Abstract
Pre-harvest male maturation is problematic for Atlantic salmon (Salmo salar) farmers and is regulated by the environment and genetics (e.g., vgll3). Five families of all-male salmon parr (produced using YY males crossed with XX females) with different vgll3 genotypes were split between three environmental regimes in January 2018. The “advanced maturation” regime used elevated temperature (16 °C) and continuous light from January 2018 with post-smolt maturation assessed in March 2018. The “extended freshwater” regime used ambient freshwater (1–16 °C) and simulated natural photoperiod (SNP) with post-smolt maturation assessed in November 2018. The “sea transfer” regime used ambient temperatures (1–14 °C) and SNP in freshwater until May 2018 when they were transferred to 9 °C seawater with natural photoperiod for 2.5 years (final mean weight of circa. 14 kg) and assessed for post-smolt maturation, 1 sea-winter (1 SW) maturation, and 2 sea-winter (2 SW) maturation in the autumn (November/December) of 2018, 2019, and 2020, respectively. Post-smolt maturation was highest in the advanced maturation and extended freshwater regimes (39–99% depending on family) and lowest in the sea transfer regime (0–95% depending on family). In the sea transfer regime, maturity incidence increased over time (0–95% post-smolt maturation, 1–100% 1 SW, and 50–90% 2 SW maturation, depending on family). In all regimes, those homozygous for the pre-designated vgll3 “early” maturing allele had the highest incidences of maturation whilst those homozygous for the “late” allele had the lowest. A low percentage of 2 SW phenotypic and genetic females were found (0–5% depending on family), one of which was successfully crossed with an XY male resulting in progeny with an approx. 50/50 sex ratio. These results show (i) post-smolt maturation varies dramatically depending on environment although genetic regulation by vgll3 was as expected, and (ii) crossing YY sperm with XX eggs can result in XX progeny which can themselves produce viable progeny with an equal sex ratio when crossed with an XY male.
Key Contribution:
These results highlight the strong role the environment and genetics play in the timing of male sexual maturation in Atlantic salmon.
1. Introduction
With 2.7 million metric tons produced in 2020 at a market value of $1.5 billion [1], Atlantic salmon (Salmo salar) is one of aquaculture’s most valuable commercial species. Yet despite the industry’s success and rapid growth since its foundations in the 1970’s, there remains significant production challenges. One of these is pre-harvest sexual maturation which leads to reductions in growth, poor seawater tolerance, and an increased likelihood of becoming sick and/or dying [2]. The lipid content of mature fish is also depleted resulting in a pale watery fillet that performs poorly in consumer preference tests [3]. This means the fillets of mature fish have a lower economic value and are either downgraded or thrown out at slaughter.
Grilsing has traditionally been the most problematic precocious phenotype in salmon farming. The term refers to individuals which mature after only one winter in seawater at around 1–5 kg [2]. However, the rise of recirculating aquaculture systems (RAS) to farm salmon outside its normal geographical range (e.g., China [4]) or to reduce the biomass at sea in locations with heavy sea lice infestations (e.g., Norway [5]) has been hindered by precocious sexual maturation with “jacking” becoming an emerging problem. This term has been taken from Pacific salmonid species (e.g., coho, chinook, sockeye) where jacks are wild males that mature after only one summer in sea and at least 1 year before the “youngest” maturing female. In Atlantic salmon, jacks are usually only a few hundred grams in body weight and are also known as mature post-smolts [6]. They have only been observed in a limited number of wild populations [7]. Changes in farming practices now mean salmon may not experience classical seasons due to the prolonged use of continuous light (e.g., [8]). Therefore, the term jack commonly refers to male salmon that mature when large enough to migrate/smoltify (>30 g), but which lack the hooked jaw (kype) and skin coloration of the larger (>1 kg) reproductive phenotypes such as grilse [9]. Jacks are also phenotypically distinct from sneaker males/mature parr, the smallest (<30 g) reproductive phenotype, as they lack parr markings having passed the threshold body size for seawater migration. Jacks are frequently observed in RAS [8,10,11,12] with incidences of ≥70% in some studies [13].
The age of puberty in salmon is known to be regulated by several environmental factors with the most studied being temperature. Like most fishes, the age/body size of salmon at maturity decreases at higher temperatures [10,14,15]. This trend is called the “temperature size rule” and is widely studied in ecology [16]. As such, the high incidence of jacking in RAS is most likely due to the relatively warm temperatures employed of between 12 and 13 °C from first feeding onwards [10]. In comparison, farmed salmon produced in more traditional flow-through systems (FTS), or those moved to sea-cages, spend a significant amount of their life experiencing winter temperatures that can be as low as 2–6 °C in FTS [17] or 0–6 °C in sea-cage facilities [18] in Norway. Previously, Fjelldal et al. [14] showed that jacking can be easily induced in 100 g salmon using elevated water temperatures of 16 °C combined with constant light after a winter signal (i.e., a short-day length). This model has made it possible to study other factors that interact with temperature to regulate jacking. However, it relies on being able to regulate water temperature for extended periods of time (at least 6–7 weeks) which is generally economically expensive. Therefore, it would be beneficial to develop other models which rely on natural temperature ranges to study jacking.
In addition to environmental factors, genetics also plays a substantial role in salmon life history. It is well established that the age of puberty is heritable in salmon [19] and has recently been explored at the level of individual genes [20]. Consequently, vgll3 has consistently been found to play a major role in regulating all the male reproductive phenotypes; parr maturation [21], jacking [22], grilsing [23], and multi-sea-winter (MSW) maturation (those that mature after two or more winters at sea [24]). Interestingly, work in wild salmon suggested the early maturing vgll3 allele was dominate in regulating the likelihood of delaying maturation from being a grilse to being a MSW salmon [20], but the relationship between jacking and grilsing has not been assessed. For the work on jacks, pubertal development was advanced by exposing salmon to a winter signal for at least 6 weeks before being put on constant light and 16 °C [22]). This leads to the advancement of puberty, evident at the pituitary, plasma hormone, and gonad level, after only 2–3 weeks of the fish receiving the constant light and elevated temperature signal [25]. However, we do not know whether vgll3 also regulates jacking initiated under more natural conditions, such as in fish moved to seawater having experienced ambient winter and spring freshwater temperatures (as observed in [6,26]) or in salmon kept in ambient freshwater for an additional summer rather than being transferred to brackish/full-strength seawater (as observed in [15,26]). This information is important for those managing broodstock, as vgll3 could be a target for those wishing to manipulate the timing of maturation for commercial, research, or stock management purposes.
In 2018, we used an advanced maturation regime to induce out-of-season sexual maturation in nine families of all-male salmon produced from a domestic strain [20]. The all-male lines are a tool to reduce the use of research animals, as female salmon rarely mature as post-smolts and are not generally considered a significant commercial problem in Norway [9]. However, five of the families used in Fjelldal et al. [22] were also reared in one to two alternative environments which relied on ambient water temperatures. In one environment, the fish were also kept until 2020 so we could assess the family links between the propensity to jack, grilse, or mature after two or more sea-winters (i.e., MSW maturation). The main objective of the current work was to i) compare the level of jacking between the three different environments in 2018 and ii) assess the extent to which it was regulated by vgll3. The hypothesis was that the family ranking for the incidence of jacking and its regulation by vgll3 would be stable across all three environments. In addition, we expected vgll3 to regulate the timing of maturation across all the regimes in accordance with the pre-designated expectations for early and late maturing alleles [20,21,22,23,24]. The results suggest family ranking is not necessarily consistent across environments, but vgll3 behaved as expected throughout.
2. Materials and Methods
2.1. Ethics
The present experiment was approved by the Norwegian Animal Research Authority (permit number 5281, 8504, and 8521) and performed according to prevailing animal welfare regulations.
