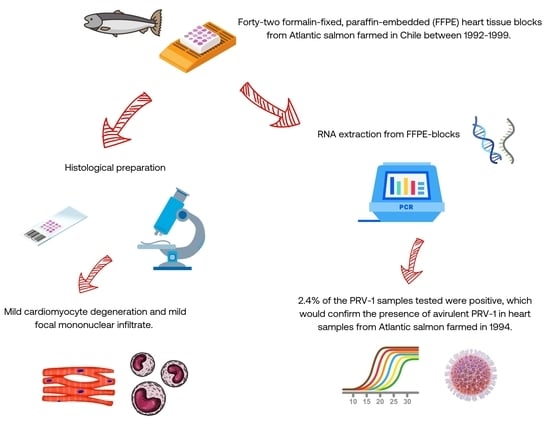
Piscine Orthoreovirus-1 (PRV-1) Has Been Present in Chilean Salmon Aquaculture since at Least 1994
Abstract
1. Introduction
2. Materials and Methodology
2.1. Fish and Tissue Samples
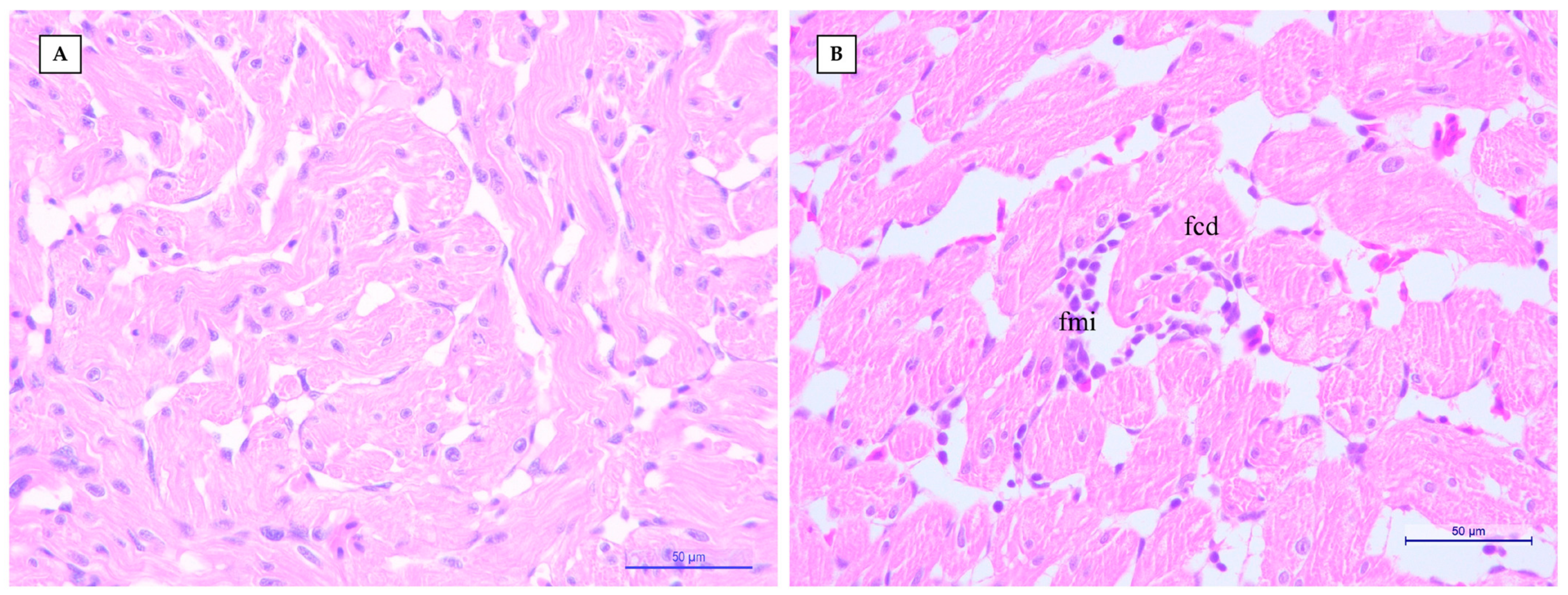
2.2. Histopathological and Immunohistochemical (IHC) Examination
2.3. RNA Extraction
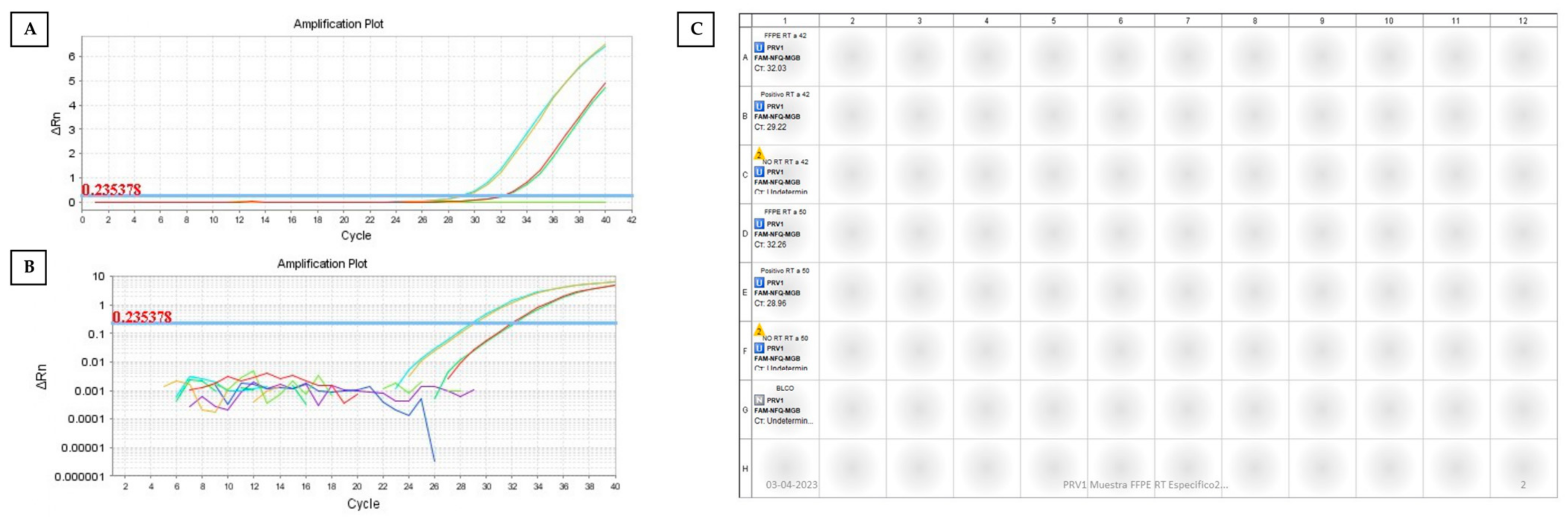
2.4. One-Step RT–qPCR
2.5. Two-Step RT–qPCR
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kongtorp, R.T.; Kjerstad, A.; Taksdal, T.; Guttvik, A.; Falk, K. Heart and skeletal muscle inflammation in Atlantic salmon, Salmo salar L.: A new infectious disease. J. Fish Dis. 2004, 27, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Palacios, G.; Lovoll, M.; Tengs, T.; Hornig, M.; Hutchison, S.; Hui, J.; Kongtorp, R.-T.; Savji, N.; Bussetti, A.V.; Solovyov, A.; et al. Heart and Skeletal Muscle Inflammation of Farmed Salmon Is Associated with Infection with a Novel Reovirus. PLoS ONE 2010, 5, e11487. [Google Scholar] [CrossRef] [PubMed]
- Di Cicco, E.; Ferguson, H.W.; Schulze, A.D.; Kaukinen, K.H.; Li, S.; Vanderstichel, R.; Wessel, Ø.; Rimstad, E.; Gardner, I.A.; Hammell, K.L.; et al. Heart and skeletal muscle inflammation (HSMI) disease diagnosed on a British Columbia salmon farm through a longitudinal farm study. PLoS ONE 2017, 12, e0171471. [Google Scholar]
- Godoy, M.G.; Kibenge, M.J.T.; Wang, Y.; Suarez, R.; Leiva, C.; Vallejos, F.; Kibenge, F.S.B. First description of clinical presentation of piscine orthoreovirus (PRV) infections in salmonid aquaculture in Chile and identification of a second genotype (Genotype II) of PRV. Virol. J. 2016, 13, 98. [Google Scholar] [CrossRef] [PubMed]
- Takano, T.; Nawata, A.; Sakai, T.; Matsuyama, T.; Ito, T.; Kurita, J.; Terashima, S.; Yasuike, M.; Nakamura, Y.; Fujiwara, A.; et al. Full-Genome Sequencing and Confirmation of the Causative Agent of Erythrocytic Inclusion Body Syndrome in Coho Salmon Identifies a New Type of Piscine Orthoreovirus. PLoS ONE 2016, 11, e0165424. [Google Scholar] [CrossRef] [PubMed]
