Temporal Pattern of the Occurrence of Japanese Glass Eels (Anguilla japonica) in the Pearl River Estuary
Abstract
1. Introduction
2. Materials and Methods
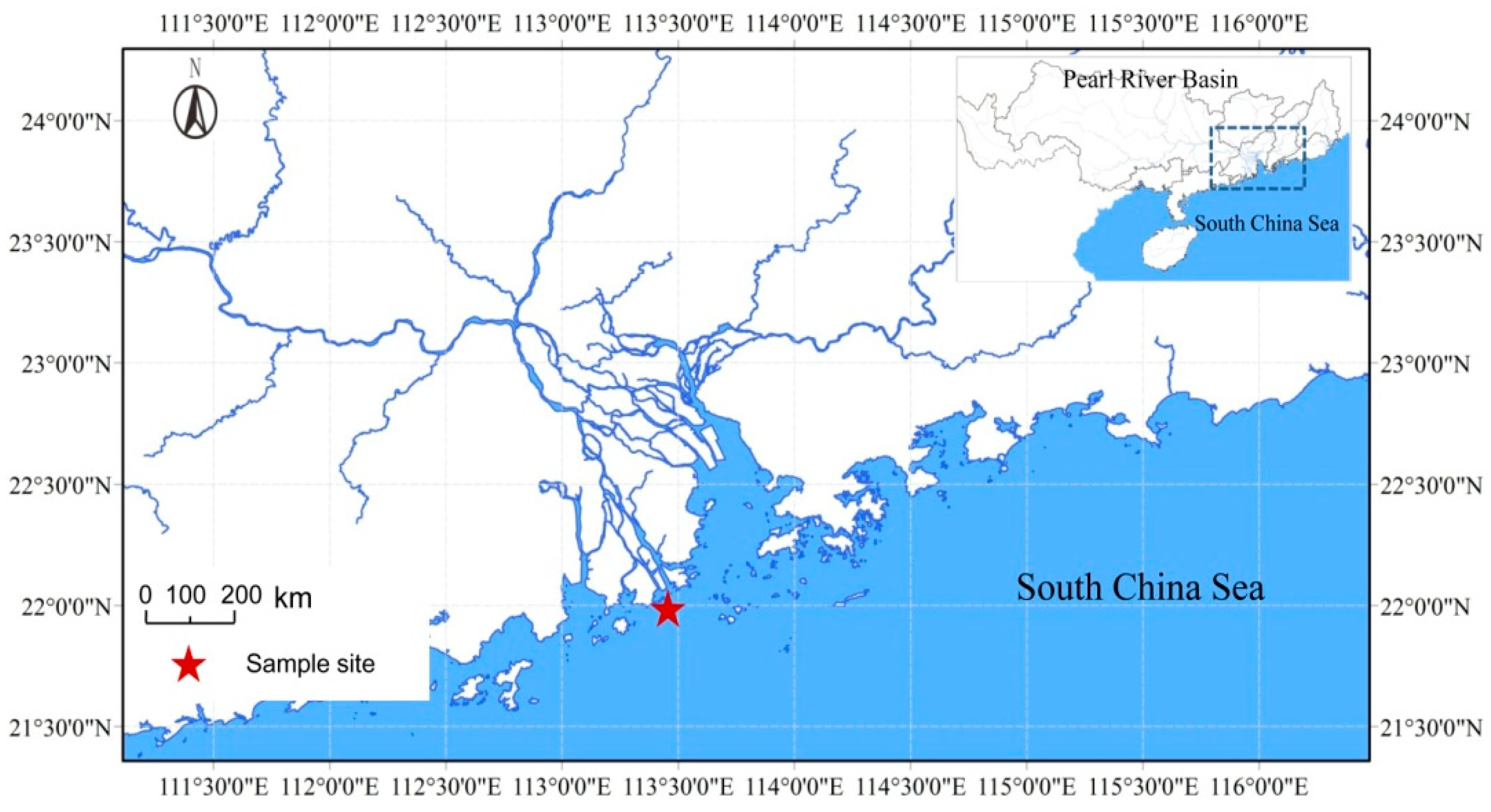
2.1. Study Site
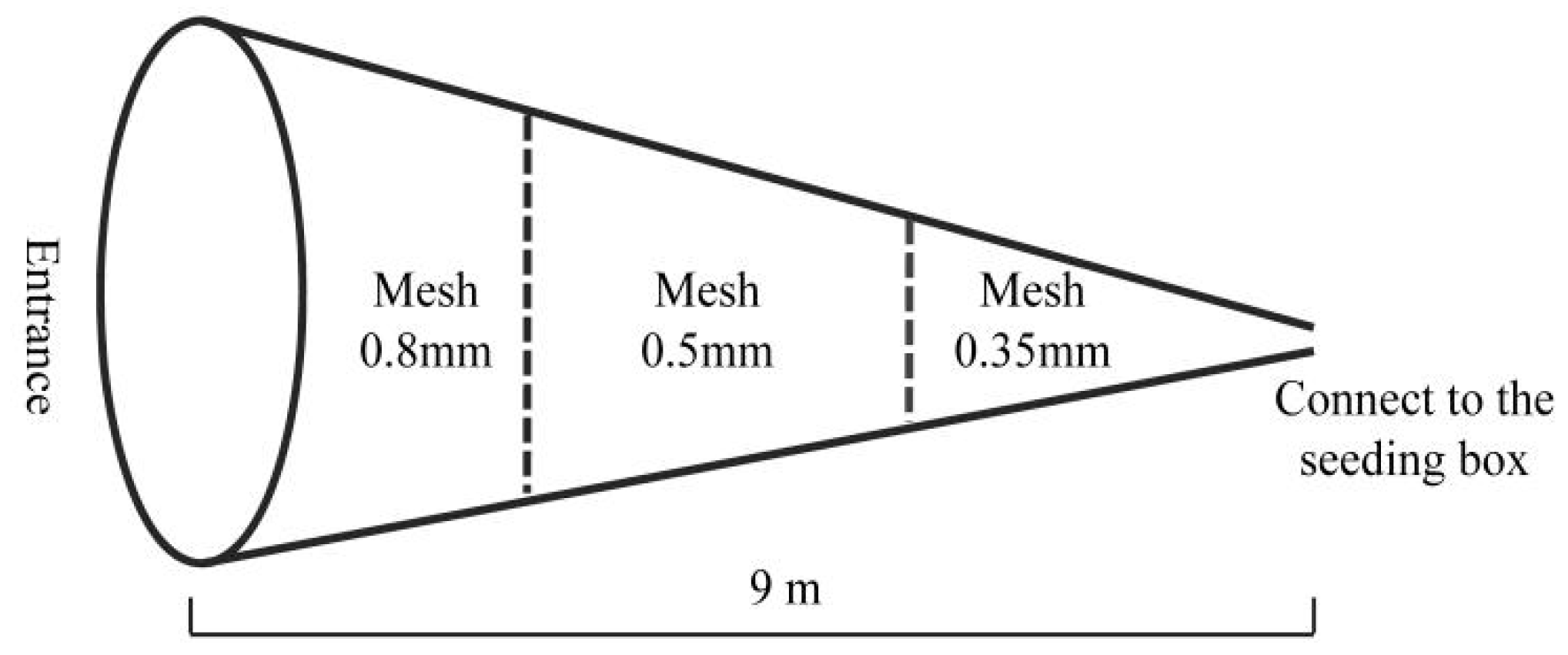
2.2. Data Collection
2.3. Data Analysis
2.4. Time Series Analyses
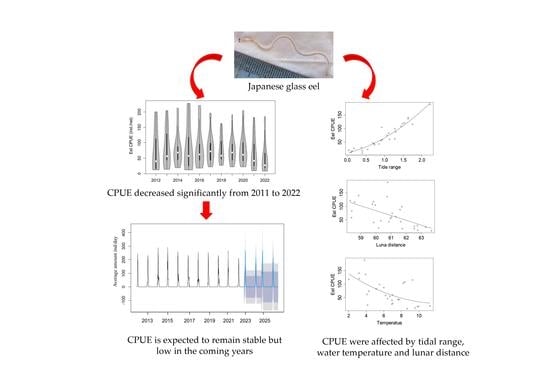
3. Results
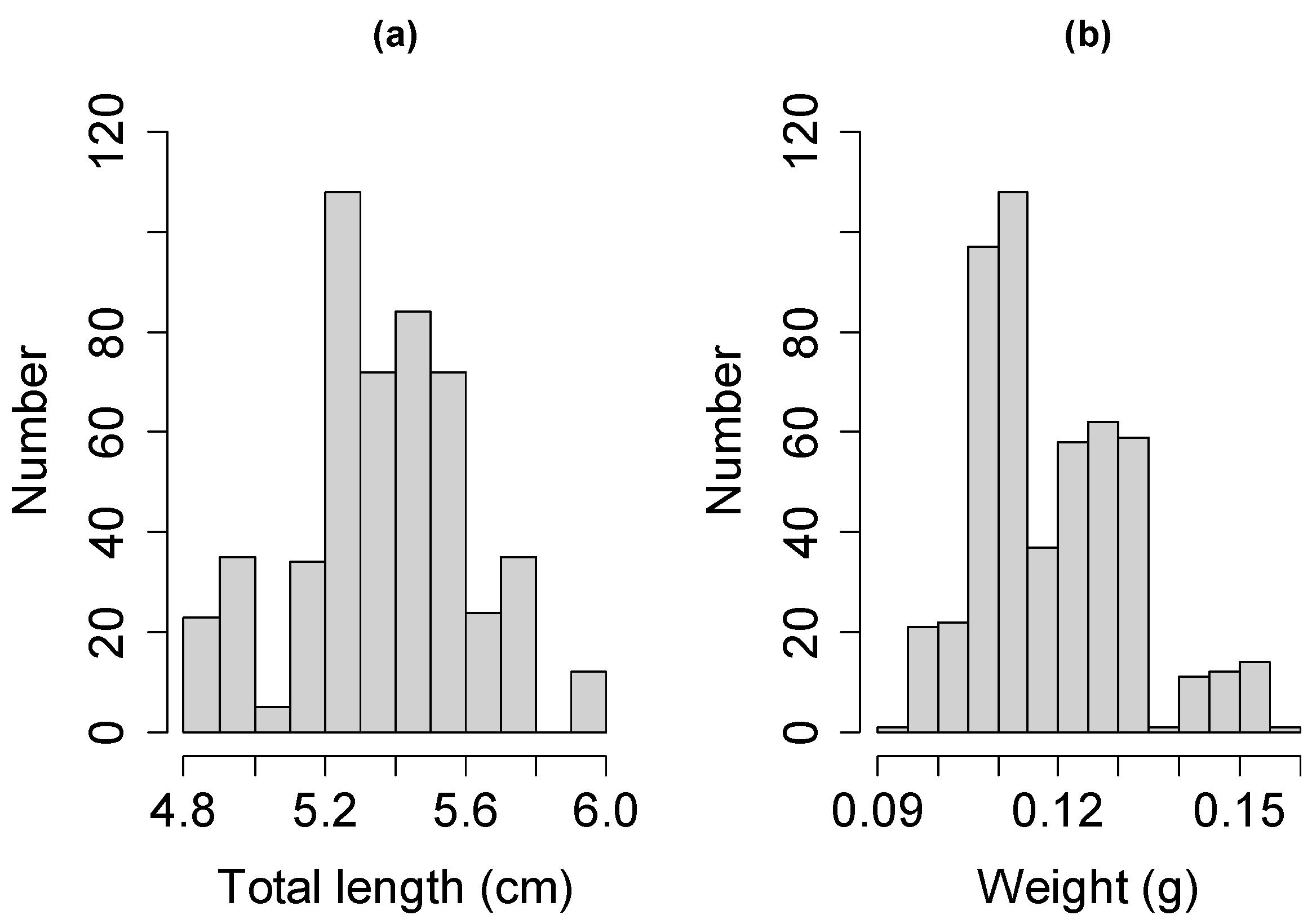
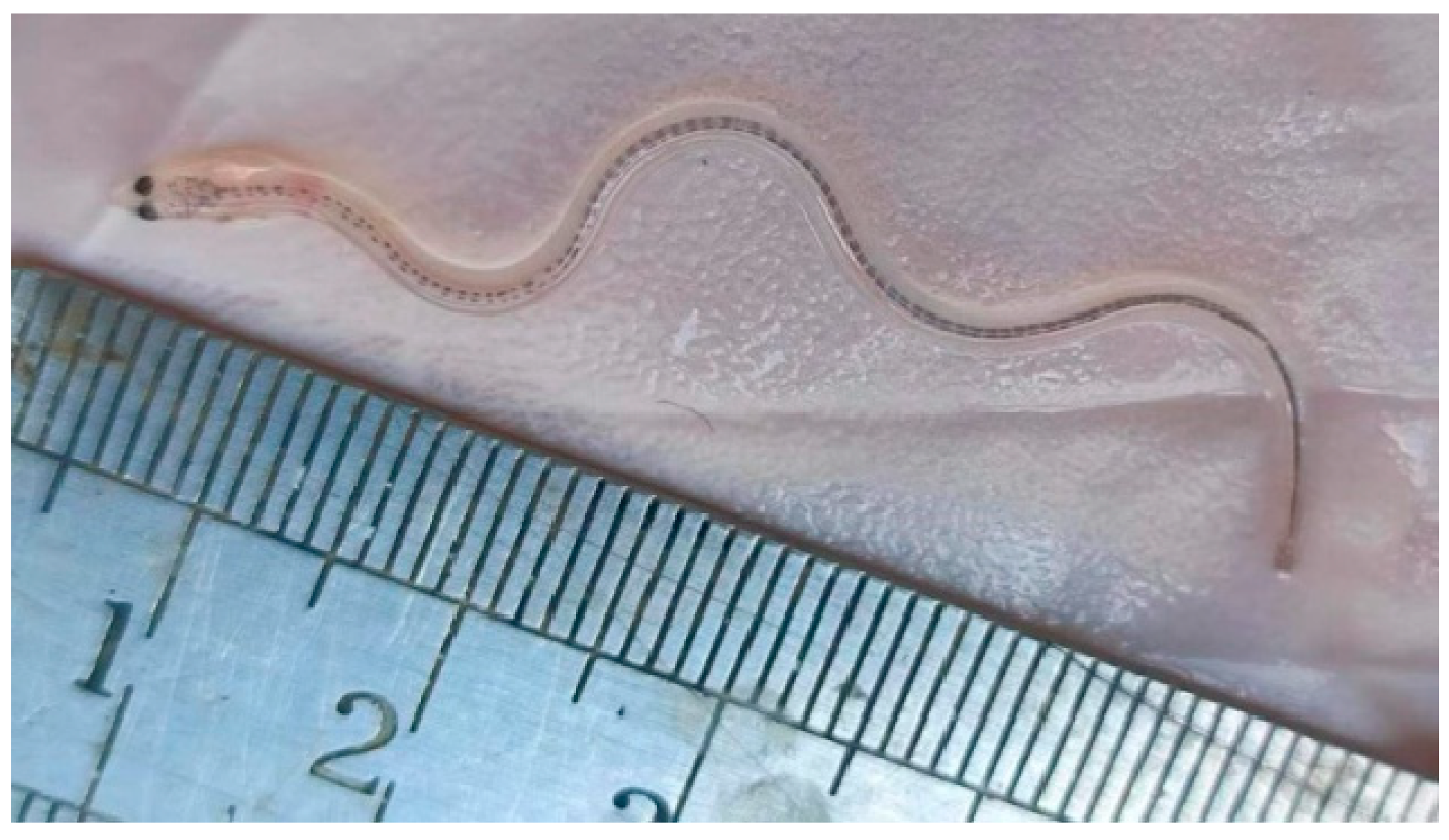
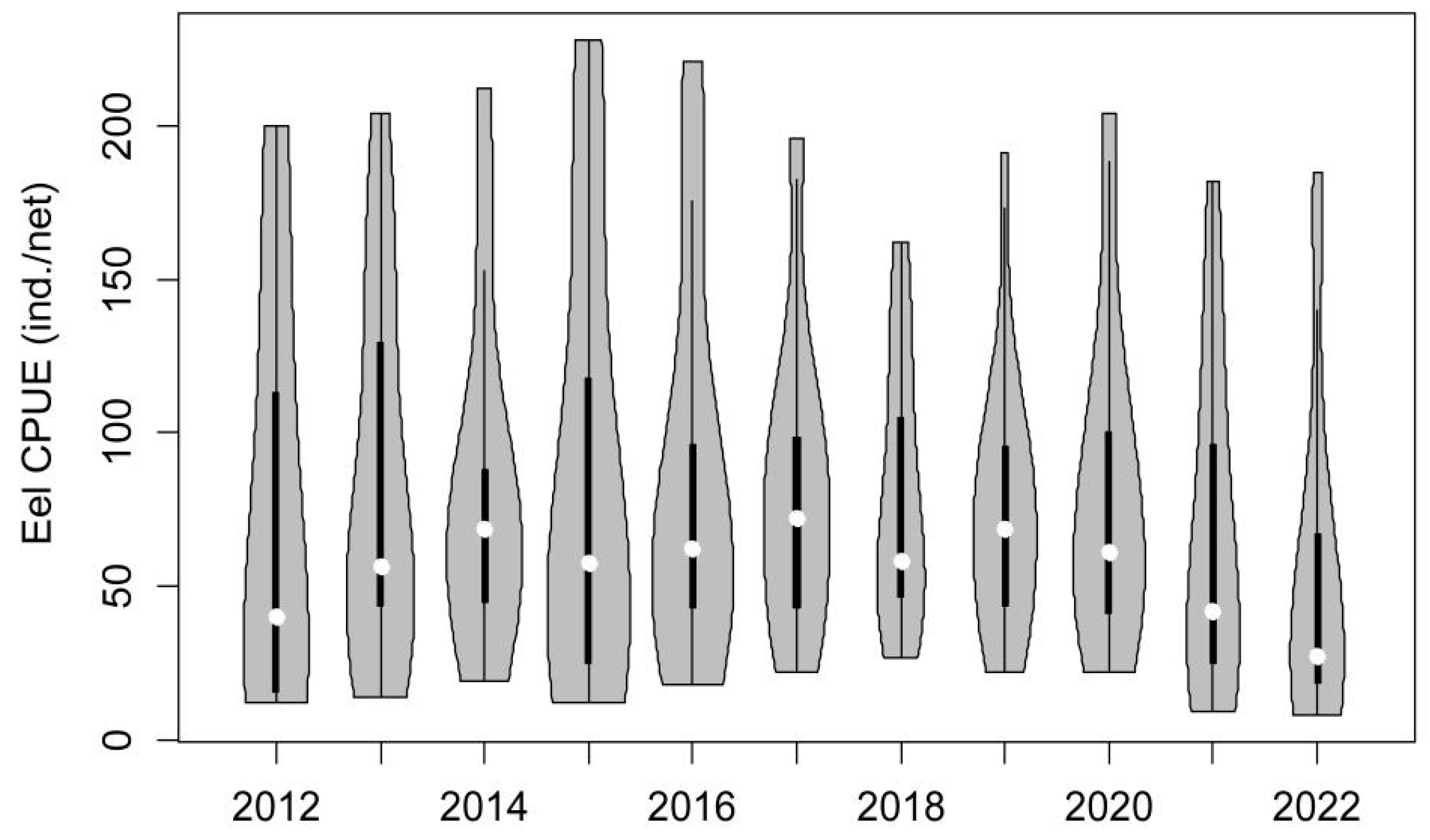
3.1. Population Characteristics of Glass Eels
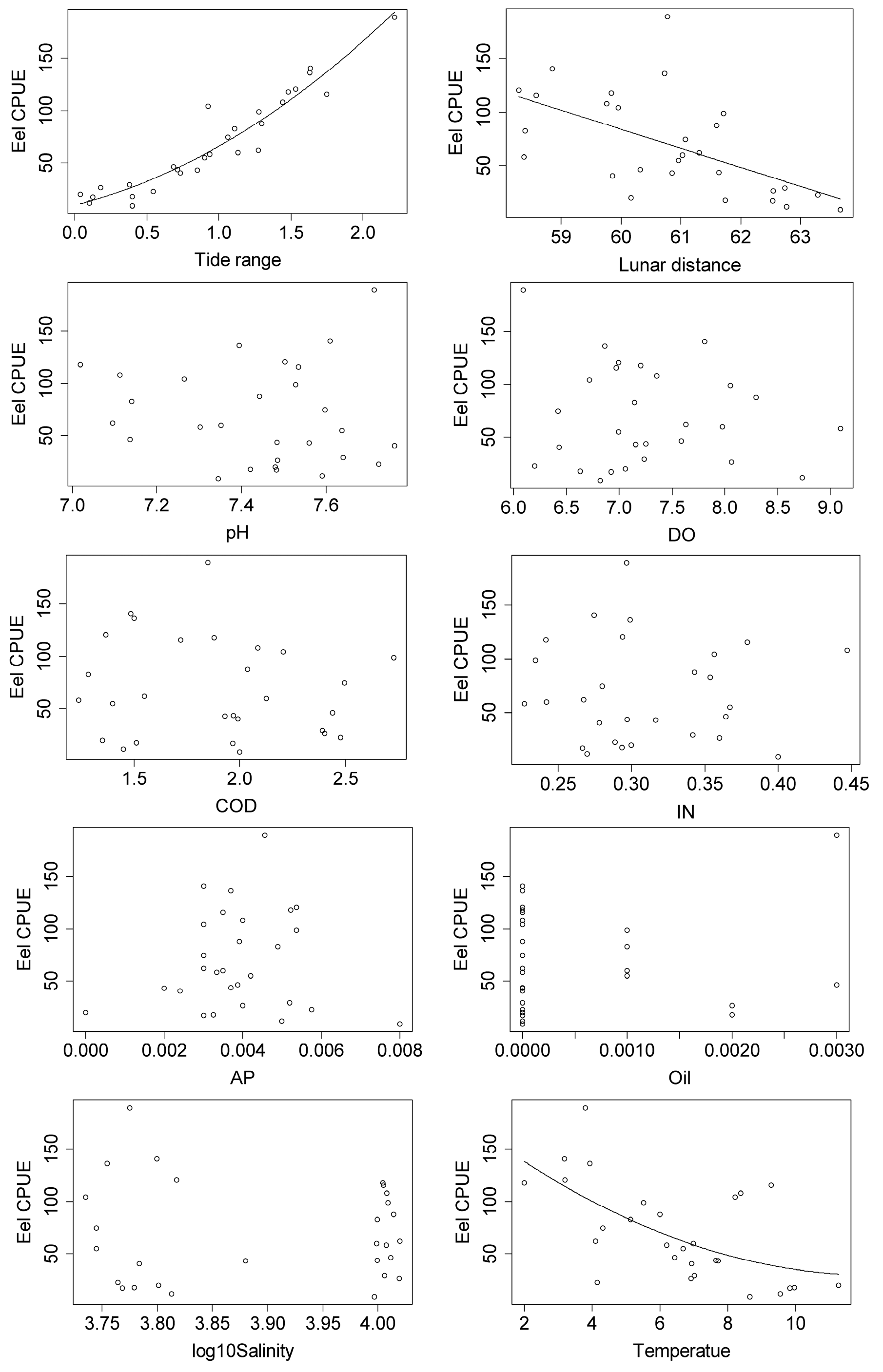
3.2. Relationships between Japanese Glass Eel CPUE and Environmental Factors
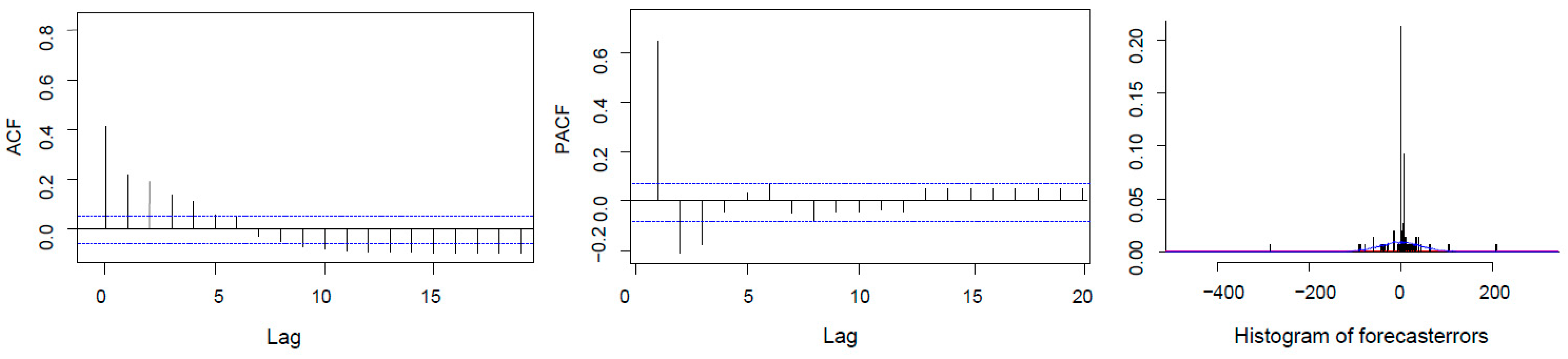
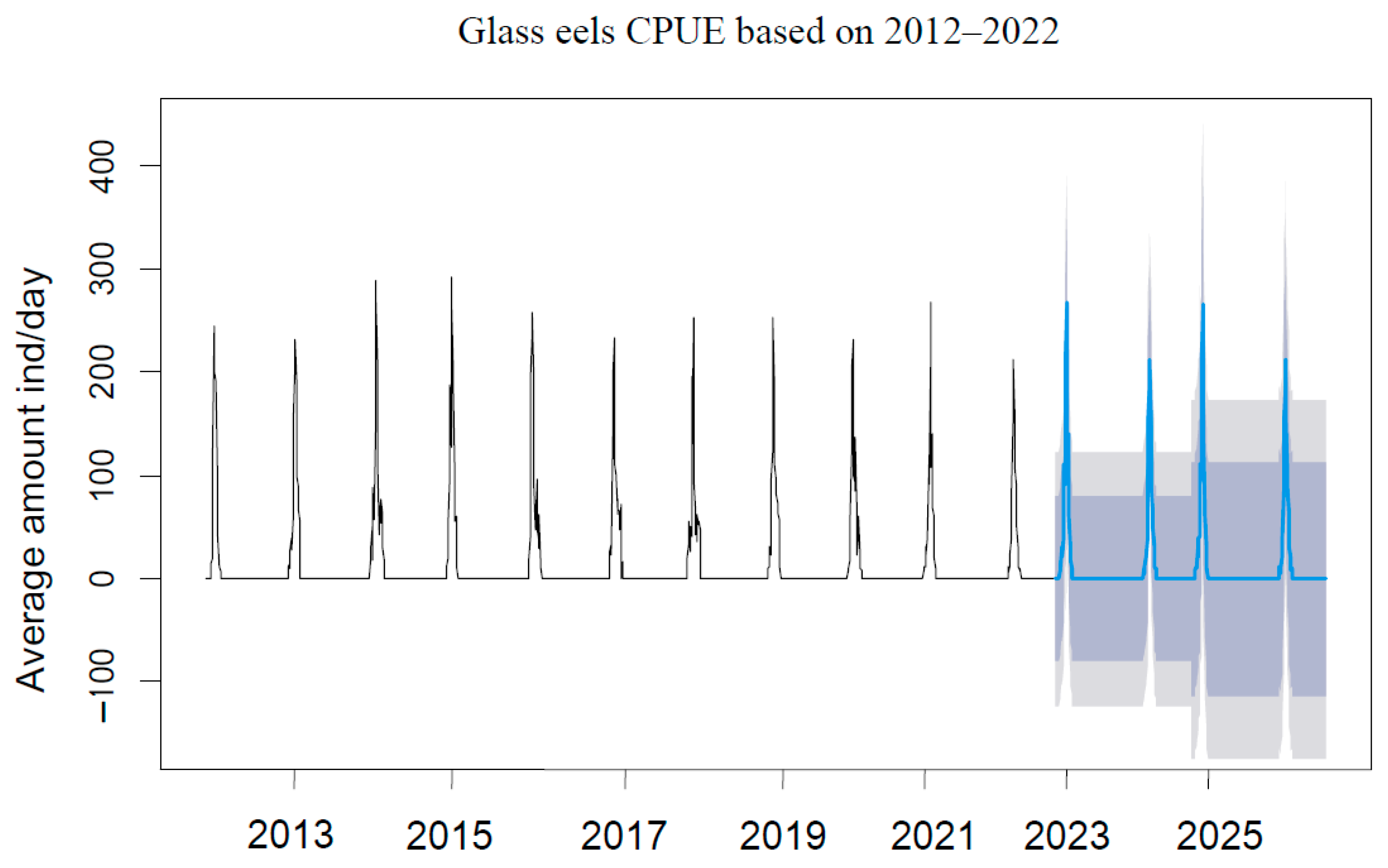
3.3. Recruitment Dynamics Forecasting