2.2. Family Material
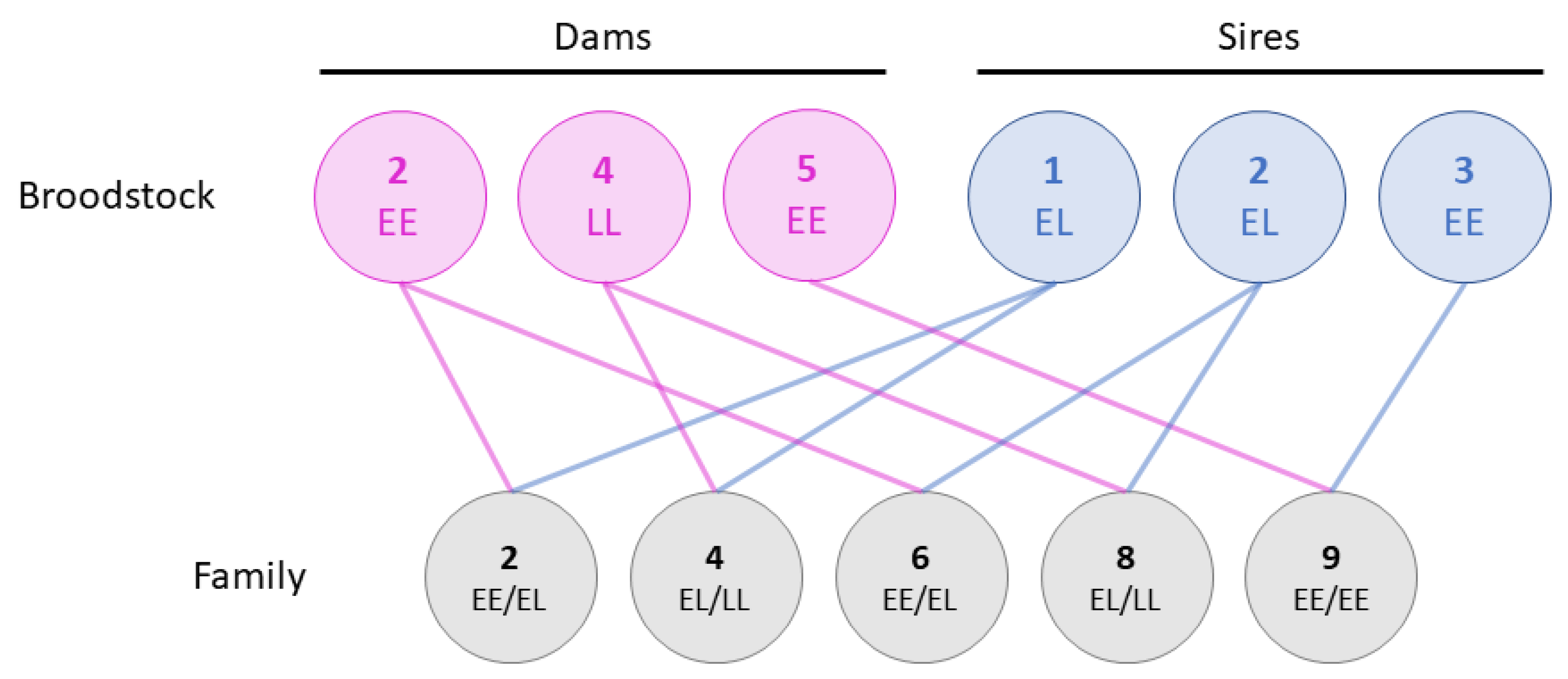
The study is a continuation of the families reported in Fjelldal et al. [22]. In brief, 9 families were originally created using 5 dams and 3 sires. However, only families 2, 4, 6, 8, and 9 from Fjelldal et al. [22] were followed up in the current study. Eggs from two double haploid females (dams 2 and 4) were each split in two equal parts and fertilized with milt from two YY males (sires 1 and 2), creating two half sibling groups per dam (Figure 1). Dam 2 was homozygous for the early maturing vgll3 allele, dam 4 was homozygous for the late maturing allele, whilst sires 1 and 2 were both heterozygous. Milt from a third YY male (sire 3) homozygous for the vgll3 early maturing allele was used to fertilize eggs from a fifth double haploid female (dam 5) also homozygous for the early allele. The YY males were initially produced from the AquaGen strain in 2012 and double haploid females from the commercial Salmobreed (now Benchmark) strain in 2012, details of which can be found in Fjelldal et al. [22] and Hansen et al. [27], respectively.
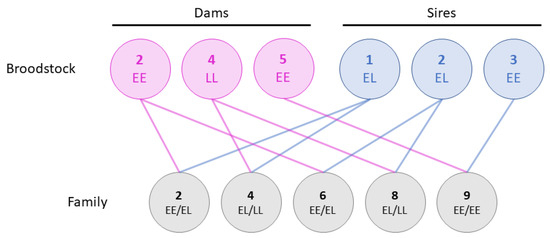
Figure 1.
An overview of the family material and the vgll3 genotype of the broodstock. In total, three dams (pink) and three sires (blue) were used to produce five families (grey), some of which were half-siblings. The broodstock were either homozygous for the early maturing vgll3 allele (EE), homozygous for the late maturing allele (LL), or heterozygous (EL). As such, each family had one to two vgll3 allele combinations. The family and broodstock names (numbers) respond to those in Fjelldal et al. [22].
2.3. Rearing Conditions
Each family was incubated in its own tray in a flow-through system at 6 °C before being moved to duplicate start feeding tanks (1 × 1 m, n = 10 tanks in total) on the 22 March 2017 under conditions of continuous light and a stable temperature of 12 °C. The fish stayed under these conditions until the 21 June 2017 when each family group was then transferred to single 3 m ø tanks (n = 9 tanks in total) and the temperature was changed to ambient. The daylength was changed to simulated natural photoperiod (SNP, civil twilight based on 61° N) on the 1 October 2017. The fish continued on these conditions until one sub-group was created on the 1 December 2017 (see “Advanced maturation” regime below), a second sub-group was created on the 1 May 2018 (see “Sea transfer” regime below), whilst the remaining fish continued on freshwater with ambient temperature and SNP (see “Extended freshwater” regime below).
2.4. Advanced Maturation Regime
The regime is described in detail in Fjelldal et al. [22]. Briefly, on the 1 December 2017, 180 fish from families 2, 4, 6, and 8, and 90 fish from family 9, were pit-tagged and distributed in common garden between six 3 m ø tanks, with the same number of individuals from each family in each tank (in total, n = 1530 fish with 255/tank; the additional fish came from families 1, 3, 5, and 7 reported in Fjelldal et al. [22]). These fish were reared under an environmental regime designed to induce post-smolt maturation (Figure 2A). They were kept under SNP and 6 °C until the 8 January 2018 when they were anesthetized (Finquel vet., 0.1 g/L), had their pit-tag number recorded, measured for fork length and body weight, and moved to six new 3 m ø tanks. On the 9 January 2018, photoperiod was shifted to continuous light and the water temperature was gradually adjusted to 16 °C over a 3-day period to induce maturation. The fish were kept under these conditions until the 6 March 2018 when they were all killed by an overdose of anesthetic (Finquel vet., 0.5 g/L). They then had their pit-tag recorded, were measured for fork length and gonad and body weight, sexed by visual examination of the gonad, and had their adipose fin preserved in ethanol for vgll3 genotyping.
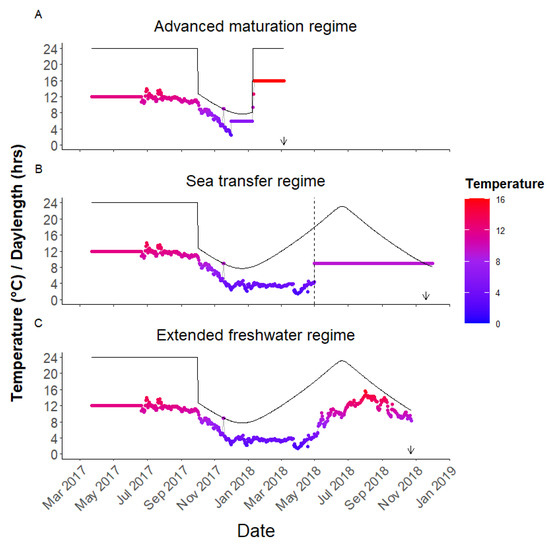
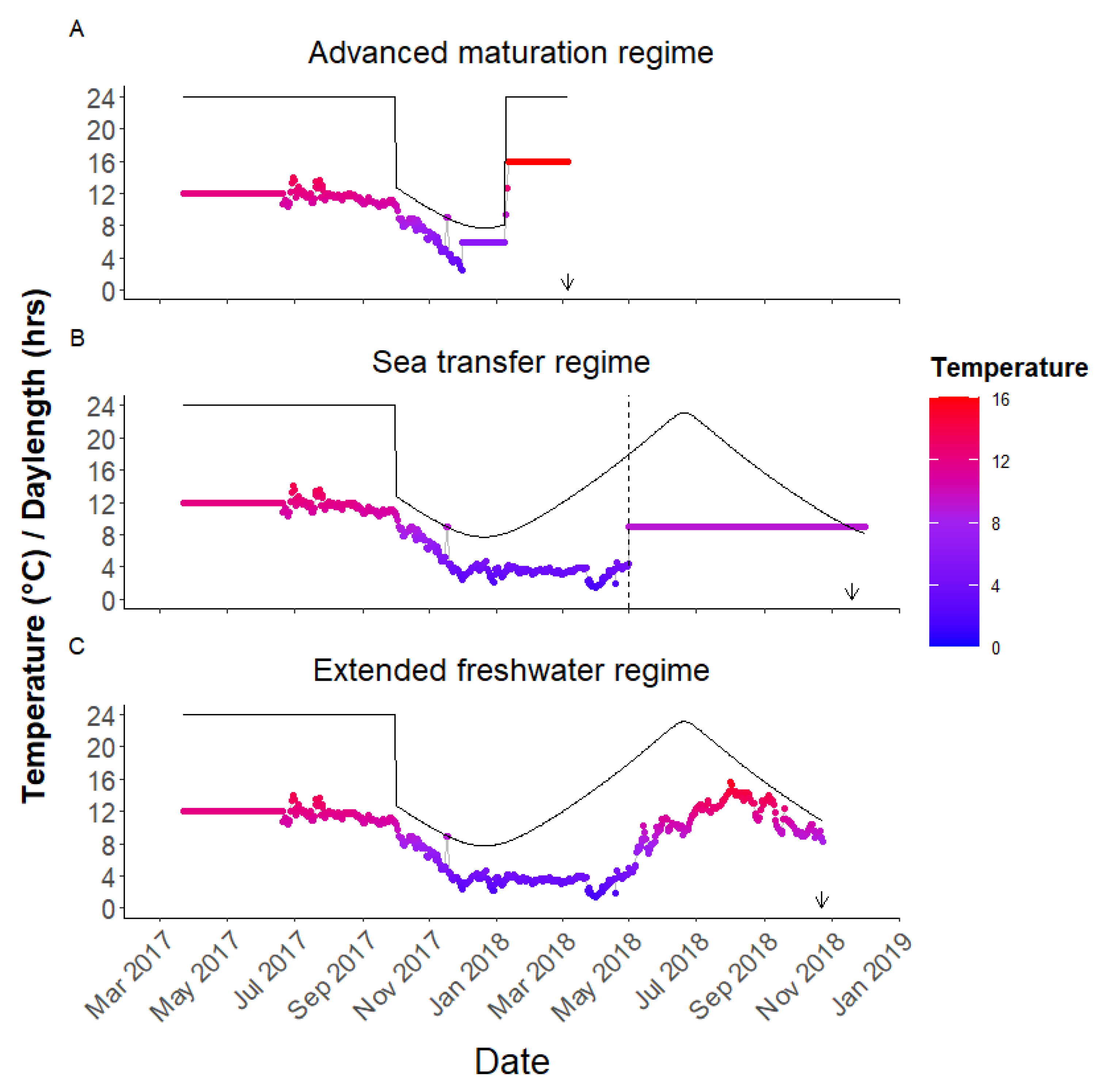
Figure 2.
