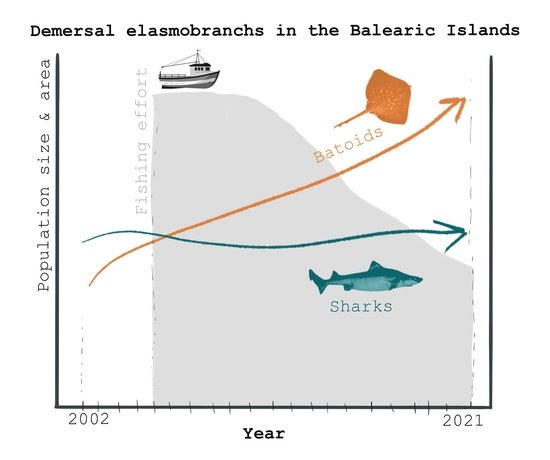
Conservation Status Assessment of Demersal Elasmobranchs in the Balearic Islands (Western Mediterranean) over the Last Two Decades
Abstract
1. Introduction
2. Materials and Methods
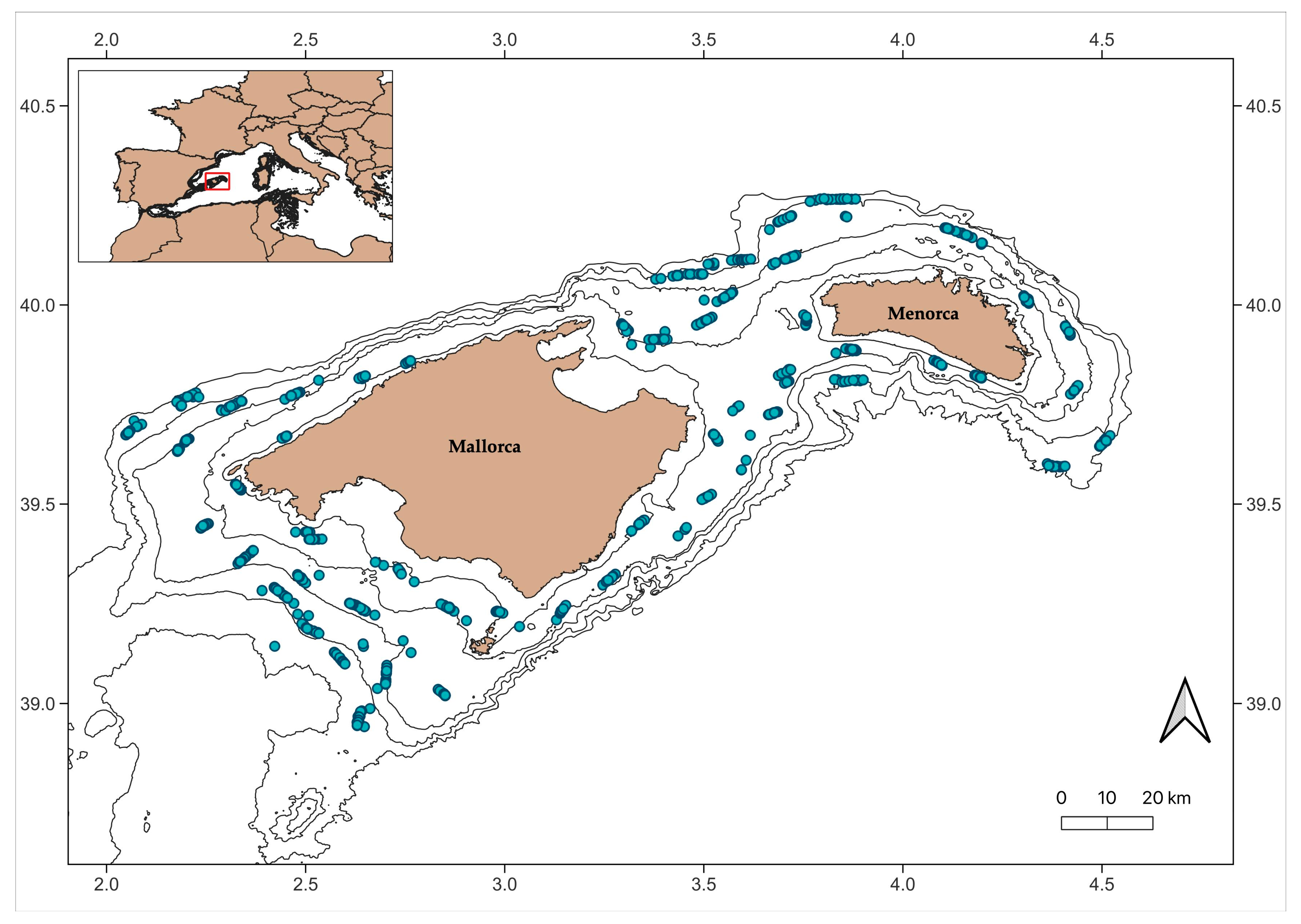
2.1. Study Area
2.2. Data Source
2.3. Species Selection and Functional Groups
2.4. Estimation of Indicators
2.5. Population Distribution Indicators
Area of Distribution
2.6. Population Size Indicators
Density and Biomass
2.7. Population Status Indicators
2.7.1. 95th Percentile of Size Distribution (P95)
2.7.2. Percentage of Mature Specimens (%Mature)
2.8. Ecosystem Structure Indicators
2.8.1. Mean Maximum Length of Fish (MMLF)
2.8.2. Large Fish Indicator (LFI)