4. Discussion
4.1. Synchronization of Spawning with the New Moon
4.2. Stable but Low Recruitment of Japanese Eel in the Pearl River Estuary
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sala, O.E.; Chapin, F.S.; Armesto, J.J.; Berlow, E.; Bloomfield, J.; Dirzo, R.; Huber-Sanwald, E.; Huenneke, L.F.; Jackson, R.B.; Kinzig, A.; et al. Global biodiversity scenarios for the year 2100. Science 2000, 287, 1770–1774. [Google Scholar] [CrossRef] [PubMed]
- Dulvy, N.K.; Sadovy, Y.; Reynolds, J.D. Extinction vulnerability in marine populations. Fish Fish. 2003, 4, 25–64. [Google Scholar] [CrossRef]
- Stenseth, N.C.; Rouyer, T. Ecology: Destabilized fish stocks. Nature 2008, 452, 825–826. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.N.; Hsieh, C.H.; Sandin, S.A.; Hewitt, R.; Hollowed, A.; Beddington, J.; May, R.M.; Sugihara, G. Why fishing magnifies fluctuations in fish abundance. Nature 2008, 452, 835–839. [Google Scholar] [CrossRef]
- Mangel, M.; Brodziak, J.; DiNardo, G. Reproductive ecology and scientific inference of steepness: A fundamental metric of population dynamics and strategic fisheries management. Fish Fish. 2010, 11, 89–104. [Google Scholar] [CrossRef]
- Righton, D.; Piper, A.; Aarestrup, K.; Amilhat, E.; Belpaire, C.; Casselman, J.; Castonguay, M.; Díaz, E.; Dörner, H.; Faliex, E.; et al. Important questions to progress science and sustainable management of anguillid eels. Fish Fish. 2021, 22, 762–788. [Google Scholar] [CrossRef]