- Dhamotharan, K.; Vendramin, N.; Markussen, T.; Wessel, Ø.; Cuenca, A.; Nyman, I.B.; Olsen, A.B.; Tengs, T.; Dahle, M.K.; Rimstad, E. Molecular and Antigenic Characterization of Piscine orthoreovirus (PRV) from Rainbow Trout (Oncorhynchus mykiss). Viruses 2018, 10, 170. [Google Scholar] [CrossRef] [PubMed]
- Kuehn, R.; Stoeckle, B.C.; Young, M.; Popp, L.; Taeubert, J.-E.; Pfaffl, M.W.; Geist, J. Identification of a piscine reovirus-related pathogen in proliferative darkening syndrome (PDS) infected brown trout (Salmo trutta fario) using a next-generation technology detection pipeline. PLoS ONE 2018, 13, e0206164. [Google Scholar] [CrossRef] [PubMed]
- Dhamotharan, K.; Tengs, T.; Wessel, Ø.; Braaen, S.; Nyman, I.B.; Hansen, E.F.; Christiansen, D.H.; Dahle, M.K.; Rimstad, E.; Markussen, T. Evolution of the Piscine orthoreovirus Genome Linked to Emergence of Heart and Skeletal Muscle Inflammation in Farmed Atlantic Salmon (Salmo salar). Viruses 2019, 11, 465. [Google Scholar] [CrossRef] [PubMed]
- Wessel, Ø.; Hansen, E.F.; Dahle, M.K.; Alarcon, M.; Vatne, N.A.; Nyman, I.B.; Soleim, K.B.; Dhamotharan, K.; Timmerhaus, G.; Markussen, T.; et al. Piscine Orthoreovirus-1 Isolates Differ in Their Ability to Induce Heart and Skeletal Muscle Inflammation in Atlantic Salmon (Salmo salar). Pathogens 2020, 9, 1050. [Google Scholar] [CrossRef] [PubMed]
- Bustos, P.; Rozas-Serri, M.; Bohle, H.; Ildefonso, R.; Sandoval, A.; Gaete, A.; Araya, C.; Grothusen, H.; Tapia, E.; Gallardo, A.; et al. Primer reporte de piscine reovirus en salmón del Atlántico, Salmo salar, cultivado en Chile. Versión Difer. 2011, 65–69. [Google Scholar]
- Sernapesca. Informe Sanitario De La Salmonicultura En Centros Agua Dulce y Mar Año 2021; Animal DdS: Valparaíso, Chile, 2022; p. 52. [Google Scholar]
- Dahle, M.K.; Wessel, Ø.; Rimstad, E. Immune Response Against Piscine orthoreovirus (PRV) in Salmonids. Princ. Fish Immunol. 2022, 445–461. [Google Scholar] [CrossRef]
- Haatveit, H.M.; Nyman, I.B.; Markussen, T.; Wessel, Ø.; Dahle, M.K.; Rimstad, E. The non-structural protein μNS of piscine orthoreovirus (PRV) forms viral factory-like structures. Veter. Res. 2016, 47, 5. [Google Scholar] [CrossRef] [PubMed]