The temperature and photoperiod of the three environments compared. The fish were reared on a common environment from hatching until December 2017 when the first subset of fish was moved to elevated temperatures and continuous light to induce maturation as part of the “Advanced maturation” regime (A). In (B), the dashed horizontal line indicates the timing when a second subset of fish were moved to 9 °C seawater in May 2018 having been on ambient freshwater from June 2017. In (C), a third subset of fish were maintained in ambient freshwater throughout. The date of the maturity assessment in 2018 for each regime is indicated by the arrow.
2.5. Sea Transfer Regime
A second subset of fish including 100 fish from families 2, 4, 6, 8, and 9 (in total n = 500) were pit-tagged on the 16 April 2018 and moved to one 7 m ø outdoor tank with natural photoperiod and 8.9 °C seawater (Figure 2B) on the 1 May 2018. The fish were kept under these conditions until the 2 December 2020. On the 18 November 2019, the fish were split between two tanks identical 7 m ø outdoor tanks. In total, 82 fish (16%) were not registered at the end of the study and were presumed to have died. Unfortunately, a predator (otter) broke into one tank in January 2019 and removed an unknown number of fish which were not recovered. Therefore, it was not possible to calculate natural mortality rates related to family. The PIT tag, length, weight, and maturity status (based on external morphology and the presence of running milt) of all the fishes were recorded on the 19 November 2018, 18 November 2019, and the 2 December 2020. At each timepoint, mature fish were removed from the experiment and had their adipose fin preserved in ethanol for vgll3 genotyping. At the final sampling, all the remaining fish were terminally anaesthetized, had their adipose fin preserved in ethanol, and their phenotypic sex assessed by visual examination of the gonads.
2.6. Extended Freshwater Regime
A third subset of fish were split between four square 1 m tanks and one 5 m ø circular tank (all indoors) with simulated SNP and ambient freshwater (Figure 2C). There were 220, 152, and 161 fish from families 6, 8, and 9 in the large circular tank with 1 unidentified fish (in total n = 534). In the smaller square tanks, 2 contained 24 fish each from family 6 (in total n = 48) with the remaining 2 having 25–26 fish from family 8 (in total n = 51). All tanks remained on ambient temperatures and SNP until the 23/24 October 2018 when they were anaesthetized, had their length and weight recorded, were examined for their maturity status, and their adipose fin preserved in ethanol for vgll3 genotyping.
2.7. Vgll3 Genotyping
Genotyping of the vgll3 locus was performed using an allelic discrimination assay for the two missense SNPs in vgll3 according to Ayllon et al. [23] and served to distinguish three different genotypes; homozygous early maturing (EE), homozygous late maturing (LL), and heterozygous early/late (EL).
2.8. Phenotypic Females
In total, the dataset included 95 (2.4%) phenotypic females and 3797 (97.6%) phenotypic males. The percentage of phenotypic females ranged from 0.0 to 4.7% within in each family. Low percentages of phenotypic females in putative “all-male” populations are commonly observed in fishes [28,29,30]. In Fjelldal et al. [22] using the same population, we found 5% of the phenotypic females were genetically male (XY, sdY-positive) whereas the remaining individuals were genetically female (XX, sdY-negative). These individuals were removed from all statistical analyses. However, 8 of the females in the sea transfer regime were sexually mature in December 2020 and their eggs were collected and crossed with sperm from 7 males (1 male per female, except for one male which was crossed with two females) from the same study population. All 8 of these females were sdY-negative (genetically female), whilst all 7 males were sdY-positive (genetically male, presumed XY) [22]. These embryos were reared under standard farm conditions in separate trays/tanks (n = 1/family) until they reached an average weight of approx. 60 g, at which point they were euthanized and sexed by visual examination of the gonads and by the sdY assay. The initial number of eggs, fertilization success, and embryo survival were unfortunately not recorded.
2.9. Statistical Analysis
The data were transferred to the R statistical software (version 4.0.4. [31]) for all analyses. Fulton’s condition (K) factor (body mass (g)/fork length (cm)3 × 100) was used as a measure of body condition. The packages “nlme” [32], “emmeans” [33], and “ggplot2” [34] were used for analysis and graphical presentation. Throughout, model diagnostics were assessed via q-q plots and standardized vs. predicted residual plots. The raw data (Fraser et al.xlsx) and R code (Fraser et al.R) used for the analysis and figures can be found in the Supplementary Material.
We fit 3 generalized linear mixed-effects models (GLMER’s) with a binomial response (matured = yes or no) to the maturity data from 2018. The first included the fixed effect of experiment (three levels, extended freshwater, advanced maturation, or sea transfer) with genotype (three levels, EE, EL, or LL) and family (five levels, family 2, 4, 6, 8, or 9) as random effects on the intercept. The second had genotype as the fixed effect and experiment and family as random effects. The third had family as a fixed effect and experiment and genotype as random effects. This approach was taken as the data showed complete separation with some groups having no maturation making more holistic models challenging (Figure S1). Based on visualizing the raw data, we fit 5 models to look at the effect of experiment within each family separately, with genotype as a random effect (expect for family 9 where all the fish were EE so the random effect was not necessary). We fit 4 models to look at the genotype effect within each family, with experiment as a random effect. Finally, we fit a final model with experiment and family as fixed effects, their interaction, and genotype as a random effect.
We fit generalized linear models (GLM’s) with a binomial response (mature = yes or no) to the maturity over time data from the sea transfer regime. Initially, we used eight models to explore the fixed effect of genotype (three levels, EE, EL, or LL) within each family (families 2, 4, 6, and 8) and year (two levels, 2019 or 2020) separately as there were many instances of complete separation which made a more holistic model challenging (Figure S2). We then fit one model to each family within grouping (10 models in total) to explore the probability of delaying maturation from 0 to 1 SW, 1 SW to 2 SW, or 2 SW to 2 SW+. Only 5 of these did not suffer from complete separation.
To assess the effects of vgll3 on body size parameters (weight, length, condition) in the extended freshwater regime, we used linear mixed effect (LME) models. We fit one model per family to each endpoint with genotype (three levels, EE, EL, or LL), maturity status (two levels, mature or immature), and their interaction included as fixed effects and tank included as a random effect on the intercept. Due to a lack of normality, a Kruskal–Wallis test was used to assess condition factors in family 8 (4 levels, EL immature, LL immature, EL mature, LL mature). To assess for effects of vgll3 and maturity on body size parameters over time (four levels, 2017, 2018, 2019, 2020) in the sea transfer data we used GLM’s. We fit one model per family per year to each endpoint with genotype (three levels, EE, EL, or LL) and maturity (two levels, yes or no) as fixed effects. Where there was a large enough sample size, we allowed genotype to interact with maturity status. For the freshwater data, 2 fish from family 6 and 9 fish from family 8 were removed due to condition factors indicative of human error or deformities (<0.8 or >1.8). Similarly for the sea transfer data, 1 fish from family 4, 1 from family 6, and 1 from family 8 were removed for the same reason.