2.8.3. Conservation Status of Fish (CSFb)
2.9. Trends Evaluation
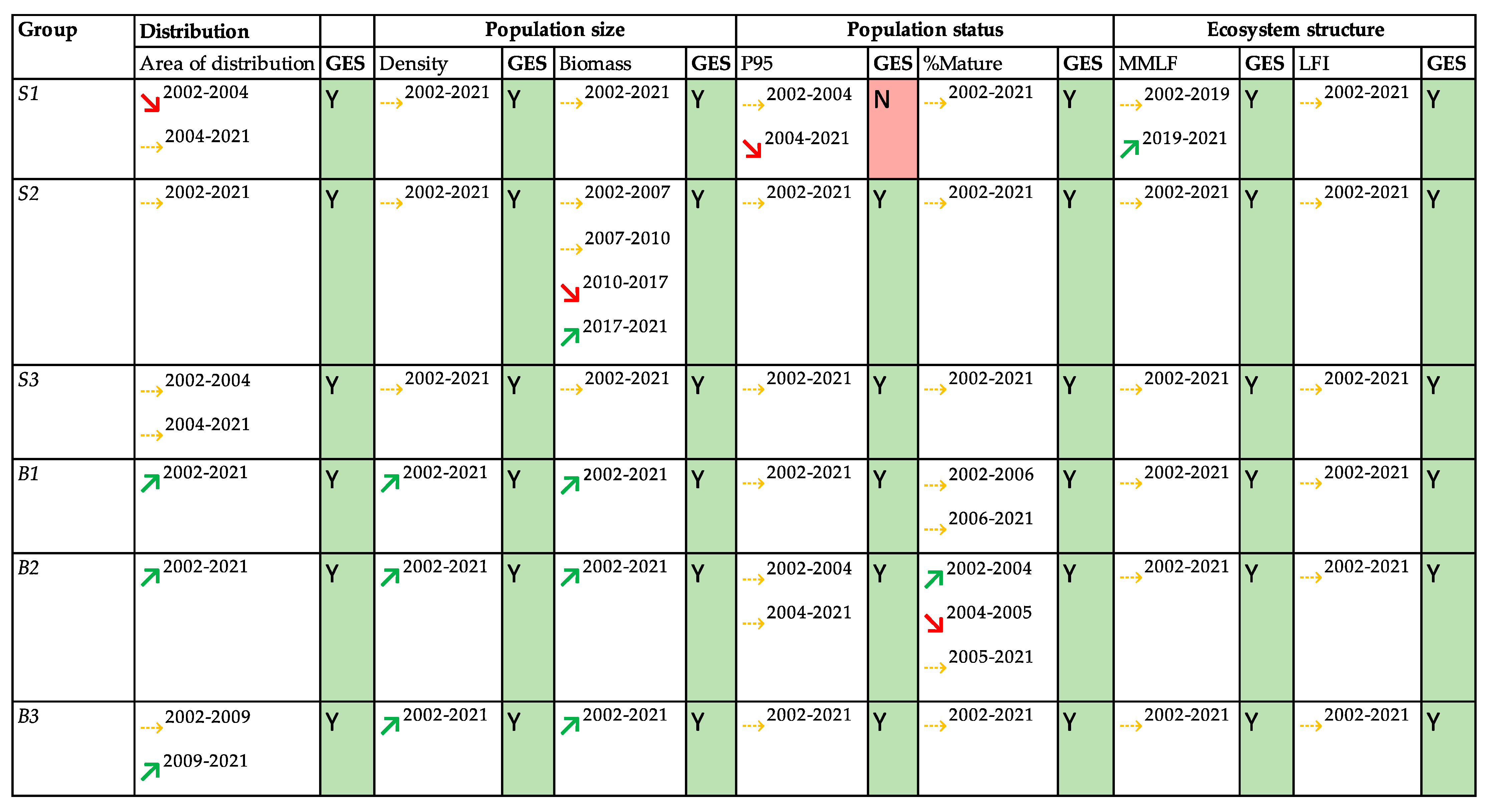
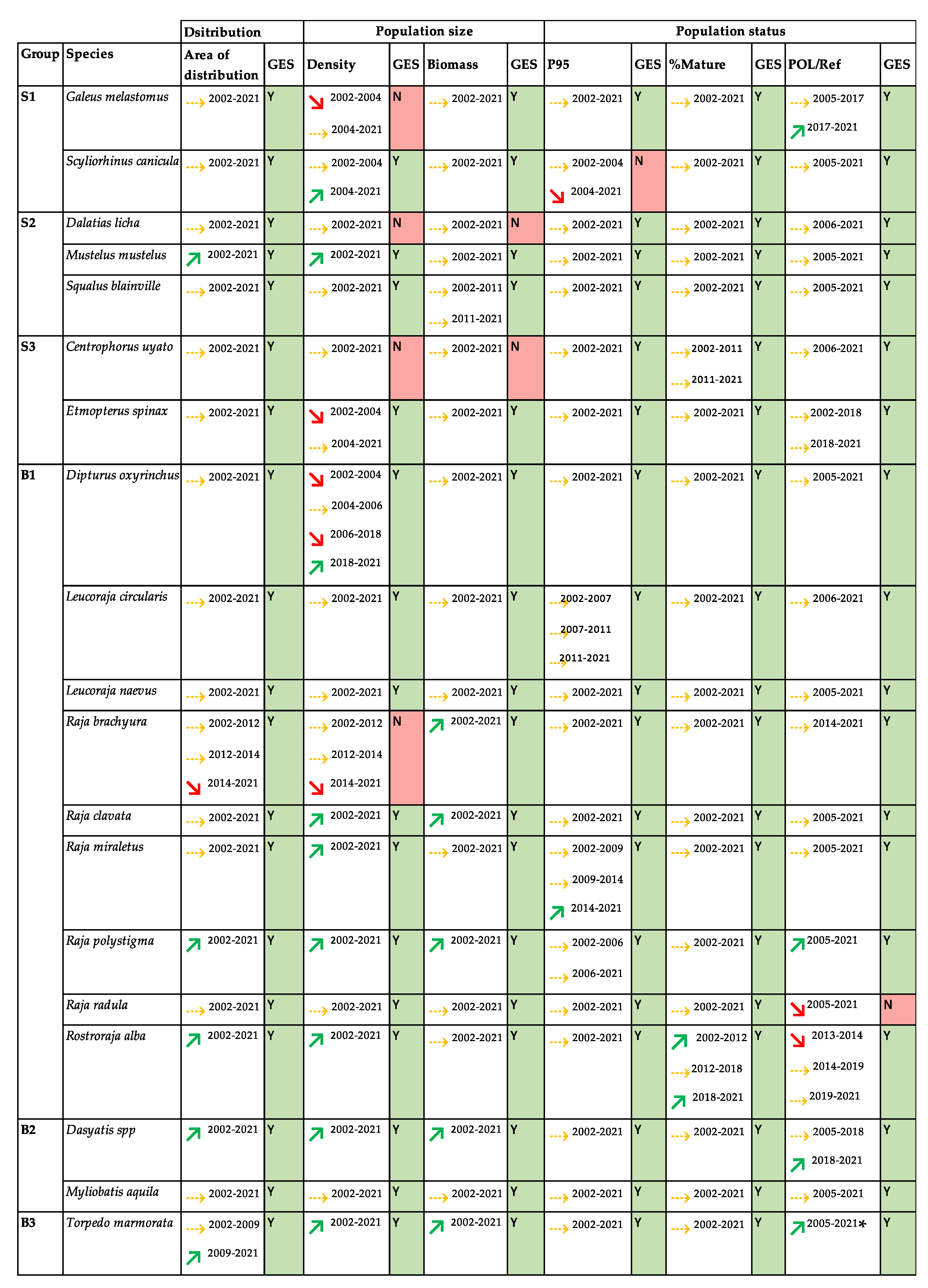
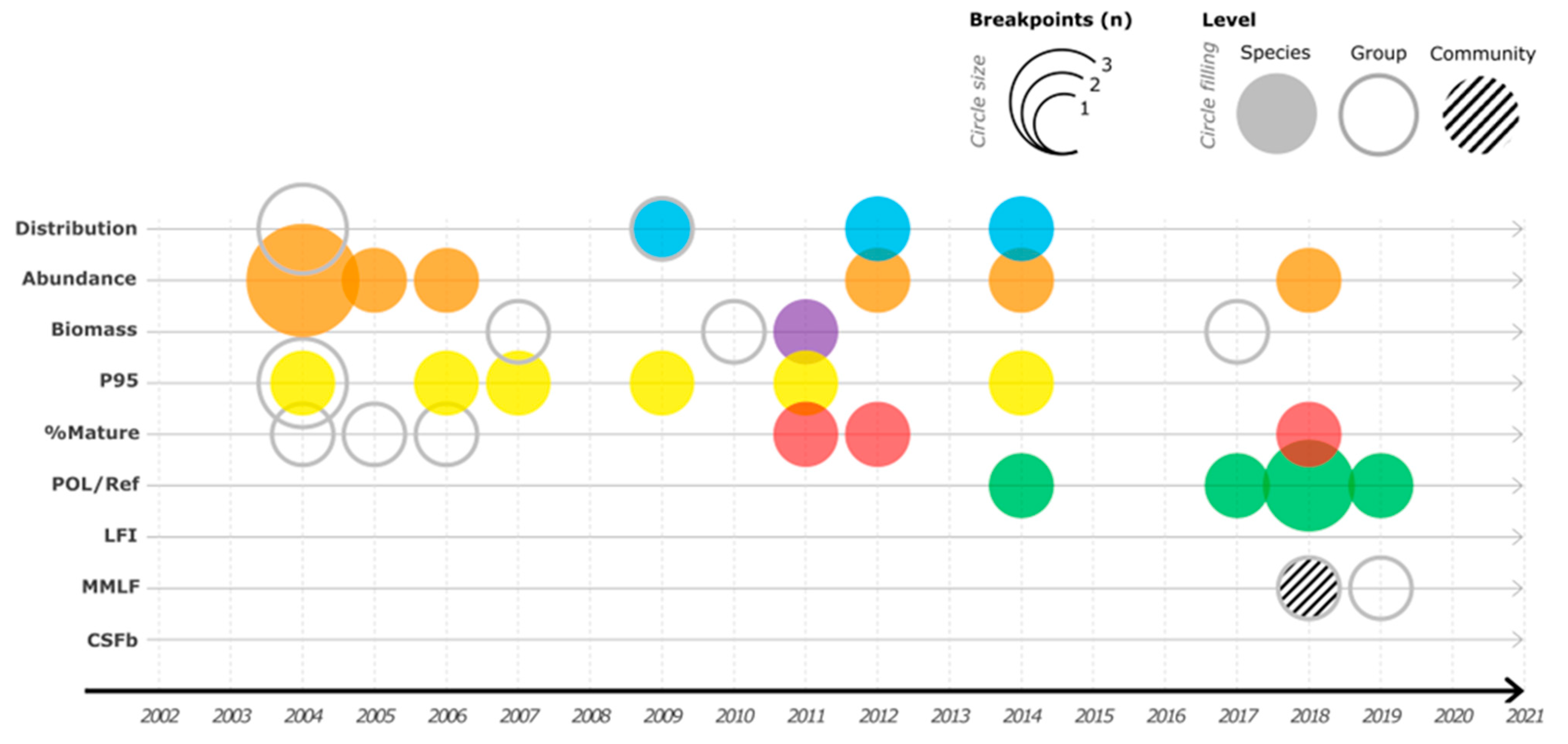
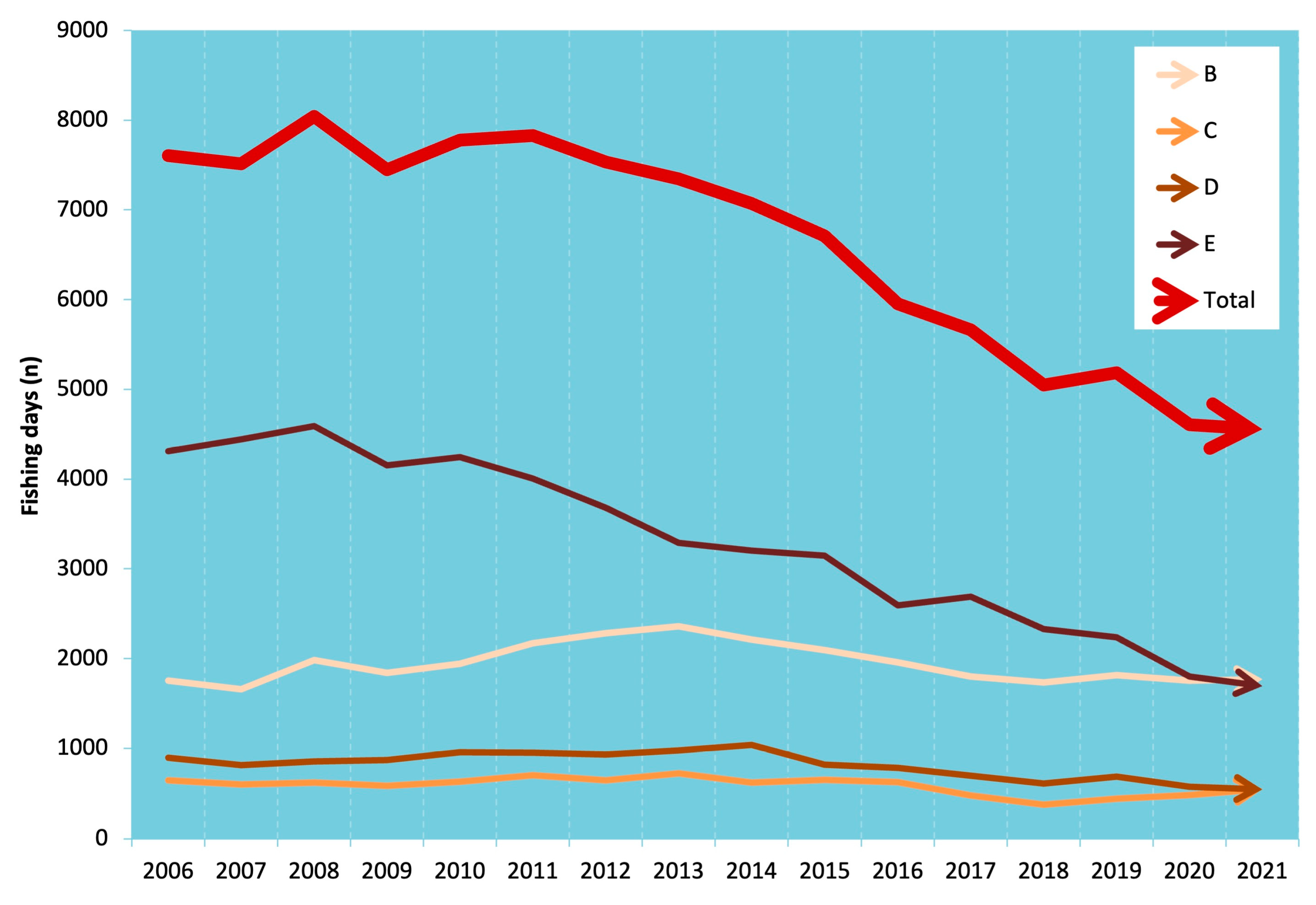
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stevens, J.D.; Bonfil, R.; Dulvy, N.K.; Walker, P.A. The Effects of Fishing on Sharks, Rays, and Chimaeras (Chondrichthyans), and the Implications for Marine Ecosystems. ICES J. Mar. Sci. 2000, 57, 476–494. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Allen, D.J.; Ralph, G.M.; Walls, R.H.L. The Conservation Status of Sharks, Rays and Chimaeras in the Mediterranean Sea; IUCN Centre for Mediterranean Cooperation: Málaga, Spain, 2016. [Google Scholar]