- Markussen, T.; Dahle, M.K.; Tengs, T.; Løvoll, M.; Finstad, Ø.W.; Wiik-Nielsen, C.R.; Grove, S.; Lauksund, S.; Robertsen, B.; Rimstad, E. Correction: Sequence Analysis of the Genome of Piscine Orthoreovirus (PRV) Associated with Heart and Skeletal Muscle Inflammation (HSMI) in Atlantic Salmon (Salmo salar). PLoS ONE 2013, 8, e70075. [Google Scholar] [CrossRef]
- Karatas, S.; Mikalsen, J.; Steinum, T.M.; Taksdal, T.; Bordevik, M.; Colquhoun, D.J. Real time PCR detection of Piscirickettsia salmonis from formalin-fixed paraffin-embedded tissues. J. Fish Dis. 2008, 31, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Snow, M.; McKay, P.; McBeath, A.J.A.; Black, J.; Doig, F.; Kerr, R.; Cunningham, C.O.; Nylund, A.; Devold, M. Development, application and validation of a Taqman real-time RT-PCR assay for the detection of infectious salmon anaemia virus (ISAV) in Atlantic salmon (Salmo salar). Dev. Biol. 2006, 126, 133–145. [Google Scholar]
- Siah, A.; Knutsen, E.; Richmond, Z.; Mills, M.; Frisch, K.; Powell, J.F.; Brevik, Ø.; Duesund, H. Real-time RT-qPCR assay to detect sequences in the Piscine orthoreovirus-1 genome segment S1 associated with heart and skeletal muscle inflammation in Atlantic salmon. J. Fish Dis. 2020, 43, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Kibenge, M.J.; Iwamoto, T.; Wang, Y.; Morton, A.; Godoy, M.G.; Kibenge, F.S. Whole-genome analysis of piscine reovirus (PRV) shows PRV represents a new genus in family Reoviridae and its genome segment S1 sequences group it into two separate sub-genotypes. Virol. J. 2013, 10, 230. [Google Scholar] [CrossRef] [PubMed]
- Kashofer, K.; Viertler, C.; Pichler, M.; Zatloukal, K. Quality Control of RNA Preservation and Extraction from Paraffin-Embedded Tissue: Implications for RT-PCR and Microarray Analysis. PLoS ONE 2013, 8, e70714. [Google Scholar] [CrossRef] [PubMed]
| Histoscore | Atrium | Ventricle | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Epicardium | Stratum Compactum | Stratum Sponsiosum | Epicardium | ||||||