3. Results
3.1. Post-Smolt Maturation across Three Environmental Regimes
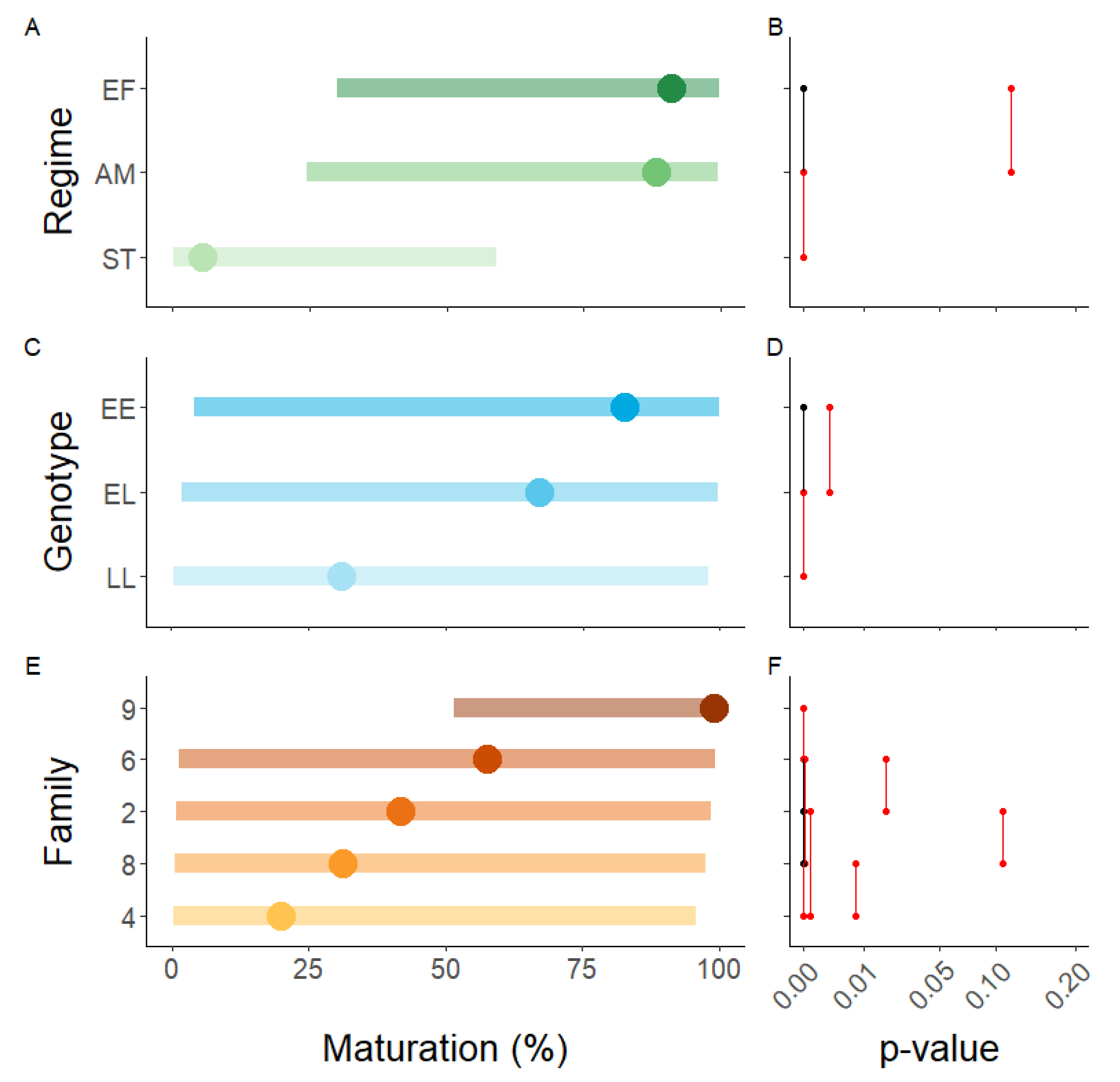
There was a general regime effect independent of vgll3 and family, with the advanced maturation and extended freshwater regimes having equal amounts of maturation whilst the sea transfer regime had significantly less (Figure 3A,B). At the family level, family 6 had a significantly higher amount of maturity in the advanced maturation regime whereas families 8 and 9 had a higher amount in the extended freshwater regime (Table 1).
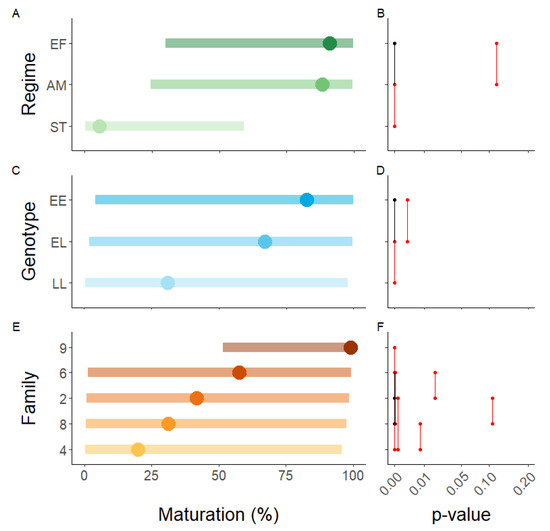
Figure 3.
Model results of the maturation data in 2018. The influence of (A,B) environment (EF = extended freshwater, AM = advanced maturation, ST = sea transfer), (C,D) genotype, and (E,F) family on the incidence of maturity. The left panels are the predicted means and 95% CI, whilst the panels to the right are pairwise p-value plots based on least square means. For the p-value plots, groups joined by black lines share the p value indicated in red.

Table 1.
Model results for the environmental effect on the incidence of sexual maturation in 2018 within families. Different superscript letters within a row indicate significant (GLM, p < 0.05) group differences. ** p < 0.01; *** p < 0.01.
There was a general effect of vgll3 independent of environment and family, with EE having the most maturation and LL the least (Figure 3C,D). The same trend was evident within every family albeit it was not significant for families 4 and 6 (p = 0.10–0.12, Table 2). The trend was also evident in each regime within every family, except in family (Figure S1). In family 6, vgll3 behaved as expected in the extended freshwater and advanced maturation regimes, but not in the sea transfer regime where a single EL male matured in seawater whereas no EE males matured.

Table 2.
Model results for the genotype and time (year) effect on the incidence of sexual maturation within families. Different superscript letters within a row indicate significant (GLM, p < 0.05) group differences. * p = <0.05; ** p < 0.01; *** p < 0.01.
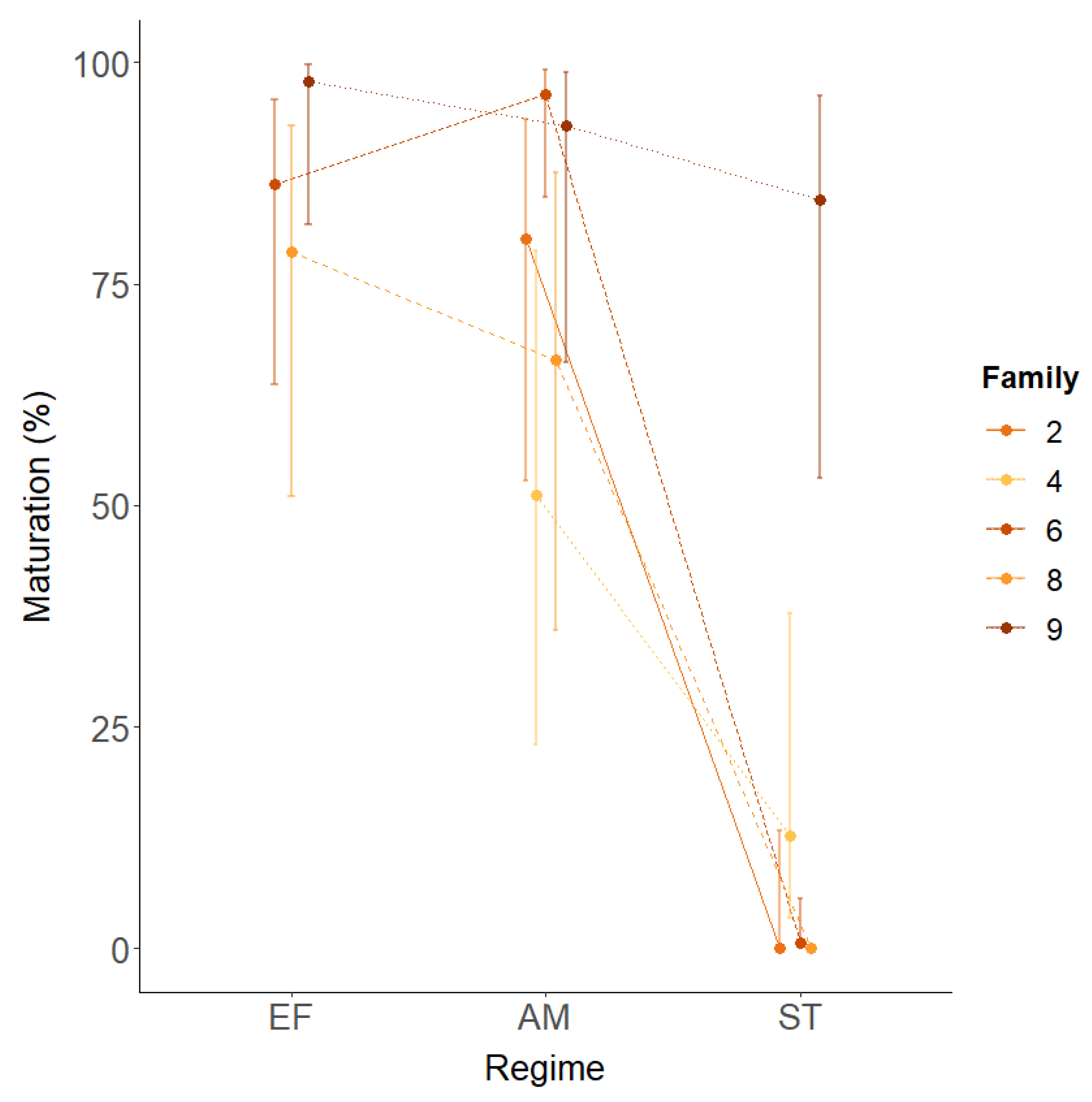
There was a strong family effect independent of vgll3 and environment (Figure 3E,F). Notably, this was not consistent across environmental regimes (Figure 4). Family 4 had the least maturity in the advanced maturation regime, but the second most in the sea transfer regime. In addition, whereas family 9 had ≥95% maturity in all regimes, family 6 had ≥91% in the advanced maturation and extended freshwater regimes but only 1% maturity in the sea transfer regime.