- Tesch, F.W. The Eel, 5th ed.; Wiley-Blackwell: Oxford, UK, 2003; p. 436. [Google Scholar]
- Tsukamoto, K. Discovery of the spawning area for Japanese eel. Nature 1992, 356, 789–791. [Google Scholar] [CrossRef]
- Kurogi, H.; Okazaki, M.; Mochioka, N.; Jinbo, T.; Hashimoto, H.; Takahashi, M.; Tawa, A.; Aoyama, J.; Shinoda, A.; Tsukamoto, K.; et al. First capture of post-spawning female of the Japanese eel Anguilla japonica at the southern West Mariana Ridge. Fish. Sci. 2011, 77, 199–205. [Google Scholar] [CrossRef]
- Cheng, P.; Tzeng, W. Timing of metamorphosis and estuarine arrival across the dispersal range of the Japanese eel Anguilla japonica. Mar. Ecol. Prog. Ser. 1996, 131, 87–96. [Google Scholar] [CrossRef]
- Tsukamoto, K. Oceanic migration and spawning of Anguillid eels. J. Fish. Biol. 2009, 74, 1833–1852. [Google Scholar] [CrossRef]
- Sullivan, M.C.; Able, K.W.; Hare, J.A.; Walsh, H.J. Anguilla rostrata glass eel ingress into two, US east coast estuaries: Patterns, processes and implications for adult abundance. J. Fish Biol. 2006, 69, 1081–1101. [Google Scholar] [CrossRef]
- Tanaka, E. Stock assessment of Japanese eels using Japanese abundance indices. Fish. Sci. 2014, 80, 1129–1144. [Google Scholar] [CrossRef]
- Kaifu, K.; Yokouchi, K. Increasing or decreasing?—Current status of the Japanese eel stock. Fish. Res. 2019, 220, 105348. [Google Scholar] [CrossRef]
- MARA (Ministry of Agriculture and Rural Affairs of the People’s Republic of China). Chinese Fishery Statistical Yearbook; China Agriculture Press: Beijing, China, 2018–2022.