| Cell Degeneration | Mononuclear Cells Infiltrate | Mononuclear Cells Infiltrate | Cell Degeneration | Mononuclear Cells Infiltrate | Mononuclear Cells Infiltrate | Cell Degeneration | Mononuclear Cells Infiltrate | Thrombosis | |
| 0 | NHC | NIC | NIC | NIC | NHC | NIC | NHC | NHC | NHC |
| 1 | FCD (<10% TS) | MiMI (<10% TS) | MiMI (<10% TS) | MiMI (<10% TS) | FCD (<10% TS) | MiMI (<10% TS) | FCD (<10% TS) | <10% TS | <10% TS |
| 2 | FCD (10–50% TS) | MoMI (10–50% TS) | MoMI (10–50% TS) | MoMI (10–50% TS) | FCD (10–50% TS) | MoMI (10–50% TS) | FCD (10–50% TS) | 10–50% TS | 10–50% TS |
| 3 | DCD (>50% TS) | SMI (>10% TS) | SMI (>10% TS) | SMI (>10% TS) | DCD (>50% TS) | SMI (>10% TS) | DCD (>50% TS) | >50% TS | >50% TS |
| Relative weighting | 5 | 5 | 18.3 | 13.3 | 13.3 | 13.3 | 13.3 | 16.5 | 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rozas-Serri, M.; Ildefonso, R.; Jaramillo, V.; Peñaloza, E.; Leiva, C.; Barrientos, S.; Coñuecar, D.; Maldonado, L.; Muñoz, A.; Peña, A.; et al. Piscine Orthoreovirus-1 (PRV-1) Has Been Present in Chilean Salmon Aquaculture since at Least 1994. Fishes 2023, 8, 229. https://doi.org/10.3390/fishes8050229
Rozas-Serri M, Ildefonso R, Jaramillo V, Peñaloza E, Leiva C, Barrientos S, Coñuecar D, Maldonado L, Muñoz A, Peña A, et al. Piscine Orthoreovirus-1 (PRV-1) Has Been Present in Chilean Salmon Aquaculture since at Least 1994. Fishes. 2023; 8(5):229. https://doi.org/10.3390/fishes8050229
Chicago/Turabian StyleRozas-Serri, Marco, Ricardo Ildefonso, Victoria Jaramillo, Estefanía Peñaloza, Camila Leiva, Soraya Barrientos, Darling Coñuecar, Lucerina Maldonado, Ariel Muñoz, Andrea Peña, and et al. 2023. "Piscine Orthoreovirus-1 (PRV-1) Has Been Present in Chilean Salmon Aquaculture since at Least 1994" Fishes 8, no. 5: 229. https://doi.org/10.3390/fishes8050229
APA StyleRozas-Serri, M., Ildefonso, R., Jaramillo, V., Peñaloza, E., Leiva, C., Barrientos, S., Coñuecar, D., Maldonado, L., Muñoz, A., Peña, A., Aranis, F., & Senn, C. (2023). Piscine Orthoreovirus-1 (PRV-1) Has Been Present in Chilean Salmon Aquaculture since at Least 1994. Fishes, 8(5), 229. https://doi.org/10.3390/fishes8050229