- Engelhard, G.H. One Hundred and Twenty Years of Change in Fishing Power of English North Sea Trawlers. In Advances in Fisheries Science: 50 Years on From Beverton and Holt; Payne, A.I.L., Cotter, J., Potter, T., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2008; pp. 1–25. [Google Scholar]
- Swartz, W.; Sala, E.; Tracey, S.; Watson, R.; Pauly, D. The Spatial Expansion and Ecological Footprint of Fisheries (1950 to Present). PLoS ONE 2010, 5, 15143. [Google Scholar] [CrossRef] [PubMed]
- Ferretti, F.; Worm, B.; Britten, G.L.; Heithaus, M.R.; Lotze, H.K. Patterns and Ecosystem Consequences of Shark Declines in the Ocean. Ecol. Lett. 2010, 13, 1055–1071. [Google Scholar] [CrossRef] [PubMed]
- Dulvy, N.K.; Fowler, S.L.; Musick, J.A.; Cavanagh, R.D.; Kyne, P.M.; Harrison, L.R.; Carlson, J.K.; Davidson, L.N.; Fordham, S.V.; Francis, M.P.; et al. Extinction Risk and Conservation of the World’s Sharks and Rays. Elife 2014, 3, e00590. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.I.; Woodley, C.M.; Brudek, R.L. A Preliminary Evaluation of the Status of Shark Species; Food and Agriculture Organization of the United Nations: Rome, Italy, 1999. [Google Scholar]
- GFCM Recommendation GFCM/44/2021/16 on Additional Mitigation Measures for the Conservation of Elasmobranchs in the Mediterranean Sea. 2021. Available online: https://www.fao.org/gfcm/decisions/en/ (accessed on 9 February 2023).