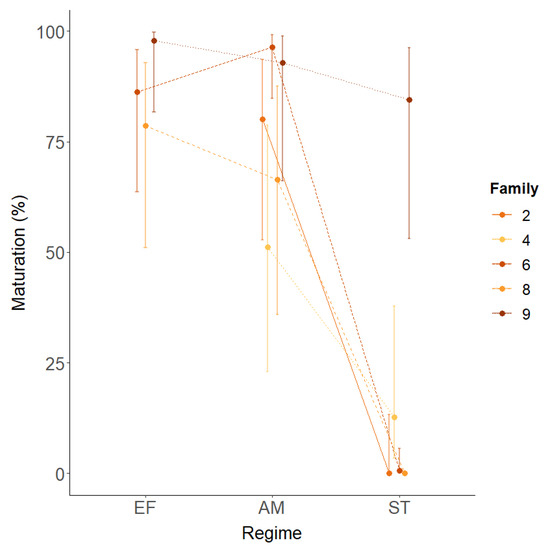
Figure 4.
Model results of the family × environment interaction on the 2018 maturation data. Data are means +/− 95% CI. Regime: EF = extended freshwater, AM = advanced maturation, ST = sea transfer.
3.2. Maturation over Time in Seawater
The probability of delaying maturation from a jack (0 SW) to grilse (1 SW) was difficult to assess due to the levels of maturity within each family (Figure S1). In families 2 and 8, no males matured before 1 SW. In family 4, those LL males that matured prior to 2 sea-winters (2 SW) did so only after 0 SW (there was no 1 SW maturation) whereas 35% of the EL males waited until they were 1 SW. In family 6, all the EE fish delayed maturing until they were 1 SW fish whereas only half the EL males delayed.
The probability of delaying maturation from 1 SW to 2 SW or from 2 SW to beyond did follow consistent trends. In families 2 and 6, EE males were less likely to delay maturation compared to EL fish in both scenarios, and in families 4 and 8, EL fish were less likely to delay maturation than LL males in both scenarios (Table 3).

Table 3.
Model results for the genotype effect on delaying maturation within families. * p = <0.05; ** p < 0.01; *** p < 0.01.
3.3. Timing of Maturation and Body Size
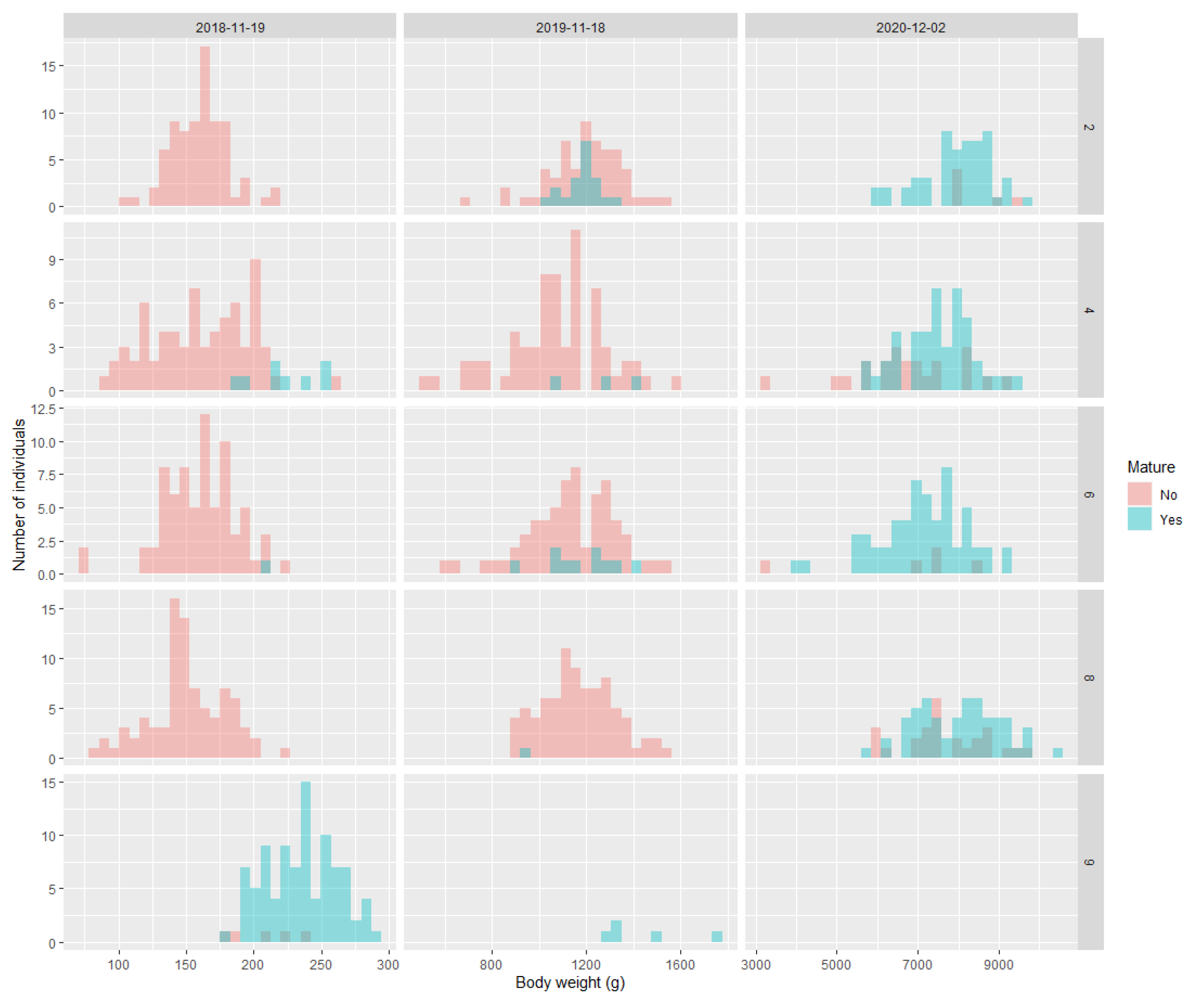
There was some evidence that body weight and length from May 2018 could predict maturation in November 2018, but the body size data from November 2018 and November 2019 showed no association with maturity in November 2019 and December 2020, respectively (Figure 5 for weight, Figure S3 for length). In 2018, family 9 was the largest and had the most maturation, whereas in family 4 there was also an indication that body size in May 2018 was related to the probability of maturation in November 2018. For all time points, body condition was not associated with the likelihood of maturation in any family (Figure S4).
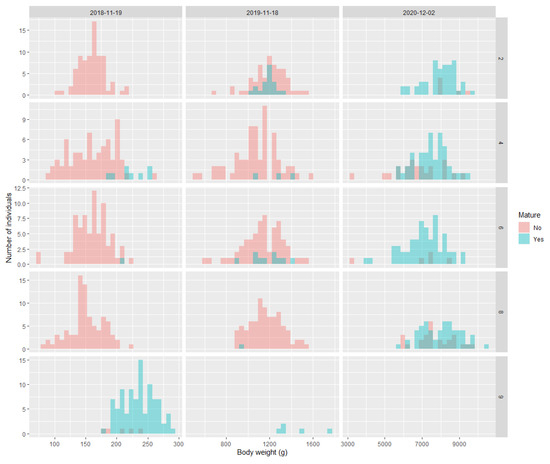
Figure 5.
Histograms of the relationship between maturity status (yes/no) and body weight at an earlier timepoint. For the 19 November 2018, the body weight is from May 2018. For the 18 November 2019, the body weight is from the 19 November 2018. For the 2 December 2020, the body weight is from the 18th of November 2019.
3.4. Vgll3 Genotype and Body Size
In the extended freshwater regime, vgll3 genotype had a significant interaction with maturity status on body condition in family 6 and length and condition in family 8 (Figure S5, Table S1). In family 6, immature EL fish had a higher body condition than immature EE fish, but there was no genotype effect in mature fish. In family 8, immature LL fish were longer with a lower condition than immature EL fish, but there was no genotype effect in mature fish. Otherwise, mature fish were significantly shorter with a higher body condition than immature fish in both families.
In the sea transfer regime, there were few genotype effects within any family, and they were transient and inconsistent (Figures S6–S17, Tables S2–S5). Mature fish were often significantly shorter and lighter than immature fish within each family, but not every year.
The data from the advanced maturation regime were previously analysed in Fjelldal et al. [22]. Briefly, mature fish were significantly larger than immature but there was no genotype effect on body size traits.
3.5. Genetic Sex of Phenotypic Females within Supposed “All-Male” Lines
Six of the eight crosses produced no viable offspring. In the remaining 2, in 1 only 6 offspring survived to 60 g whereas in the other 23 survived. Of these, 33 (2/6) and 52% (12/23) were both phenotypically and genetically female, whereas the others were both phenotypically and genetically male.
4. Discussion
Our main objective was to compare jacking across multiple environments varying in their practicality for use in research. Of particular interest was the association between maturation levels in the advanced maturation regime with its high manipulation of both temperature and photoperiod to induce out-of-season maturation in small fish, compared to the levels in the extended freshwater and sea transfer regimes which received less environmental manipulation and relied on in-season maturation. In this respect, the extended freshwater regime produced comparable maturity levels as the advanced maturation regime and would be a useful tool for further research into precocious maturation. Our second objective was to see whether vgll3 consistently regulated maturation across environmental regimes and over time. We found vgll3 did control the timing of maturation, but neither allele appeared to be strongly dominant.
We describe differences in maturation levels in the same population of fish split between different environments. These different environments were set up to understand whether we could find a feasible alternative to the advanced maturation model used in Fjelldal et al. [22]. In this sense, keeping fish on ambient freshwater for an additional summer led to a high incidence of sexual maturation with the same family ranking as in the advanced maturation model. However, the work described herein is not a strict comparison of different environments due to differences in the tank size, number of replicates per treatment, whether each family was represented within each tank, light source (natural vs. SNP), and stocking densities. Similar inconsistencies have also hampered previous studies [15,26,35,36] due to the expense of maintaining fish under different environments for extended periods of time as is necessary when studying salmon reproduction. For instance, at our facility in 2022, temperature control costs 25% more per day than without and these tanks are limited in number and in high demand. It is certainly feasible the uncontrolled factors may explain some of the variation in maturation between our environments. However, the variables discussed below (temperature, salinity, growth, and genetics) have previously been identified as major regulators.