- Masuda, Y.; Imaizumi, H.; Oda, K. Artificial completion of the Japanese eel, Anguilla japonica, life cycle: Challenge to mass production. Bull. Fish. Res. Agency 2012, 35, 111–117. [Google Scholar]
- Tanaka, H. Progression in artificial seedling production of Japanese eel Anguilla japonica. Fish. Sci. 2015, 81, 11–19. [Google Scholar] [CrossRef]
- Shuai, F.M.; Li, Z.Q.; Liu, G.W.; Li, X.H.; Li, Y.F.; Yang, J.P.; Li, J. Resource status of Japanese eel (Anguilla japonica) in the Pearl River Estuary. South China Fish. Sci. 2015, 11, 85–89. (In Chinese) [Google Scholar]
- Tatsukawa, K. Eel Resources in East Asia. Eel Biology; Springer: Berlin/Heidelberg, Germany, 2003; pp. 293–298. [Google Scholar]
- Harrison, A.J.; Walker, A.M.; Pinder, A.C.; Briand, C.; Aprahamian, M.W. A review of glass eel migratory behaviour, sampling techniques and abundance estimates in estuaries: Implications for assessing recruitment, local production and exploitation. Rev. Fish Biol. Fish. 2014, 24, 967–983. [Google Scholar] [CrossRef]
- Jacoby, D.M.P.; Casselman, J.M.; Crook, V.; DeLucia, M.B.; Ahn, H.; Kaifu, K.; Kurwie, T.; Sasal, P.; Silfvergrip, A.M.C.; Smith, K.G.; et al. Synergistic patterns of threat and the challenges facing global anguillid eel conservation. Glob. Ecol. Conserv. 2015, 4, 321–333. [Google Scholar] [CrossRef]
- Lu, K.X. Fishery Resources in the Pearl River System; Guangdong Science and Technology Press: Guangzhou, China, 1990. [Google Scholar]
- Tesch, F.W.; White, R.J. The Eel; Blackwell Publishing: London, UK, 2008; pp. 9–24. [Google Scholar]
- Fukuda, N.; Miller, M.J.; Aoyama, J.; Shinoda, A.; Tsukamoto, K. Evaluation of the pigmentation stages and body proportions from the glass eel to yellow eel in Anguilla japonica. Fish. Sci. 2013, 79, 425–438. [Google Scholar] [CrossRef]
- Kim, J.Y.; Jeong, H.C.; Kim, H.; Kang, S. Forecasting the monthly abundance of anchovies in the South Sea of Korea using a univariate approach. Fish. Res. 2015, 161, 293–302. [Google Scholar] [CrossRef]
- Box, G.E.; Jenkins, G.M.; Reinsel, G.C.; Ljung, G.M. Time Series Analysis: Forecasting and Control, 5th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Tsitsika, E.V.; Maravelias, C.D.; Haralabous, J. Modeling and forecasting pelagic fish production using univariate and multivariate ARIMA models. Fish. Sci. 2007, 73, 979–988. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: http://www.r-project.org/ (accessed on 1 January 2022).