- Browman, H.I.; Sterigou, K.I. Perspectives on Ecosystem-Based Approaches to the Management of Marine Resources. Mar. Ecol. Prog. Ser. 2004, 274, 269–303. [Google Scholar] [CrossRef]
- Lleonart, J.; Maynou, F. Fish Stock Assessments in the Mediterranean: State of the Art. Sci. Mar. 2003, 67, 37–49. [Google Scholar] [CrossRef]
- Carbonell, A.; Alemany, F.; Merella, P.; Quetglas, A.; Román, E. The By-Catch of Sharks in the Western Mediterranean (Balearic Islands) Trawl Fishery. Fish. Res. 2003, 61, 7–18. [Google Scholar] [CrossRef]
- Guijarro, B.; Quetglas, A.; Moranta, J.; Ordines, F.; Valls, M.; González, N.; Massutí, E. Inter- and Intra-Annual Trends and Status Indicators of Nektobenthic Elasmobranchs off the Balearic Islands (Northwestern Mediterranean). Sci. Mar. 2012, 76, 87–96. [Google Scholar] [CrossRef]
- Ellis, J.R.; Dulvy, N.K.; Jennings, S.; Parker-Humphreys, M.; Rogers, S.I. Assessing the Status of Demersal Elasmobranchs in UK Waters: A Review. J. Mar. Biol. Assoc. United Kingd. 2005, 85, 1025–1047. [Google Scholar] [CrossRef]
- Juan-Jordá, M.J.; Murua, H.; Arrizabalaga, H.; Merino, G.; Pacoureau, N.; Dulvy, N.K. Seventy Years of Tunas, Billfishes, and Sharks as Sentinels of Global Ocean Health. Science 2022, 378, eabj0211. [Google Scholar] [CrossRef]
- Walls, R.H.L.; Dulvy, N.K. Tracking the Rising Extinction Risk of Sharks and Rays in the Northeast Atlantic Ocean and Mediterranean Sea. Sci. Rep. 2021, 11, 15397. [Google Scholar] [CrossRef] [PubMed]
- Massutí, E.; Moranta, J. Demersal Assemblages and Depth Distribution of Elasmobranchs from the Continental Shelf and Slope off the Balearic Islands (Western Mediterranean). ICES J. Mar. Sci. 2003, 60, 753–766. [Google Scholar] [CrossRef]
- Ordines, F.; Massutí, E.; Moranta, J.; Quetglas, A.; Guijarro, B.; Fliti, K. Balearic Islands vs Algeria: Two Nearby Western Mediterranean Elasmobranch Assemblages with Different Oceanographic Scenarios and Fishing Histories. Sci. Mar. 2011, 75, 707–717. [Google Scholar] [CrossRef]
- Ramírez-Amaro, S.; Ordines, F.; Esteban, A.; García, C.; Guijarro, B.; Salmerón, F.; Terrasa, B.; Massutí, E. The Diversity of Recent Trends for Chondrichthyans in the Mediterranean Reflects Fishing Exploitation and a Potential Evolutionary Pressure towards Early Maturation. Sci. Rep. 2020, 10, 547. [Google Scholar] [CrossRef]
- Acosta, J.; Canals, M.; López-Martínez, J.; Muñoz, A.; Herranz, P.; Urgeles, R.; Palomo, C.; Casamor, J.L. The Balearic Promontory Geomorphology (Western Mediterranean): Morphostructure and Active Processes. Geomorphology 2003, 49, 177–204. [Google Scholar] [CrossRef]
- Canals, M.; Ballesteros, E. Production of Carbonate Particles by Phytobenthic Communities on TheMallorca–Menorca Shelf, Northwestern Mediterranean Sea. Deep-Sea Res. II 1997, 44, 611–629. [Google Scholar] [CrossRef]
- Group, E. Progress from 1989 to 1992 in Understanding the Circulation of the Western Mediterranean. Oceanol. Acta 1995, 18, 255–271. [Google Scholar]
- Arnone, R.A.; Wiesenburg, D.A.; Saunders, K.D. The Origin and Characteristics of the Algerian Current. J. Geophys. Res. 1990, 95, 1587–1598. [Google Scholar] [CrossRef]
- Pinot, J.M.; López-Jurado, J.L.; Riera, M. The CANALES Experiment (1996–1998). Interannual, Seasonal, and Mesoscale Variability of the Circulation in the Balearic Channels. Prog. Oceanogr. 2022, 55, 335–370. [Google Scholar] [CrossRef]
- Bosc, E.; Bricaud, A.; Antoine, D. Seasonal and Interannual Variability in Algal Biomass and Primary Production in the Mediterranean Sea, as Derived from 4 Years of SeaWiFS Observations. Glob. Biogeochem. Cycles 2004, 18, GB1005. [Google Scholar] [CrossRef]
- Estrada, M. Primary Production in the Northwestern Mediterranean. Sci. Mar. 1996, 60, 55–64. [Google Scholar]
- Ballesteros, E. Els Fons Rocosos Profunds Amb Osmundaria Volubilis (Linné) R.E. Norris a Les Balears. Bol. Soc. Hist. Nat. Balear. 1992, 35, 33–49. [Google Scholar]
- Ballesteros, E. The Deep-Water Peyssonnelia Beds from the Balearic Islands (Western Mediterranean). Mar. Ecol. 1994, 15, 233–253. [Google Scholar] [CrossRef]
- Joher, S.; Ballesteros, E.; Cebrian, E.; Sánchez, N.; Rodríguez-Prieto, C. Deep-Water Macroalgal-Dominated Coastal Detritic Assemblages on the Continental Shelf off Mallorca and Menorca (Balearic Islands, Western Mediterranean). Bot. Mar. 2012, 55, 485–497. [Google Scholar] [CrossRef]
- Joher, S.; Ballesteros, E.; Rodríguez-Prieto, C. Contribution to the Study of Deep Coastal Detritic Bottoms: The Algal Communities of the Continental Shelf off the Balearic Islands, Western Mediterranean. Medit. Mar. Sci. 2015, 16, 573–590. [Google Scholar] [CrossRef]
- Fernández de Puelles, M.L.; Valencia, J.; Jansá, J.; Morillas, A. Hydrographical Characteristics and Zooplankton Distribution in the Mallorca Cannel (Western Mediterranean): Spring 2001. ICES J. Mar. Sci. 2004, 61, 654–666. [Google Scholar] [CrossRef]
- Quetglas, A.; Guijarro, B.; Ordines, F.; Massutí, E. Stock Boundaries for Fisheries Assessment and Management in the Mediterranean: The Balearic Islands as a Case Study. Sci. Mar. 2012, 76, 17–28. [Google Scholar] [CrossRef]
- Spedicato, M.T.; Massutí, E.; Mérigot, B.; Tserpes, G.; Jadaud, A.; Relini, G. The MEDITS Trawl Survey Specifications in an Ecosystem Approach to Fishery Management. Sci. Mar. 2019, 83, 9–20. [Google Scholar] [CrossRef]
- Bertrand, J.A.; Gil de Sola, L.; Papaconstantinou, C.; Relini, G.; Souplet, A. The General Specifications of the MEDITS Surveys. Sci. Mar. 2002, 66, 9–17. [Google Scholar] [CrossRef]
- Farriols, M.T.; Ordines, F.; Somerfield, P.J.; Pasqual, C.; Hidalgo, M.; Guijarro, B.; Massutí, E. Bottom Trawl Impacts on Mediterranean Demersal Fish Diversity: Not so Obvious or Are We Too Late? Cont. Shelf Res. 2017, 137, 84–102. [Google Scholar] [CrossRef]
- Clarke, K.; Gorley, R.; Somerfield, P.; Warwick, R. Change in Marine Communities: An Approach to Statistical Analysis and Interpretation, 3rd ed.; PRIMER-E: Plymouth, UK, 2014. [Google Scholar]
- Available online: Https://Www.Miteco.Gob.Es/Es/Biodiversidad/Servicios/Banco-Datos-Naturaleza/Informacion-Disponible/Bdn-Cart-Aux-Malla-Marina.Aspx (accessed on 25 August 2022).
