Abstract
Nitrite is a common pollutant encountered in aquaculture systems. During intensive hatchery, accumulation of nitrite can cause massive mortality of juvenile crustaceans. However, the nitrite toxicity and cellular stress responses in juvenile crustaceans is not clearly understood. Here, we investigate the survival, energy metabolism, and cellular stress responses in juvenile P. trituberculatus, an important aquaculture species in China, under acute nitrite stress. The results revealed nitrite resulted in a significant decrease in survival rate of juvenile swimming crab. After nitrite exposure, the activity of catabolic enzymes, such as HK, PK, CS, and CPT-1, were initially enhanced, and then they showed significant decrease at the late stage of exposure, accompanied by reduction in ATP and adenylate energy charge (AEC). The impaired energy homeostasis was possibly associated with disturbed AMPK signaling and enhanced anaerobic metabolism, which was indicated by the high levels of LDH activity and HIF-1α expression. Furthermore, we found that nitrite stress can depress antioxidant systems and unfold protein responses, causing oxidative damage and endoplasmic reticulum (ER) stress, and this, in turn, can trigger autophagy and apoptosis through both caspase-dependent and caspase-independent pathways. The results of the present study improve our understanding regarding adverse effects of nitrite on P. trituberculatus and provide valuable information for hatchery management.
Key Contribution:
In this study, we investigated the survival and cellular stress responses of early juvenile Portunus trituberculatus (C2) under acute nitrite stress. We found that acute nitrite can impair energy homeostasis by disturbing AMPK signaling, cause oxidative damage and endoplasmic reticulum stress, induce apoptotic and autophagic cell death, and result in significant decrease in survival.
1. Introduction
The swimming crab Portunus trituberculatus is widely distributed in the coastal waters of Korea, Japan, China, and Southeast Asian countries, and it is an important aquaculture species in China. In 2020, its production reached 100,895 tons [1]. During recent years, because of the continuous rise of market demand and rapid expansion of aquaculture industry, the production of high-quality seeds of the swimming crab cannot meet the increasing needs, which have become important factors restricting the sustainable development of the industry [2]. At larval and juvenile stages, the swimming crabs are very sensitive to changes in the environmental conditions. In high-density intensive hatchery system, decomposition of residual feeds and excretion of the animals, particularly at the late stage of hatchery, may result in rapid accumulation of nitrogenous inorganic substances, such as ammonia and nitrite, causing massive mortality of juvenile crabs and significant economic loss. Many studies have been carried out on the toxic effects of ammonia on P. trituberculatus [3,4,5,6]. However, there is limited information available regarding nitrite toxicity in this species, particularly for early juvenile individuals. Therefore, it is of great importance to study the effects of nitrite on the early juvenile P. trituberculatus.
Environmental stressors, including nitrite, can disturb cellular processes and result in deformation of/damage to proteins, DNA, or other essential macromolecules, and this can trigger cellular stress responses (CSR), as well as defense reactions to counteract stress-induced damage and restore cellular homeostasis, which is critical for animals to survive stressful conditions [7]. Previous studies in juvenile and adult crustaceans have shown that nitrite exposure can result in over-production of reactive oxygen species (ROS), inducing oxidative stress [8,9,10]. To prevent oxidative damage, organisms can activate antioxidant defense systems to alleviate oxidative stress [11,12]. However, high levels of nitrite may overwhelm antioxidant defense capacity, causing damage to cellular macromolecules. For example, a recent study in the mud crab Scylla paramamosain found that nitrite exposure over 10 mg/L suppresses activities of antioxidant enzymes, superoxide dismutase (SOD) and catalase (CAT), and significantly increased the levels of malondialdehyde (MDA), an indicator of lipid peroxidation [13]. Oxidative stress is often accompanied by the accumulation of unfolded or misfolded proteins in the endoplasmic reticulum (ER), defined as ER stress [14,15]. ER stress can activate unfolded protein response (UPR), a highly conserved pathway that allows the cell to handle ER stress and maintain the protein-folding capacity of ER [16]. A transcriptome study in the Pacific white shrimp, Litopenaeus vannamei, reported significant difference in expression of the genes involved in UPR between nitrite-tolerant and -sensitive families, suggesting the importance of UPR in resisting nitrite stress [17]. In addition, modulation of energy metabolism is also a critical component of CSR, as energy demand associated with cellular protection could be increased substantially under environmental stresses [10,18]. Li et al., 2019, reported that nitrite exposure could lead to hepatopancreas glycogen mobilization, as well as increased levels of hemolymph glucose and activity of glycolytic enzymes in L. vannamei [19]. Thus, multiple CSR pathways could be triggered to protect against nitrite-induced damage in crustaceans.