The extended freshwater regime strongly promoted early maturation over the sea transfer regime as in other regions. In Baltic (Swedish) salmon, maintaining previously mature male parr in freshwater for another summer led to 100% maturation (n = 80) the following autumn, whereas only 7% (n = 3/43) of those moved to brackish (5 ppt) water the previous spring matured [26]. Similarly, maintaining previously immature Saint Johns River (Canada) smolts in freshwater over the summer resulted in 100% (n = 8) male maturation in the autumn compared to 0% (n = 20) in those transferred to full-strength seawater in the spring [15]. Conversely, Duston and Saunders [35] and Duston [36] found no maturation in the same Canadian stock reared for up to 2 years in freshwater. Duston and Saunders [15] theorized this was because their population was produced using early maturing parr as their sires, whereas Duston and Saunders [35] and Duston [36] had used later maturing grilse as sires. The nature of the broodstock used in Lundqvist and Fridberg [26] is not described. The age of puberty is heritable in salmon and may explain some of the discrepancy between the three Canadian studies. Our male broodstock were also all mature parr but came from the domesticated AquaGen (Norwegian) strain which has been selected against early maturation since the 1980’s (Thomas Moen, personal communication).
Higher temperatures lead to earlier maturation in Atlantic salmon [10,14]. Therefore, one could expect this may explain the higher levels of precocious maturity in the extended freshwater (peak 16 °C) over the sea transfer (constant 8.9 °C) regime. The same trend was also observed by Duston and Saunders [15] as their freshwater values peaked at 21 °C whilst the seawater temperature only reached 15 °C, and this was associated with more maturation in the former than the latter. However, in Lundqvist and Fridberg [26], the freshwater and brackish (5 ppt) water treatments had more similar temperature profiles peaking at around 20–21 °C in both yet precocious maturation was much more prevalent in freshwater. Family 9 in our experiment showed high rates of jacking (≥95%) in both the extended freshwater and sea transfer regime despite the large difference in temperature profiles. Therefore, although our data fit the general trend that higher temperatures led to earlier maturation, we are unable to separate this from other uncontrolled factors such as photoperiod/light source (natural vs. SNP) or salinity.
How migration/salinity regulates life history decisions in salmon is relatively unexplored from an experimental viewpoint. It is generally thought that smoltification and sexual maturation are in developmental conflict with the two processes unable to occur simultaneously [37]. However, post-smolt maturation following the first summer at sea does occur in both farming [38] and in the wild [7]. Furthermore, the two processes can be readily initiated simultaneously in fish of >90 g although they can show signs of conflict. In smolts for instance, puberty can suppress indicators of seawater tolerance [9,39] or increase the likelihood of dying in fish moved abruptly from freshwater to seawater [40]. Earlier work has also shown that sex steroid implants can impair the development of hypo-osmoregulatory ability during smoltification [41]. Work on salinity exposure in cultured salmon suggests it stimulates early maturation in those parr able to survive the transition from freshwater to seawater [36], it speeds up the maturation process in smolts that had already initiated puberty [42], and it reduces the likelihood of jacking in previously mature smolts [26] but has no effect on grilsing rates [43]. Ytrestøyl et al. [12] found an elevation in jacking in post-smolts kept in RAS on constant light and brackish water (12 ppt) compared to freshwater controls, but the opposite occurred in another group that had been given a square-wave photoperiod (long-short-long day length) to induce smoltification. In this study, the sea transfer regime reduced maturation levels in all families but had only a minor effect in the fastest growing/largest family. This suggests that body size/growth rate may have played an important role in regulating maturation rates between the different regimes.
Growth rate, body size, and/or body condition are often positively associated with precocious maturation [44]. The theory behind this is that all are regarded as proxies for energy reserves [45], maturation is energetically expensive [46], and salmon are expected to mature as soon as they reach an energy threshold [37]. In the current study, those in seawater were 57% heavier than those kept on freshwater (albeit the seawater fish had been allowed to grow for 26 days longer) but showed less maturation. As discussed above, this could be because the seawater was colder or that salinity exposure has a suppressive effect on the decision to mature. Nevertheless, the quickest growing family had the highest levels of sexual maturation. Following on from this, body size in May 2018 showed some positive associations with maturation the same year. However, the size data from November 2018 and 2019 showed no association with the tendency to mature the following year. Body condition showed no associations with the timing of puberty as found previously [22,39]. This may be because its relevance is restricted to a narrow developmental window not covered in the current design or that the strength of the correlation between Fulton’s K factor and energy density can be context dependent [47]. The high maturation rates in family 9 even in seawater may suggest the size/energy threshold for maturation was higher in this scenario compared to the extended freshwater regime. Notably, family 4 had the second highest prevalence of jacking in the sea transfer regime and was on average the second largest behind family 9.
One factor not explored in the current work is when male salmon take the decision to mature. Females initiate puberty in the autumn when daylength is decreasing, one year prior to maturing the next autumn [48]. If this is the case in males, then all the males were on a common environment at this time and the decision would have been made simultaneously across the whole population. It would then suggest the advanced maturation regime merely advances the pubertal process, whereas in the extended freshwater regime the fish followed the natural progression. This may explain why similar levels of maturity were seen between the two regimes. In contrast, it would suggest males in the sea transfer regime may have needed to terminate the maturation process having already decided to initiate it. We have previously provided evidence that males can terminate post-smolt maturation [49], although how common this phenomenon is has not been explored. Why family 9 still went on to almost unanimously mature is unclear, but it was the largest family followed by family 4 which also showed a relatively high incidence of jacking in seawater. As such, it could be that larger fish are able to migrate in the spring and then mature after only one summer at sea.
Vgll3 behaved as expected during the current experiment. The EE males matured earlier than EL males, and EL males matured earlier than LL males, irrespective of the environment or family. Unfortunately, the direct comparison of EE to LL males in the current setup is compromised as they come from half-siblings due to broodstock limitations. We found no strong evidence that the E allele is dominant as suggested in wild fish [20], although this analysis was hampered by a lack of all phenotypes in each family. Nevertheless, others have also found no indication of allele dominance [24] or evidence that the L allele shows slight dominance for parr maturation [21]. How vgll3 regulates the timing of maturation is not fully understood, but it is associated with elevated markers of pituitary gonadotropin levels prior to testes growth [50]. Vgll3 has also been found to be associated with small but significant differences in body condition, with the EE genotype having higher values than LL fish, with EL fish intermediate [21]. We found little evidence vgll3 regulated growth or condition in immature fish in this study or in Fjelldal et al. [22]. However, both these studies are unable to directly compare EE to LL fish. However, the fact that vgll3 behaved consistently over the three regimes matches recent work on parr maturation under two temperatures [51].
Of future interest, the family ranking for the prevalence of maturation did show one inconsistency in that family 4 had the second highest proportion of fish maturing in sea transfer regime, but the lowest prevalence in the advanced maturation regime. This suggests genes other than vgll3 may be important with regards to the timing of precocious maturation in freshwater versus seawater as reported in Tasmania salmon [43]. We also observed several individuals which had not matured after 2 sea-winters even though 93% (55/59) of these attained body weights of between 11 and 21 kg and lengths of 96–115 cm. These individuals are notably larger than the median harvest weight in Norway of 4.25 kg [52] and already within the range of returning MSW spawners in Norwegian rivers with a sea-age of 3–4 years (e.g., 88–131 cm and 6.5–22.5 kg for a mixed sex population [53] or 80–100 cm in 3 SW males [20]). In comparison, 2 SW wild salmon in Norway are typically reported to be around 65–75 cm [20,53,54,55] or 2.5–3.8 kg [53,54] whereas our 2 SW salmon were on average 105 cm and 13.6 kg. This would suggest the domesticated strains we used show a higher size threshold for maturation than wild fish as expected. This is despite our seawater fish being kept at a constant 8.9 °C which is generally higher than what Norwegian salmon experience in the wild during winter (e.g., 3.4–5.0 °C), and at the high end of what they experience in the summer (e.g., 6.7–8.9 °C) [56]. It is also warmer than mean daily temperatures found in Norwegian sea-cages during the winter (e.g., 1–7 °C), but lower than those experienced in the summer (e.g., 11–18 °C) [18].
By chance, we found eight ripe phenotypic females in the sea transfer regime in December 2020, and they were also genetically female. The occurrence of females in supposed “all-male” lines is not uncommon [22,28,29,30]. Why this occurs is unknown, given crosses between XX eggs with YY sperm are all expected to be XY. We opportunistically crossed these females with presumed XY males. Although survival was generally very poor for unknown reasons, the sex ratio of the one cross with 23 survivors was approximately 50/50 which is what would be expected from crossing an XX female with a XY male and would support the assumption the female was XX and the male XY.
5. Conclusions
In conclusion, we describe the prevalence of precocious maturation in the same population of domesticated all-male salmon split between multiple environments. All environments produced jacks to various levels, with more in the advanced maturation and extended freshwater regimes than the sea transfer regime. As such, the extended freshwater regime could be a useful tool for those wishing to work on understanding the factors that influence jacking without the need to artificially heat water for extensive periods of time. Vgll3 behaved as expected based on previous studies across all three environments.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes8050275/s1, Figures S1–S17: Raw maturation data, histograms of body length and body condition, and split box/violin plots of body size within each family in the EF and ST regimes. Tables S1–S5: Model results for body size data in the EF and ST regimes.