- Cochrane, S.K.J.; Connor, D.W.; Nilsson, P.; Mitchell, I.; Reker, J.; Franco, J.; Valavanis, V.; Moncheva, S.; Ekebom, J.; Nygaard, K.; et al. Marine strategy framework directive Task Group 1 Report Biological Diversity. EUR 2010, 24337, 110. [Google Scholar] [CrossRef]
- Piet, G.J.; Albella, A.J.; Aro, E.; Farrugio, H.; Lleonart, J.; Lordan, C.; Mesnil, B.; Petrakis, G.; Pusch, C.; Radu, G.; et al. Marine Strategy Framework Directive Task Group 3 Report Commercially Exploited Fish and Shellfish. 2010. Available online: https://publications.jrc.ec.europa.eu/repository/handle/JRC57750 (accessed on 25 August 2022).
- Shin, Y.J.; Rochet, M.J.; Jennings, S.; Field, J.G.; Gislason, H. Using Size-Based Indicators to Evaluate the Ecosystem Effects of Fishing. ICES J. Mar. Sci. 2005, 62, 384–396. [Google Scholar] [CrossRef]
- Muggeo, V.M.R. Segmented: An R Package to Fit Regression Models with Broken-Line Relationships. R News 2008, 8, 20–25. [Google Scholar]
- Muggeo, V.M.R. Estimating Regression Models with Unknown Break-Points. Stat. Med. 2003, 22, 3055–3071. [Google Scholar] [CrossRef]
- Vito, R.; Muggeo, M.M. Package “segmented” Type Package Title Regression Models with Break-Points/Change-Points (with Possibly Random Effects) Estimation. 2022. Available online: http://dirk.eddelbuettel.com/cranberries/2023/03/26/#segmented_1.6-3 (accessed on 1 February 2023).
- Muggeo, V.M.R. Selecting Number of Breakpoints in Segmented Regression: Implementation in the R Package Segmented. 2020, pp. 1–3. Available online: https://www.researchgate.net/publication/343737604 (accessed on 20 September 2022).
- Morey, G.; Moranta, J.; Massutí, E.; Grau, A.; Linde, M.; Riera, F.; Morales-Nin, B. Weight-Length Relationships of Littoral to Lower Slope Fishes from the Western Mediterranean. Fish. Res. 2003, 62, 89–96. [Google Scholar] [CrossRef]
- Pallaoro, A.; Jardas, I.; SANTIC, M. Weight-Length Relationships for 11 Chondrichthyan Species in the Eastern Adriatic Sea. Cybium 2005, 29, 93–96. [Google Scholar]
- Bonfil, R. Overview of World Elasmobranch Fisheries; Food and Agriculture Organization of the United Nations: Rome, Italy, 1994. [Google Scholar]
- Bertrand, J.; Gil-de-Sola-Simarro, L.; Papakonstantinou, C.; Relini, G.; Souplet, A. Contribution on the Distribution of Elasmobranchs in the Mediterranean (from MEDITS Surveys). Biol. Mar. Medit. 2000, 7, 1–15. [Google Scholar]
- IEO. Estrategia Marina. Demarcación Marina Levantino-Balear. Parte IV. Descriptores Buen Estado Ambiental. Descriptor 1: Biodiversidad. Evaluación Inicial y Buen Estado Ambiental. 2012. Available online: https://www.miteco.gob.es/es/costas/temas/proteccion-medio-marino/IV_D6_Levantino-Balear_tcm30-130922.pdf (accessed on 10 July 2022).