When stress stimuli exceed cellular tolerance limits, programmed cell death will be elicited to eliminate damaged cells [20]. It has been reported that acute nitrite stress can induce apoptosis, a major cell death mechanism, in crustaceans [21]. Apoptosis is mainly mediated by two signaling routes, namely, the extrinsic death receptor pathway and the intrinsic mitochondrial pathway [22]. Previous studies showed that acute nitrite stress triggers apoptosis in decapods primarily through the intrinsic mitochondrial pathway [23]. Recently, a study in L. vannamei found that sub-lethal nitrite exposure can trigger autophagy, a conserved process that delivers components of the cytoplasm to lysosomes for degradation in the hepatopancreas [24]. Autophagy is considered both a pro-survival mechanism and a type of cell death [25,26,27]. The role of autophagy in nitrite stress response in crustaceans is still unclear.
Although a number of studies on nitrite toxicity have been reported in variety of crustacean species, these studies were mainly focused on the survival of the early juvenile or the stress responses in the late-juvenile and adult individuals [12,28,29,30], and there has been limited knowledge regarding the stress responses in early juveniles during hatchery stages. Considering the early juvenile crustaceans are much more sensitive to nitrite than the late-juveniles and adults, the stress responses may be of significant differences in the individuals at different developmental stages. Hence, it is important to study the nitrite stress responses in early juveniles. Here, we accessed the survival rate, as well as the temporal variation of adenylate energy charge (AEC), which are related to the activity of the enzymes implicated in energy metabolism and antioxidant defense, as well as expression of the genes involved in UPR, apoptosis, and autophagy, in early juvenile swimming crab P. trituberculatus under different nitrite nitrogen concentrations (<0.1 mg/L, 20 mg/L, and 40 mg/L). The results of this study will improve our understanding of nitrite toxicity in early juvenile crustaceans and provide valuable information for hatchery management of the swimming crab.
2. Materials and Methods
2.1. Experimental Animal
The juvenile P. trituberculatus at stage Ⅱ (C2) were obtained from Haifeng company (Weifang, China). Prior to the experiment, a total of 3000 swimming crabs were acclimated in 40-L tanks under laboratory conditions for three days, where aeration was provided continuously, temperature was maintained at 26 °C ± 0.5 °C, salinity was 30.2 ± 0.5 psu, nitrite nitrogen concentration was below 0.1 mg/L, pH was 7.7 ± 0.1, and photoperiod was set as 12 h of light: 12 h of dark. One third of the rearing water was exchanged every day using fresh equi-temperature seawater. The juvenile crabs were fed with adult Artemia ad libitum every 8 h, and the leftover feed was removed before feeding.
2.2. Survival Experiment
Based on the results of our preliminary experiments, three nitrite nitrogen concentrations were chosen for this study, including the control group (natural seawater, below 0.1 mg/L), the LN group (the low-nitrite group, 20 mg/L), and the HN group (the high-nitrite group, 40 mg/L). The nitrite nitrogen concentration in the different experimental groups was prepared with 1 M sodium nitrite (NaNO2) stock solution (Sinopharm Chemical Reagent Co., Ltd., Qingdao, China; purity ≥ 99.0%), and the culture conditions were the same as those during acclimation period. A total of 90 juvenile crabs were equally and randomly allocated into the three groups, and there were three replicates for each group. The survival rate of different groups was recorded every 12 h after nitrite exposure.
2.3. Sample Collection
Following acclimation, 2600 juvenile swimming crabs were equally allocated into nine tanks, in which the filtered seawater has been adjusted to the selected concentrations for the control, LN, and HN groups, and there are three tanks for each group. At 12 h, 24 h, and 48 h after nitrite exposure, 150 crabs from randomly collected from each group, immediately frozen in liquid nitrogen, and stored at −80 °C for the subsequent biochemical assays and gene expression analysis. The animal experiment was approved by the Institutional Animal Care and Use Committee of Yellow Sea Fisheries Research Institute.
2.4. Gene Expression Analysis
Considering the juvenile crabs are very small, five individuals were pooled together as one replicate, and there were three replicates for each group at the same sample point. Total RNA of the samples was extracted using TransZol Up Plus RNA Kit (TransGen Biotech, Beijing, China), following the manufacturer’s protocols. RNA integrity was assessed using agarose gel electrophoresis (Bio-Rad, Hercules, CA, USA), and RNA quantity was determined using Nanodrop (Thermo Fisher Scientific, Waltham, MA, USA). Reverse transcription of the total RNA into complementary DNA (cDNA) was carried out using Evo M-MLV RT mix Kit with gDNA Clean for qPCR (Accurate Biology, Hunan, China).
