CrossRef]
| Electrofishing | Paired Fyke Net | Bottom Trawl | |
|---|---|---|---|
| Years | 2014, 2016 | 2013–2016 | 2013, 2015, 2016 |
| # Sites | 24 | 60 | 45 |
| Range of Species | 0–17 | 4–25 | 3–11 |
| Mean of Species (SD) | 4.2 (4.2) | 10.2 (4.0) | 7.4 (1.7) |
| Moran’s I | p = 0.08 | p = 0.02 | p < 0.01 |
| First Peak (m) | 3490.55 | 1627.78 | 2547.81 |
| Electrofishing | Paired Fyke Net | Bottom Trawl | |||||
|---|---|---|---|---|---|---|---|
| Fish Species | Scientific Name | Traditional | Hot spot | Traditional | Hot spot | Traditional | Hot spot |
| Black Crappie * | Pomoxis nigromaculatus | X | |||||
| Bluegill | Lepomis macrochirus | X | X | X | |||
| Bluntnose Minnow | Pimephales notatus | X | X | X | |||
| Bowfin ** | Amia calva | X | |||||
| Brook Silverside | Labidesthes sicculus | X | X | ||||
| Brown Bullhead | Ameiurus nebulosus | X | X | X | X | ||
| Channel Catfish | Ictalurus punctatus | X | X | X | |||
| Common Carp * | Cyprinus carpio carpio | X | |||||
| Common Shiner | Luxilus cornutus | X | |||||
| Emerald Shiner | Notropis atherinoides | X | X | X | X | X | |
| Freshwater Drum | Aplodinotus grunniens | X | X | X | X | X | |
| Gizzard Shad | Dorosoma cepedianum | X | X | X | X | X | X |
| Golden Shiner ** | Notemigonus crysoleucas | X | X | ||||
| Goldfish | Carassius auratus auratus | X | X | X | |||
| Johnny Darter | Etheostoma nigrum | X | X | ||||
| Largemouth Bass | Micropterus salmoides | X | X | X | |||
| Logperch | Percina caprodes | X | X | X | X | X | |
| Longnose Gar * | Lepisosteus osseus | X | |||||
| Mimic Shiner | Notropis volucellus | X | X | X | X | X | X |
| Northern Pike ** | Esox lucius | X | X | ||||
| Pumpkinseed | Lepomis gibbosus | X | X | X | X | ||
| Rock Bass * | Ambloplites rupestris | X | |||||
| Round Goby | Neogobius melanostomus | X | X | X | X | ||
| Sand Shiner | Notropis stramineus | X | X | X | |||
| Shorthead Redhorse ** | Moxostoma macrolepidotum | X | X | ||||
| Silver Chub ** | Macrhybopsis storeriana | X | X | X | |||
| Smallmouth Bass * | Micropterus dolomieu | X | |||||
| Spottail Shiner | Notropis hudsonius | X | X | X | X | ||
| Tadpole Madtom * | Noturus gyrinus | X | |||||
| Trout-Perch | Percopsis omiscomaycus | X | X | ||||
| Walleye | Sander vitreus | X | X | X | X | X | |
| White Bass | Morone chrysops | X | X | X | X | X | |
| White Perch | Morone americana | X | X | X | X | X | |
| White Sucker * | Catostomus commersonii | X | |||||
| Yellow Perch | Perca flavescens | X | X | X | X | X | X |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bowser, J.; Briggs, A.S.; Thompson, P.; McLean, M.; Bowen, A. A Geospatial Approach to Improving Fish Species Detection in Maumee Bay, Lake Erie. Fishes 2023, 8, 3. https://doi.org/10.3390/fishes8010003
Bowser J, Briggs AS, Thompson P, McLean M, Bowen A. A Geospatial Approach to Improving Fish Species Detection in Maumee Bay, Lake Erie. Fishes. 2023; 8(1):3. https://doi.org/10.3390/fishes8010003
Chicago/Turabian StyleBowser, Jessica, Andrew S. Briggs, Patricia Thompson, Matthew McLean, and Anjanette Bowen. 2023. "A Geospatial Approach to Improving Fish Species Detection in Maumee Bay, Lake Erie" Fishes 8, no. 1: 3. https://doi.org/10.3390/fishes8010003
APA StyleBowser, J., Briggs, A. S., Thompson, P., McLean, M., & Bowen, A. (2023). A Geospatial Approach to Improving Fish Species Detection in Maumee Bay, Lake Erie. Fishes, 8(1), 3. https://doi.org/10.3390/fishes8010003