- Cortés, E. Standardized Diet Compositions and Trophic Levels of Sharks. ICES J. Mar. Sci. 1999, 56, 707–717. [Google Scholar] [CrossRef]
- Guijarro, B.; Massutí Guijarro, E.; Guijarro, B.; Massutí, E. Selectivity of Diamond- and Square-Mesh Codends in the Deepwater Crustacean Trawl Fishery off the Balearic Islands (Western Mediterranean). ICES J. Mar. Sci. 2006, 63, 52–67. [Google Scholar] [CrossRef]
- Ordines, F.; Massutí, E.; Guijarro, B.; Mas, R. Diamond vs. Square Mesh Codend in a Multi-Species Trawl Fishery of the Western Mediterranean: Effects on Catch Composition, Yield, Size Selectivity and Discards. Aquat. Living Resour. 2006, 19, 329–338. [Google Scholar] [CrossRef]
- Quetglas, A.; Merino, G.; González, J.; Ordines, F.; Garau, A.; Grau, A.M.; Guijarro, B.; Oliver, P.; Massutí, E. Harvest Strategies for an Ecosystem Approach to Fisheries Management in Western Mediterranean Demersal Fisheries. Front. Mar. Sci. 2017, 4, 106. [Google Scholar] [CrossRef]
- Company, J.B.; Puig, P.; Sardà, F.; Palanques, A.; Latasa, M.; Scharek, R. Climate Influence on Deep Sea Populations. PLoS ONE 2008, 3, e1431. [Google Scholar] [CrossRef] [PubMed]
- Amores, A.; Rueda, L.; Monserrat, S.; Guijarro, B.; Pasqual, C.; Massutí, E. Influence of the Hydrodynamic Conditions on the Accessibility of Aristeus Antennatus and Other Demersal Species to the Deep Water Trawl Fishery off the Balearic Islands (Western Mediterranean). J. Mar. Syst. 2014, 138, 203–210. [Google Scholar] [CrossRef]
- Mas, I.M. The Fishing Footprint of a Tourism-Based Economy: Displacing Seafood Consumption from Local to Distant Waters in the Balearic Islands. J. Polit. Ecol. 2015, 22, 211–238. [Google Scholar] [CrossRef]
- del Valle, I.; Guillen, J.; Astorkiza, K. Substituting Hake with Sardines? Economic Crisis and Fish Demand in Spain. Agribusiness 2017, 33, 600–610. [Google Scholar] [CrossRef]
- Moranta, J.; Quetglas, A.; Massutí, E.; Guijarro, B.; Hidalgo, M.; Diaz, P. Spatio-Temporal Variations in Deep-Sea Demersal Communities off the Balearic Islands (Western Mediterranean). J. Mar. Syst. 2008, 71, 346–366. [Google Scholar] [CrossRef]
- Hidalgo, M.; Tomás, J.; Moranta, J.; Morales-Nin, B. Intra-Annual Recruitment Events of a Shelf Species around an Island System in the NW Mediterranean. Estuar. Coast. Shelf Sci. 2009, 83, 227–238. [Google Scholar] [CrossRef]
- Quetglas, A.; Rueda, L.; Alvarez-Berastegui, D.; Guijarro, B.; Massutí, E. Contrasting Responses to Harvesting and Environmental Drivers of Fast and Slow Life History Species. PLoS ONE 2016, 11, e0148770. [Google Scholar] [CrossRef]
- Planque, B.; Fromentin, J.M.; Cury, P.; Drinkwater, K.F.; Jennings, S.; Perry, R.I.; Kifani, S. How Does Fishing Alter Marine Populations and Ecosystems Sensitivity to Climate? J. Mar. Syst. 2010, 79, 403–417. [Google Scholar] [CrossRef]
- Osgood, G.J.; White, E.R.; Baum, J.K. Effects of Climate-Change-Driven Gradual and Acute Temperature Changes on Shark and Ray Species. J. Anim. Ecol. 2021, 90, 2547–2559. [Google Scholar] [CrossRef]
- Dolgov, A.V.; Drevetnyak, K.V.; Gusev, E.V. The Status of Skate Stocks in the Barents Sea. J. Northw. Atl. Fish. Sci. 2005, 35, 249–260. [Google Scholar] [CrossRef]
- Vedor, M.; Queiroz, N.; Mucientes, G.; Couto, A.; da Costa, I.; dos Santos, A.; Vandeperre, F.; Fontes, J.; Afonso, P.; Rosa, R.; et al. Climate-Driven Deoxygenation Elevates Fishing Vulnerability for the Ocean’s Widest Ranging Shark. Elife 2021, 10, e62508. [Google Scholar] [CrossRef] [PubMed]
- Brander, K. Disappearance of Common Skate Raia Batis from Irish Sea. Nature 1981, 290, 48–49. [Google Scholar] [CrossRef]
- Farrugio, H.; Oliver, P.; Biagi, F. An Overview of the History, Knowledge, Recent and Future Research Trends in Mediterranean Fisheries. Sci. Mar. 1993, 57, 105–119. [Google Scholar]
- Olaso, I.; Velasco, F.; Sánchez, F.; Serrano, A.; Rodríguez-Cabello, C.; Cendrero, O. Trophic Relations of Lesser-Spotted Catshark (Scyliorhinus canicula) and Blackmouth Catshark (Galeus melastomus) in the Cantabrian Sea. J. Northw. Atl. Fish. Sci 2005, 35, 481–494. [Google Scholar] [CrossRef]
- Olaso, I.; Sánchez, F.; Rodríguez-Cabello, C.; Velasco, F. The Feeding Behaviour of Some Demersal Fish Species in Response to Artificial Discarding. Sci. Mar. 2002, 66, 301–311. [Google Scholar] [CrossRef]
- Olaso, I.; Velasco, F.; Pérez, N. Importance of Discarded Blue Whiting (Micromesistius poutassou) in the Diet of Lesser Spotted Dogfish (Scyliorhinus canicula) in the Cantabrian Sea. ICES J. Mar. Sci. 1998, 55, 331–341. [Google Scholar] [CrossRef]
- Valls-Mir, M.; Quetglas, A.; Ordines, F.; Moranta, J. Feeding Ecology of Demersal Elasmobranchs from the Shelf and Slope off the Balearic Sea (Western Mediterranean). Sci. Mar. 2011, 75, 633–639. [Google Scholar] [CrossRef]
- Capape, C.; Zaouali, J. Contribution À La Biologie Des Scyliorhinidae Des Côtes Tunisiennes. V. Galeus Melastomus Rafinesque, 1810. Régime Alimentaire. Arch. Inst. Pasteur. 1976, 53, 281–292. [Google Scholar]
- Macpherson, E. Régime Alimentaire de Galeus Melastomus Rafinesque, 1810 Etmopterus spinax (L., 1758) et Scymnorhinus licha (Bonnaterre, 1788) En Méditerranée Occidentale. Vie Milieu/Life Environ. 1980, 30, 139–148. Available online: https://hal.sorbonne-universite.fr/hal-03008169v1/file/VOLUME_1980_30_fasc2_06_p139-148.pdf (accessed on 9 February 2023).