Author Contributions
Conceptualization, P.G.F. and T.J.H.; formal analysis, T.W.K.F.; investigation, P.G.F., T.J.H. and T.W.K.F.; resources, T.J.H.; data curation, T.W.K.F.; writing—original draft preparation, T.W.K.F.; writing—review and editing, T.W.K.F., P.G.F. and T.J.H.; visualization, T.W.K.F.; project administration, T.W.K.F. and T.J.H.; funding acquisition, P.G.F., T.J.H. and T.W.K.F. All authors have read and agreed to the published version of the manuscript.
Funding
The current project has been funded by the Research Council of Norway (RCN) projects “POSTSMOLTMAT: Understanding postsmolt maturation in Atlantic salmon in the context of new, closed production systems” (#254870) and “ENVGEN: Towards the sustainable production of male Atlantic salmon: The balance between genetic and environmental control for age at maturity” (#295100), and internal funding through the “STORSMOLT” (#15832) project at the Institute of Marine Research (IMR).
Institutional Review Board Statement
The animal experiments were approved by the Norwegian Animal Research Authority (permit number 5281, 8504, and 8521) and performed according to prevailing animal welfare regulations.
Data Availability Statement
The raw data (Fraser et al.xlsx) and R code (Fraser et al.R) used for the analysis and figures can be found in the Supplementary Material.
Acknowledgments
The authors thank the technical staff at IMR Matre for help with fish husbandry and sampling.
Conflicts of Interest
The authors declare no conflict of interest. The RCN had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results. IMR provided some funding for the project, including the salary of the all the authors.
References
- FAO [FISHSTAT]. License: CC BY-NC-SA 3.0 IGO. Available online: https://www.fao.org/fishery/en/collection/aquaculture?lang=en (accessed on 23 January 2023).
- Taranger, G.L.; Carrillo, M.; Schulz, R.W.; Fontaine, P.; Zanuy, S.; Felip, A.; Weltzien, F.A.; Dufour, S.; Karlsen, Ø.; Norberg, B.; et al. Control of puberty in fish. Gen. Comp. Endocrinol. 2010, 165, 483–515. [Google Scholar] [CrossRef] [PubMed]
- Aksnes, A.; Gjerde, B.; Roald, S.O. Biological, chemical and organoleptic changes during maturation of farmed Atlantic salmon. Salmo Salar. Aquac. 1986, 53, 7–20. [Google Scholar] [CrossRef]
- Qui, D.; Xu, S.; Song, C.; Chi, L.; Li, X.; Sun, G.; Liu, Y. Effects of spectral composition, photoperiod and light intensity on the gonadal development of Atlantic salmon Salmo salar in recirculating aquaculture systems (RAS). Chin. J. Oceanol. Limnol. 2015, 33, 45–56. [Google Scholar]
- Bjørndal, T.; Tusvik, A. Economic analysis of on-growing of salmon post-smolts. Aquac. Econ. Manag. 2020, 24, 355–386. [Google Scholar] [CrossRef]
- Stefansson, S.O.; Hansen, T.J.; Taranger, G.L. Growth and parr-smolt transformation of Atlantic salmon (Salmo salar L.) under different light intensities and subsequent survival and growth in seawater. Aquac. Eng. 1993, 13, 231–243. [Google Scholar] [CrossRef]
- Klemetsen, A.; Amundsen, P.A.; Dempson, J.B.; Jonsson, B.; Jonsson, N.; O’Connell, M.F.; Mortensen, E. Atlantic salmon Salmo salar L., brown trout Salmo trutta L. and Arctic charr Salvelinus alpinus (L.): A review of aspects of their life histories. Ecol. Freshw. Fish 2003, 12, 1–59. [Google Scholar] [CrossRef]
- Crouse, C.; Davidson, J.; May, T.; Summerfelt, S.; Good, C. Production of market size European strain Atlantic salmon (Salmo salar) in land-based freshwater closed containment aquaculture systems. Aquac. Eng. 2021, 92, 102138. [Google Scholar] [CrossRef]
- Fjelldal, P.G.; Schulz, R.; Nilsen, T.O.; Andersson, E.; Norberg, B.; Hansen, T.J. Sexual maturation and smoltification in domesticated Atlantic salmon (Salmo salar L.)—Is there a developmental conflict? Physiol. Rep. 2018, 6, e13809. [Google Scholar] [CrossRef]
- Crouse, C.; Davidson, J.; Good, C. The effects of two water temperature regimes on Atlantic salmon (Salmo salar) growth performance and maturation in freshwater recirculating aquaculture systems. Aquaculture 2022, 553, 738063. [Google Scholar] [CrossRef]
- Martinez, E.P.; Balseiro, P.; Pedrosa, C.; Haugen, T.S.; Fleming, M.S.; Handeland, S.O. The effect of photoperiod manipulation on Atlantic salmon growth, smoltification and sexual maturation: A case study of a commercial RAS. Aquac. Res. 2021, 52, 2593–2608. [Google Scholar] [CrossRef]
- Ytrestøyl, T.; Hjelle, E.; Kolarevic, J.; Takle, H.; Rebl, A.; Afanasyev, S.; Krasnov, A.; Brunsvik, P.; Terjesen, B.F. Photoperiod in recirculation aquaculture systems and timing of seawater transfer affect seawater growth performance of Atlantic salmon (Salmo salar). J. World Aquac. Soc. 2023, 54, 73–95. [Google Scholar] [CrossRef]
- Good, C.; Davidson, J.; Iwanowicz, L.; Meyer, M.; Dietze, J.; Kolpin, D.W.; Marancik, D.; Birkett, J.; Williams, C.; Summerfelt, S. Investigating the influence of nitrate nitrogen on post-smolt Atlantic salmon Salmo salar reproductive physiology in freshwater recirculation aquaculture systems. Aquac. Eng. 2017, 78, 2–8. [Google Scholar] [CrossRef]
- Fjelldal, P.G.; Hansen, T.; Huang, T.-S. Continuous light and elevated temperature can trigger maturation both during and immediately after smoltification in male Atlantic salmon (Salmo salar). Aquaculture 2011, 321, 93–100. [Google Scholar] [CrossRef]
- Duston, J.; Saunders, R.L. Life histories of Atlantic salmon altered by winter temperature and summer rearing in fresh- or sea-water. Environ. Biol. Fishes 1997, 50, 149–166. [Google Scholar] [CrossRef]
- Atkinson, D. Temperature and organism size: A biological law for ectotherms? Adv. Ecol. Res. 1994, 25, 1–58. [Google Scholar]
- Fraser, T.W.K.; Hansen, T.; Skjæraasen, J.E.; Mayer, I.; Sambraus, F.; Fjelldal, P.G. The effect of triploidy on the culture performance, deformity prevalence, and heart morphology in Atlantic salmon. Aquaculture 2013, 416–417, 255–264. [Google Scholar] [CrossRef]
- Falconer, L.; Hjøllo, S.S.; Telfer, T.C.; McAdam, B.J.; Hermansen, Ø.; Ytteborg, E. The importance of calibrating climate change projections to local conditions at aquaculture sites. Aquaculture 2020, 514, 734487. [Google Scholar] [CrossRef]
- Nævdal, G.; Holm, M.; Ingebrigtsen, O.; Møller, D. Variation in age at fist spawning in Atlantic salmon (Salmo salar). J. Fish. Board Can. 1978, 35, 145–147. [Google Scholar] [CrossRef]
- Barson, N.J.; Aykanat, T.; Hindar, K.; Baranski, M.; Bolstad, G.H.; Fiske, P.; Jacq, C.; Jensen, A.J.; Johnston, S.E.; Karlsson, S.; et al. Sex-dependent dominance at a single locus maintains variation in age at maturity in salmon. Nature 2015, 528, 405–408. [Google Scholar] [CrossRef]
- Debes, P.V.; Piavchenko, N.; Ruokolainen, A.; Ovaskainen, O.; Moustakas-Verho, J.E.; Parre, N.; Aykanat, T.; Erkinaro, J.; Primmer, C.R. Polygenic and major-locus contributions to sexual maturation timing in Atlantic salmon. Mol. Ecol. 2021, 30, 4505–4519. [Google Scholar] [CrossRef]
- Fjelldal, P.G.; Hansen, T.J.; Wargelius, A.; Ayllon, F.; Glover, K.A.; Schulz, R.W.; Fraser, T.W.K. Development of supermale and all-male Atlantic salmon to research the vgll3 allele-puberty link. BMC Genet. 2020, 21, 123. [Google Scholar] [CrossRef]
- Ayllon, F.; Kjærner-Semb, E.; Furmanek, T.; Wennevik, V.; Solberg, M.F.; Dahle, G.; Taranger, G.L.; Glover, K.A.; Almén, S.; Rubin, C.J.; et al. The vgll3 locus controls age at maturity in wild and domesticated Atlantic salmon (Salmo salar L.) males. PLoS Genet. 2015, 11, e1005628. [Google Scholar] [CrossRef]
- Ayllon, F.; Solberg, M.F.; Glover, K.A.; Mohammadi, F.; Kjærner-Semb, E.; Fjelldal, P.G.; Andersson, E.; Hansen, T.; Edvardsen, E.; Wargelius, A. The influence of vgll3 genotypes on sea age at maturity is altered in farmed mowi strain Atlantic salmon. BMC Genet. 2019, 20, 44. [Google Scholar] [CrossRef]
- Crespo, D.; Skaftnesmo, K.O.; Kjærner-Semb, E.; Yilmaz, O.; Norberg, B.; Olausson, S.; Vogelsang, P.; Bogerd, J.; Kleppe, L.; Edvardsen, R.B.; et al. Pituitary gonadotropin gene expression during induced onset of postsmolt maturation in male Atlantic salmon: In vivo and tissue culture studies. Front. Endocrinol. 2022, 13, 826920. [Google Scholar] [CrossRef] [PubMed]
- Lundqvist, H.; Fridberg, G. Sexual maturation versus immaturity: Different tactics with adaptive values in Baltic salmon (Salmo salar L.) male smolts. Can. J. Zool. 1982, 60, 1822–1827. [Google Scholar] [CrossRef]
- Hansen, T.J.; Penman, D.; Glover, K.A.; Fraser, T.W.K.; Vågseth, T.; Thorsen, A.; Sørvik, A.G.E.; Fjelldal, P.G. Production and verification of the first Atlantic salmon (Salmo salar L.) clonal lines. BMC Genet. 2020, 21, 71. [Google Scholar] [CrossRef]
- Quillet, E.; Aubard, G.; Queau, I. Mutation in a sex-determining gene in rainbow trout: Detection and genetic analysis. J. Hered. 2002, 93, 91–99. [Google Scholar] [CrossRef]
- Komen, J.; De Boer, P.; Richter, C. Male sex reversal in gynogenetic XX females of common carp (Cyprinus carpio L.) by a recessive mutation in a sex-determining gene. J. Hered. 1992, 83, 431–434. [Google Scholar] [CrossRef]
- Mair, G.; Abucay, J.; Abella, T.; Beardmore, J.; Skibinski, D. Genetic manipulation of sex ratio for the large-scale production of all-male tilapia Oreochromis niloticus. Can. J. Fish Aquat. Sci. 1997, 54, 396–404. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing. In R Foundation for Statistical Computing; R Core Team: Vienna, Austria, 2021; Available online: https://www.R-project.org/ (accessed on 15 February 2021).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; R Core Team. Nlme: Linear and Nonlinear Mixed Effects Models, R Package Version 3.1–155; R Core Team: Vienna, Austria, 2022; Available online: https://CRAN.R-project.org/package=nlme (accessed on 15 February 2021).