- Tzeng, W.N. Immigration timing and activity rhythms of the eel, Anguilla japonica, elvers in the estuary of northern Taiwan, with emphasis on environmental influences. Bull. Jap. Soc. Fish. Oceanogr. 1985, 47, 11–28. [Google Scholar]
- Hwang, S.D.; Lee, T.W.; Choi, I.S.; Hwang, S.W. Environmental factors affecting the daily catch levels of Anguilla japonica glass eels in the Geum River estuary, South Korea. J. Coast. Res. 2014, 297, 954–960. [Google Scholar] [CrossRef]
- Jellyman, D.; Lambert, P. Factors affecting recruitment of glass eels into the Grey River, New Zealand. J. Fish Biol. 2003, 63, 1067–1079. [Google Scholar] [CrossRef]
- Tsukamoto, K.; Otake, T.; Mochioka, N.; Lee, T.W.; Fricke, H.; Inagaki, T.; Aoyama, J.; Ishikawa, S.; Kimura, S.; Miller, M.J.; et al. Seamounts, new moon and eel spawning: The search for the spawning site of the Japanese eel. Environ. Biol. Fish. 2003, 66, 221–229. [Google Scholar] [CrossRef]
- Han, Y.S.; Wu, C.R.; Iizuka, Y. Batch-like Arrival Waves of Glass Eels of Anguilla japonica in Offshore Waters of Taiwan. Zool. Stud. 2016, 55, e36. [Google Scholar]
- Cresci, A.A. Comprehensive hypothesis on the migration of European glass eels (Anguilla anguilla). Biol. Rev. 2020, 95, 1273–1286. [Google Scholar] [CrossRef]
- Yamamoto, T.; Mochioka, N.; Nakazono, A. Seasonal occurrence of anguillid glass eels at Yakushima Island, Japan. Fish. Sci. 2001, 67, 530–532. [Google Scholar] [CrossRef]
- Helme, H.; Bertucci, F.; Moussa, R.M.; Wolff, Y.; Sasal, P. Temporal dynamics of the recruitment of glass eels in two valleys of French Polynesia (Tahiti and Moorea Islands). Cybium 2018, 42, 341–348. [Google Scholar]
- Chow, S.; Masuda, Y.; Satomi, M.; Kamoshida, M.; Takahashi, M. A method to separate eel leptocephalus larvae from turbid water by controlling the light environment. Nippon Suisan Gakkaishi 2019, 85, 585–590. [Google Scholar] [CrossRef]
- Yamada, Y.; Okamura, A.; Mikawa, N.; Utoh, T.; Horie, N.; Tanaka, S.; Miller, M.J.; Tsukamoto, K. Ontogenetic changes in phototactic behavior during metamorphosis of artificially reared Japanese eel Anguilla japonica larvae. Mar. Ecol. Prog. Ser. 2009, 379, 241–251. [Google Scholar] [CrossRef]
- Laffaille, P.; Caraguel, J.M.; Legault, A. Temporal patterns in the upstream migration of European glass eels (Anguilla anguilla) at the Couesnon estuarine dam. Estuar. Coast. Shelf Sci. 2007, 73, 81–90. [Google Scholar] [CrossRef]
- Guo, H.Y.; Zhang, X.G.; Zhang, Y.; Tang, W.Q.; Wu, J.M. Effects of environmental variables on recruitment of Anguilla japonica glass eels in the Yangtze Estuary, China. Fish. Sci. 2017, 83, 333–341. [Google Scholar] [CrossRef]
- August, S.M.; Hicks, B.J. Water temperature and upstream migration of glass eels in New Zealand: Implications of climate change. Environ. Biol. Fishes 2008, 81, 195–205. [Google Scholar] [CrossRef]
- Jellyman, D.; Booker, D.; Watene, E. Recruitment of Anguilla spp. glass eels in the Waikato River, New Zealand. Evidence of declining migrations? J. Fish Biol. 2009, 74, 2014–2033. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; He, W.; Tong, C.; Lu, J. The effect of fishing the anguillid elver (Anguilla japonica) on the fishery of the Yangtze estuary. Estuar. Coast. Shelf Sci. 2008, 76, 902–908. [Google Scholar] [CrossRef]
- Belkin, I.M. Rapid warming of large marine ecosystems. Prog. Oceanogr. 2009, 81, 207–213. [Google Scholar] [CrossRef]
- Yao, Y.; Wang, C. Variations in summer marine heatwaves in the South China Sea. J. Geophys. Res. Oceans 2021, 126, e2021JC017792. [Google Scholar] [CrossRef]
- Rozenfeld, C.; García-Carpintero, V.; Pérez, L.; Gallego, V.; Herranz-Jusdado, J.G.; Tveiten, H.; Johnsen, H.K.; Fontaine, R.; Weltzien, F.; Cañizares, J.; et al. Cold seawater induces early sexual developmental stages in the BPG axis of European eel males. BMC Genom. 2019, 20, 597. [Google Scholar] [CrossRef]
- Sudo, R.; Yada, T. Anguillid eels as a model species for understanding endocrinological influences on the onset of spawning migration of fishes. Biology 2022, 11, 934. [Google Scholar] [CrossRef]
- Desaunay, Y.; Guerault, D. Seasonal and long-term changes in biometrics of eel larvae: A possible relationship between recruitment variation and North Atlantic ecosystem productivity. J. Fish. Biol. 1997, 51, 317–339. [Google Scholar] [CrossRef]
- Kawakami, Y.; Mochioka, N.; Kimura, R.; Nakazono, A. Seasonal changes of the RNA/DNA ratio, size and lipid contents and immigration adaptability of Japanese glass-eels, Anguilla japonica, collected in northern Kyushu, Japan. J. Exp. Mar. Biol. Ecol. 1999, 238, 1–19. [Google Scholar] [CrossRef]
- Kearney, M.; Jeffs, A.; Lee, P. Seasonal differences in the quality of shortfin glass eel, Anguilla australis, and subsequent effects on growth and survival in captivity. N. Z. Nat. Sci. 2011, 36, 45–55. [Google Scholar]
- Belpaire, C.G.J.; Goemans, G.; Geeraerts, C.; Quataert, P.; Parmentier, K.; Hagel, P.; De Boer, J. Decreasing eel stocks: Survival of the fattest? Ecol. Freshw. Fish 2009, 18, 197–214. [Google Scholar] [CrossRef]
- Arai, T. Do we protect freshwater eels or do we drive them to extinction? Springerplus 2014, 3, 534. [Google Scholar] [CrossRef]
- Kahn, D.M. Trends in abundance and fishing mortality of american eel. Fisheries 2018, 44, 129–136. [Google Scholar] [CrossRef]
- Sonne, C.; Peng, W.X.; Alstrup, A.K.O.; Lam, S.S. European eel population at risk of collapse. Science 2021, 372, 1271. [Google Scholar] [CrossRef]
- Feunteun, E. Management and restoration of european eel population (Anguilla anguilla): An impossible bargain. Ecol. Eng. 2002, 18, 575–591. [Google Scholar] [CrossRef]
- Dekker, W. Did lack of spawners cause the collapse of the European eel, Anguilla anguilla? Fish. Manag. Ecol. 2003, 10, 365–376. [Google Scholar] [CrossRef]