Real-time PCR (RT-PCR) was used to detect the expression levels of the genes involved in energy metabolism: four subunits of AMP-activated protein kinase (AMPKα, AMPKβ1, AMPKβ2, AMPKγ); UPR: activated transcription factor-4 (ATF4), activated transcription factor-6 (ATF6), X-box binding protein 1 (XBP1); apoptosis: B-cell lymphoma-2 (Bcl-2), cysteinyl aspartate specific proteinase (Caspase-3), apoptosis inducing factor (AIF) and autophagy: Beclin1, autophagy related gene (Atg3), microtubule-associated proteins light chain 3 (LC3). The RT-PCR was performed using SYBR Green Pro Taq HS premixed qPCR kit (Accurate Biology, Hunan, China) in ABI 7500 fast qPCR system (Applied biosystems, Foster City, CA, USA). The reaction system was 10 μL, the system was 1 μL cDNA template, 5 μL SYBR reagent, 0.4 μL upstream primer, 0.4 μL downstream primer, 0.2 μL ROX, and 3 μL ddH2O. The reaction program was set as follows: 95 °C for 30 s, 5 s at 95 °C, and 30 s at 60 °C for 40 cycles. β-actin was used as the reference gene to normalize expression levels of the target genes. The relative expression levels were calculated using the 2−ΔΔCT method. All the specific primers used in this study are listed in Table 1.

Table 1.
The primers used in this study.
2.5. Biochemical Assays
The contents of adenosine 5′-triphosphate (ATP), adenosine 5′-diphosphate (ADP), and adenosine 5′-monophosphate (AMP) in the samples were detected by high performance liquid chromatography, as described in our study [31]. Ten juveniles were pooled as replicates, and there were three replicates for each group at the same sample point. The samples were grounded to powder in liquid nitrogen and homogenized in 9 volumes of 0.9 mol/L perchloric acid. The homogenate was centrifuged at 7000× g and 4 °C for 5 min, and then the supernatant was collected, and the pH was adjusted to 6.5–7.0 with 3.75 mol/L K2CO3 solution, followed by a centrifugation at 7000× g and 4 °C for 10 min. The supernatant was filtered with a 0.45 μm HV-Millipore filter. The aliquots of 20 µL were injected into an Agilent 1100 high-performance liquid chromatography (Agilent Corp., California, USA) system for analyzing ATP, ADP, and AMP contents with an Ultimate AQ-C18 column (4.6 × 250 mm) at 254 nm, using phosphate buffer (40 mmol L mmol/L KH2PO4 and 60 mmol/L K2HPO4, pH 6.50) as the mobile phases. The flow rate was 1.0 mL/min, and column temperature was at 35 °C. The elution time was 24 min. The concentration of the adenylate was calculated from the measured peak areas and standard curves made with the standards of known concentrations. TAN was calculated as:
TAN = [ATP] + [ADP] + [AMP],
Adenylate energy charge (AEC) was calculated as:
AEC = ([ATP] + 0.5 × [ADP])/([ATP] + [ADP] + [AMP])
Activity of the enzymes, including superoxide dismutase (SOD), catalase (CAT), hexokinase (HK), pyruvate kinase (PK), citrate synthase (CS), and succinate dehydrogenase (SDH) in the crabs were determined using detection kits purchased from Nanjing Jiancheng Institute of Bioengineering (Nanjing, China). The activity of superoxide dismutase (SOD), catalase (CAT), lactate dehydrogenase (LDH), and the content of malondialdehyde (MDA) were detected with commercial kits (Solarbio Science & Technology Co., Ltd., Beijing, China). Fatty acid synthase (FAS) activity was analyzed using the procedures described by Tian et al., 1985 [32], and carnitine palmitoyl transferase-1 (CPT-1) was analyzed using the method by Bieber and Fiol, 1986 [33].
2.6. Statistical Analysis
The data in this study were analyzed using IBM SPSS Statistics 26. The levels of survival rate, enzyme activity, MDA, adenylate, AEC, and gene expression among the different groups at each time point were analyzed with one-way ANOVA after checking for normality and homogeneity of variance of the data. If significant differences were found, Duncan’s post hoc test was used to identify the differences between the treatments. The statistically significant level was set as p < 0.05.