- Grau, A.M.; Mayol, J.; Oliver, J.; Riera, M.A. Llibre Vermell Dels Peixos de Les Illes Balears; Conselleria de Medi Ambient, Agricultura i Pesca: Palma, Spain, 2015. Available online: http://www.caib.es/govern/organigrama/area.do?lang=ca&coduo=3329 (accessed on 9 February 2023).
- Pauly, D. Anecdotes and the Shifting Baseline Syndrome of Fisheries. Trends Ecol. Evol. 1995, 10, 430. [Google Scholar] [CrossRef] [PubMed]
- Aldebert, Y. Demersal resources of the gulf of lions (nw mediterranean). Impact of exploitation on fish diversity. Vie Milieu/Life Environ. 1997, 47, 275–284. [Google Scholar]
- Coelho, R.; Erzini, K. Length at First Maturity of Two Species of Lantern Sharks (Etmopterus spinax and Etmopterus pusillus) off Southern Portugal. J. Mar. Biol. Assoc. United Kingd. 2005, 85, 1163–1165. [Google Scholar] [CrossRef]
- Coelho, R.; Erzini, K. Effects of Fishing Methods on Deep Water Shark Species Caught as By-Catch off Southern Portugal. Hydrobiologia 2008, 606, 187–193. [Google Scholar] [CrossRef]
- Villagra, D.; van Bogaert, N.; Ampe, B.; Walker, P.; Uhlmann, S.S. Life-History Traits of Batoids (Superorder batoidea) in the Northeast Atlantic and the Mediterranean. Rev. Fish Biol. Fish. 2022, 32, 473–495. [Google Scholar] [CrossRef]



| Family | Species | Group | Y | N | D | Lmax | IUCN |
|---|---|---|---|---|---|---|---|
| Pentanchidae | Galeus melastomus | Sharks 1 (S1) | 100 | 35425 | 54–795 | 69 | LC |
| Scyliorhinidae | Scyliorhinus canicula | 100 | 43310 | 43–762 | 77 | LC | |
| Dalatiidae | Dalatias licha | Sharks 2 (S2) | 40 | 8 | 492–717 | 100 | VU |
| Triakidae | Mustelus mustelus | 95 | 88 | 43–140 | 160 | VU | |
| Squalidae | Squalus blainville | 70 | 718 | 77–762 | 81.5 | DD | |
| Centrophoridae | Centrophorus uyato | Sharks 3 (S3) | 35 | 9 | 636–764 | 119 | CR |
| Etmopteridae | Etmopterus spinax | 100 | 544 | 319–795 | 49 | LC | |
| Rajidae | Dipturus oxyrinchus | Batoidea 1 (B1) | 100 | 451 | 136–717 | 128 | NT |
| Leucoraja circularis | 55 | 22 | 132–605 | 87.5 | CR | ||
| Leucoraja naevus | 100 | 574 | 43–759 | 61 | NT | ||
| Raja brachyura | 50 | 160 | 43–189 | 119 | NT | ||
| Raja clavata | 100 | 3554 | 43–751 | 128 | NT | ||
| Raja miraletus | 100 | 802 | 43–189 | 69.5 | NT | ||
| Raja polystigma | 100 | 940 | 43–758 | 65 | LC | ||
| Raja radula | 100 | 454 | 43–185 | 66.5 | EN | ||
| Rostroraja alba | 55 | 21 | 45–251 | 142 | EN | ||
| Dasyatidae | Dasyatis spp. | Batoidea 2 (B2) | 100 | 155 | 43–99 | 127 | VU |
| Mylobatidae | Myliobatis aquila | 85 | 213 | 43–138 | 142 | VU | |
| Torpedinidae | Torpedo marmorata | Batoidea 3 (B3) | 90 | 54 | 43–251 | 39 | LC |
| Group | LFTS (cm) | % |
|---|---|---|
| Whole community | 60 | 2.01 |
| Reduced community | 95 | 1.84 |
| S1 | 50 | 1.45 |
| S2 | 80 | 2.37 |
| S3 | 50 | 1.28 |
| B1 | 85 | 1.86 |
| B2 | 115 | 1.94 |
| B3 | 35 | 1.89 |
| Trend | Slope | p-Value * | 95% CI Range | Symbol |
|---|---|---|---|---|
| Increasing | Positive (+) | ≤0.05 | Over or below zero | ↗ |
| Decreasing | Negative (−) | ≤0.05 | Over or below zero | ↘ |
| Uncertain | Positive or negative (+/−) | >0.05 | Includes zero | → |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrat, A.; Farriols, M.T.; Ramírez-Amaro, S.; Ordines, F.; Guijarro, B.; Ferragut-Perello, F.; Massutí, E. Conservation Status Assessment of Demersal Elasmobranchs in the Balearic Islands (Western Mediterranean) over the Last Two Decades. Fishes 2023, 8, 230. https://doi.org/10.3390/fishes8050230
Serrat A, Farriols MT, Ramírez-Amaro S, Ordines F, Guijarro B, Ferragut-Perello F, Massutí E. Conservation Status Assessment of Demersal Elasmobranchs in the Balearic Islands (Western Mediterranean) over the Last Two Decades. Fishes. 2023; 8(5):230. https://doi.org/10.3390/fishes8050230
Chicago/Turabian StyleSerrat, Alba, Maria Teresa Farriols, Sergio Ramírez-Amaro, Francesc Ordines, Beatriz Guijarro, Francesca Ferragut-Perello, and Enric Massutí. 2023. "Conservation Status Assessment of Demersal Elasmobranchs in the Balearic Islands (Western Mediterranean) over the Last Two Decades" Fishes 8, no. 5: 230. https://doi.org/10.3390/fishes8050230
APA StyleSerrat, A., Farriols, M. T., Ramírez-Amaro, S., Ordines, F., Guijarro, B., Ferragut-Perello, F., & Massutí, E. (2023). Conservation Status Assessment of Demersal Elasmobranchs in the Balearic Islands (Western Mediterranean) over the Last Two Decades. Fishes, 8(5), 230. https://doi.org/10.3390/fishes8050230