- Leone, C.; Zucchetta, M.; Capoccioni, F.; Gravina, M.F.; Franzoi, P.; Ciccotti, E. Stage-specific distribution models can predict eel (Anguilla anguilla) occurrence during settlement in coastal lagoons. Estuar. Coast. Shelf Sci. 2016, 170, 123–133. [Google Scholar] [CrossRef]
- Shuai, F.M.; Liu, Q.F.; Liu, Y.Q.; Wu, Z.; Lek, S.; Chen, Y.S. Spatial Distribution Patterns of Japanese Eel (Anguilla japonica) in a Large Subtropical River (Pearl), China. N. Am. J. Fish. Manag. 2021, 41, 929–938. [Google Scholar] [CrossRef]
- Tzeng, W.N.; Lin, H.R.; Wang, C.H.; Xu, S.N. Differences in size and growth rates of male and female migrating Japanese eels in Pearl River, China. J. Fish Biol. 2000, 57, 1245–1253. [Google Scholar] [CrossRef]
- Podda, C.; Palmas, F.; Pusceddu, A.; Sabatini, A. When the Eel Meets Dams: Larger Dams’ Long-Term Impacts on Anguilla anguilla (L., 1758). Front. Environ. Sci. 2022, 10, 876369. [Google Scholar] [CrossRef]
- Acou, A.; Laffaille, P.; Legault, A.; Feunteun, E. Migration pattern of silver eel (Anguilla anguilla, L.) in an obstructed river system. Ecol. Freshw. Fish 2008, 17, 432–442. [Google Scholar] [CrossRef]
- Trancart, T.; Teichert, N.; Lamoureux, J.; Gharnit, E.; Acou, A.; Oliveira, E.D.; Roy, R.; Feunteun, E.; Feunteun, E. A possible strong impact of tidal power plant on silver eels’ migration. Estuar. Coast. Shelf Sci. 2022, 278, 108116. [Google Scholar] [CrossRef]
- Robinet, T.T.; Feunteun, E.E. Sublethal effects of exposure to chemical compounds: A cause for the decline in atlantic eels? Ecotoxicology 2002, 11, 265–277. [Google Scholar] [CrossRef]
- Corsi, I.; Mariottini, M.; Badesso, A.; Caruso, T.; Borghesi, N.; Bonacci, S.; Iacocca, A.; Focardi, S. Contamination and sub-lethal toxicological effects of persistent organic pollutants in the European eel (Anguilla anguilla) in the Orbetello lagoon (Tuscany, Italy). Hydrobiologia 2005, 550, 237–249. [Google Scholar] [CrossRef]
- Sancho, E.; Fernandez-Vega, C.; Sanchez, M.; Ferrando, M.D.; Andreu-Moliner, E. Alterations on ache activity of the fish Anguilla anguilla as response to herbicide-contaminated water. Ecotoxicol. Environ. Saf. 2000, 46, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Pierron, F.; Baudrimont, M.; Bossy, A.; Bourdineaud, J.; Brèthes, D.; Elie, P.; Massabuau, J. Impairment of lipid storage by cadmium in the european eel (Anguilla anguilla). Aquat. Toxicol. 2007, 81, 304–311. [Google Scholar] [CrossRef]
- Belpaire, C.; Reyns, T.; Geeraerts, C.; Loco, J.V. Toxic textile dyes accumulate in wild european eel Anguilla anguilla. Chemosphere 2015, 138, 784–791. [Google Scholar] [CrossRef]
- Maes, G.E.; Raeymaekers, J.A.M.; Pampoulie, C.; Seynaeve, A.; Goemans, G.; Belpaire, C.; Volckaert, F.A.M. The catadromous European eel Anguilla anguilla (L.) as a model for freshwater evolutionary ecotoxicology: Relationship between heavy metal bioaccumulation, condition and genetic variability. Aquat. Toxicol. 2005, 73, 99–114. [Google Scholar] [CrossRef] [PubMed]
- Tao, W.; Niu, L.; Dong, Y.; Fu, T.; Lou, Q. Nutrient Pollution and Its Dynamic Source-Sink Pattern in the Pearl River Estuary (South China). Front. Mar. Sci. 2021, 8, 713907. [Google Scholar] [CrossRef]
- Niu, L.; Cai, H.; Jia, L.; Luo, X.; Tao, W.; Dong, Y.; Yang, Q. Metal pollution in the Pearl River Estuary and implications for estuary management: The influence of hydrological connectivity associated with estuarine mixing. Ecotoxicol. Environ. Saf. 2021, 225, 112747. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Vazquez, S.; Linde, A.R.; Ayllon, F.; Garcia-Vazquez, E. Induction of micronuclei in eel (Anguilla anguilla L.) by heavy metals. Ecotox Environ. Saf. 2001, 49, 139–143. [Google Scholar]
- Pujolar, J.M.; Marino, I.A.; Milan, M.; Coppe, A.; Maes, G.E.; Capoccioni, F.; Ciccotti, E.; Bervoets, L.; Covaci, A.; Belpaire, C.; et al. Surviving in a toxic world: Transcriptomics and gene expression profiling in response to environmental pollution in the critically endangered European eel. BMC Genom. 2012, 13, 507. [Google Scholar] [CrossRef]
- Palstra, A.P.; Ginneken, V.; Murk, A.J.; Thillart, G.E.E.J.M. Are dioxin-like contaminants responsible for the eel (Anguilla anguilla) drama? Naturwissenschaften 2006, 93, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Dekker, W.; Casselman, J.M.; Cairns, D.K.; Tsukamoto, K.; Jellyman, D.; Lickers, H. Worldwide decline of eel resources necessitates immediate action: Québec Declaration of Concern. Fisheries 2003, 28, 28–30. [Google Scholar]
- Shuai, F.M.; Li, H.Y.; Li, J.; Jiang, T.; Yang, J.; Yang, W.L. Unravelling the life-history patterns and habitat preferences of the Japanese eel (Anguilla japonica) in the Pearl River, China. J. Fish Biol. 2023. early view. [Google Scholar] [CrossRef]
- Jellyman, D.J. Status of New Zealand fresh-water eel stocks and management initiatives. ICES J. Mar. Sci. 2007, 64, 1379–1386. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shuai, F.; Li, J.; Yu, S.; Yang, J. Temporal Pattern of the Occurrence of Japanese Glass Eels (Anguilla japonica) in the Pearl River Estuary. Fishes 2023, 8, 256. https://doi.org/10.3390/fishes8050256
Shuai F, Li J, Yu S, Yang J. Temporal Pattern of the Occurrence of Japanese Glass Eels (Anguilla japonica) in the Pearl River Estuary. Fishes. 2023; 8(5):256. https://doi.org/10.3390/fishes8050256
Chicago/Turabian StyleShuai, Fangmin, Jie Li, Shunchao Yu, and Jian Yang. 2023. "Temporal Pattern of the Occurrence of Japanese Glass Eels (Anguilla japonica) in the Pearl River Estuary" Fishes 8, no. 5: 256. https://doi.org/10.3390/fishes8050256
APA StyleShuai, F., Li, J., Yu, S., & Yang, J. (2023). Temporal Pattern of the Occurrence of Japanese Glass Eels (Anguilla japonica) in the Pearl River Estuary. Fishes, 8(5), 256. https://doi.org/10.3390/fishes8050256