- Lenth, R.V. Emmeans: Estimated Marginal Means, Aka Least-Squares Means, R Package Version 1.7.0; R Core Team: Vienna, Austria, 2021; Available online: https://CRAN.R-project.org/package=emmeans (accessed on 15 February 2021).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978–3–319-24277-4. Available online: https://ggplot2.tidyverse.org (accessed on 15 February 2021).
- Duston, J.; Saunders, R.L. Effect of 6-, 12-, and 18-month photoperiod cycles on smolting and sexual maturation in juvenile Atlantic Salmon (Salmo salar). Can. J. Fish. Aquat. Sci. 1992, 49, 2273–2280. [Google Scholar] [CrossRef]
- Duston, J. Effect of salinity on survival and growth of Atlantic salmon (Salmo salar) parr and smolts. Aquaculture 1994, 121, 115–124. [Google Scholar] [CrossRef]
- Thorpe, J.E. Maturation responses of salmonids to changing developmental opportunities. Mar. Ecol. Prog. Ser. 2007, 335, 285–288. [Google Scholar] [CrossRef]
- Thrush, M.A.; Duncan, N.J.; Bromage, N.R. The use of photoperiod in the production of out-of-season Atlantic salmon (Salmo salar) smolts. Aquaculture 1994, 121, 29–44. [Google Scholar] [CrossRef]
- Fraser, T.W.K.; Hansen, T.J.; Norberg, B.; Nilsen, T.O.; Schulz, R.W.; Fjelldal, P.G. Atlantic salmon male post-smolt maturation can be reduced by using a 3-hour scotophase when inducing smoltification. Aquaculture 2023, 562, 738772. [Google Scholar] [CrossRef]
- Fjelldal, P.G.; Fraser, T.W.K.; Hansen, T.J.; Karlsen, Ø.; Bui, S. Effects of laboratory salmon louse infection on mortality, growth and sexual maturation in Atlantic salmon. ICES J. Mar. Sci. 2022, 79, 1530–1583. [Google Scholar] [CrossRef]
- Lundqvist, H.; Borg, B.; Berglund, I. Androgens impair seawater adaptability in smolting Baltic salmon (Salmo salar). Can. J. Zool. 1989, 67, 1733–1736. [Google Scholar] [CrossRef]
- Melo, M.C.; Andersson, E.; Fjelldal, P.G.; Bogerd, J.; França, L.R.; Taranger, G.L.; Schulz, R.W. Salinity and photoperiod modulate pubertal development in Atlantic salmon (Salmo salar). J. Endocrinol. 2014, 220, 319–332. [Google Scholar] [CrossRef]
- Mohamed, A.R.; Verbyla, K.L.; Al-Mamun, H.A.; McWilliam, S.; Evans, B.; King, H.; Kube, P.; Kijas, J.W. Polygenic and sex specific architecture for two maturation traits in farmed Atlantic salmon. BMC Genom. 2019, 20, 139. [Google Scholar] [CrossRef]
- Bohlin, T.; Dellefors, C.; Faremo, U. Probability of first sexual maturation of male parr in wild sea-run brown trout (Salmo trutta) depends on condition factor 1 yr in advance. Can. J. Fish. Aquat. Sci. 1994, 51, 1920–1926. [Google Scholar] [CrossRef]
- Herbinger, C.M.; Friars, G.W. Correlation between condition factor and total lipid content in Atlantic salmon, Salmo salar L., parr. Aquac. Fish. Manag. 1991, 22, 527–529. [Google Scholar] [CrossRef]
- Jonsson, N.; Jonsson, B.; Hansen, L.P. Changes in proximate composition and estimates of energetic costs during upstream migration and spawning in Atlantic salmon Salmo salar. J. Anim. Ecol. 1997, 66, 425–436. [Google Scholar] [CrossRef]
- Trudel, M.; Tucker, S.; Morris, J.F.T.; Higgs, D.A.; Welch, D.W. Indicators of energetic status in juvenile coho salmon and chinook salmon. North Am. J. Fish. Manag. 2004, 25, 374–390. [Google Scholar] [CrossRef]
- Andersson, E.; Schulz, R.W.; Male, R.; Bogerd, J.; Patiña, P.; Benedet, S.; Norberg, B.; Taranger, G.L. Pituitary gonadotropin and ovarian gonadotropin receptor transcript levels: Seasonal and photoperiod-induced changes in the reproductive physiology of female Atlantic salmon (Salmo salar). Gen. Comp. Endocrinol. 2013, 191, 247–258. [Google Scholar] [CrossRef]
- Fraser, T.W.K.; Fjelldal, P.G.; Schulz, R.W.; Norberg, B.; Hansen, T.J. Termination of puberty in out-of-season male Atlantic salmon smolts. Comp. Biochem. Physiol. Part A 2019, 232, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Ahi, E.P.; Sinclair-Waters, M.; Moustakas-Verho, J.; Jansouz, S.; Primmer, C.R. Strong regulatory effects of vgll3 genotype on reproductive axis gene expression in juvenile male Atlantic salmon. Gen. Comp. Endocrinol. 2022, 325, 114055. [Google Scholar] [CrossRef]
- Åsheim, E.R.; Debes, P.V.; House, A.; Liljeström, P.; Niemelä, P.T.; Siren, P.T.; Erkinaro, J.; Primmer, C.R. Atlantic salmon (Salmo salar) age at maturity is strongly affected by temperature, population and age-at-maturity genotype. Conserv. Physiol. 2023, 11, coac086. [Google Scholar] [CrossRef]
- Barrett, L.T.; Oldham, T.; Kristiansen, T.S.; Oppedal, F.; Stien, L.H. Declining size-at-harvest in Norwegian salmon aquaculture: Lice, disease, and the role of stunboats. Aquaculture 2022, 559, 738440. [Google Scholar] [CrossRef]
- Borgstrøm, R.; Opdahl, J.; Svenning, M.-A.; Länsman, M.; Orell, P.; Niemelä, E.; Erkinaro, J.; Dempson, J.B. Temporal changes in ascendance and in-season exploitation of Atlantic salmon, Salmo salar, inferred by a video camera array. Fish. Manag. Ecol. 2010, 17, 454–463. [Google Scholar] [CrossRef]
- Besnier, F.; Ayllon, F.; Skaala, Ø.; Favnbøe, M.F.; Fjeldheim, P.T.; Anderson, K.; Knutar, S.; Glover, K.A. Introgression of domesticated salmon changes life history and phenology of a wild salmon population. Evol. Appl. 2022, 15, 853–864. [Google Scholar] [CrossRef]
- Besnier, F.; Skaala, Ø.; Wennevik, V.; Ayllon, A.; Utne, R.; Fjeldheim, P.T.; Andersen-Fjeldheim, K.; Knutar, S.; Glover, K.A. Overruled by nature: A plastic response to an ecological regime shift disconnects a gene and its trait. Mol. Ecol. 2022, in press. [Google Scholar]
- Strøm, J.F.; Thorstad, E.B.; Rikardsen, A.H. Thermal habitat of adult Atlantic salmon Salmo salar in a warming ocean. J. Fish Biol. 2020, 96, 327–336. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).