3. Results
3.1. Survival Rate
The survival rate of the LN and HN groups decreased gradually as the nitrite exposure extended and became significantly lower than that of the control group at 36 h (p = 0.013) and 24 h (p = 0.003), respectively (Figure 1). At the end of the exposure, the survival rates for the LN and HN groups were 56.67% and 40.00%, respectively, which were 30% and 46.67% lower than the control group. The LD50 of 48-h nitrite exposure in early juvenile swimming crab was 27.97 mg/L.
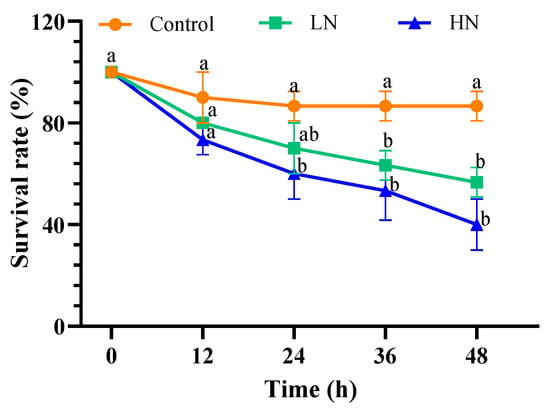
Figure 1.
Survival rate in different groups of juvenile P. trituberculatus under nitrite stress. At each time point, the letters indicate significant differences between experimental groups (p < 0.05).
3.2. Energy Metabolism
As shown in Table 2, the levels of ATP, ADP/ATP, AMP/ATP, and AEC significantly changed after nitrite exposure. The contents of ATP in the LN and HN groups significantly increased at 12 h, and they decreased at 24 h and 48 h compared to those in the control group. Significantly lower levels of AEC were observed in the LN group at 24 h and 48 h, as well as in the HN group at 24 h. The LN and HN groups showed higher ADP/ATP and AMP/ATP after 24 h.

Table 2.
Energy substance content in juvenile P. trituberculatus under nitrite stress.
The expression of the genes encoding four different subunits of AMPK in P. trituberculatus under nitrite stress was shown in Figure 2. The four genes in the nitrite-treated group showed significant difference in expression after exposure, and all of them were down-regulated at 12 h. AMPKα and AMPKγ in the LN and HN groups were significantly up-regulated at 24 h. At 48 h, AMPKα, AMPKβ1, and AMPKγ in the LN group were down-regulated, while AMPKβ1, AMPKβ2, and AMPKγ in the HN group were up-regulated.
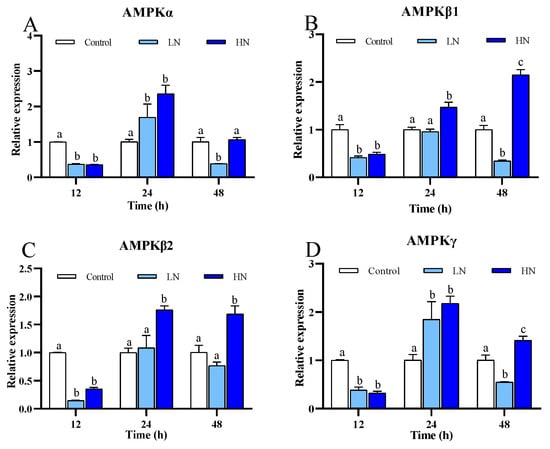
Figure 2.
mRNA expression of four genes of AMPK of juvenile P. trituberculatus under nitrite stress. (A) AMPKα, (B) AMPKβ1, (C) AMPKβ2, (D) AMPKγ. At each time point, the letters indicate significant differences between experimental groups (p < 0.05).
Nitrite stress caused significant changes in the activity of the enzymes involved in glycolysis, Krebs cycle, and lipid metabolism (Figure 3A). HK activity in both the LN and HN groups reached a peak value at 24 h, and the activity in the LN group returned to the control level at 48 h, while the activity in the HN group became lower than the control. The variation of PK activity in the LN and HN groups showed a similar trend (Figure 3B), and both had significantly higher activity at 12 h and 24 h, as well as lower activity at 48 h, compared to the control.
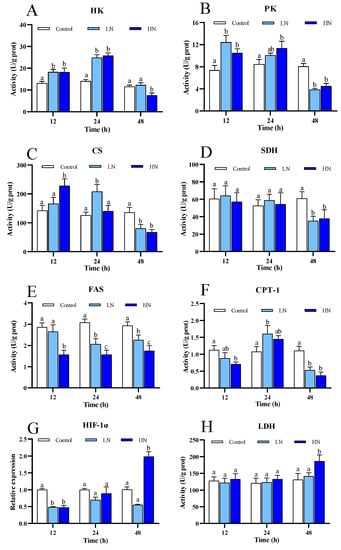
Figure 3.
The enzymes activities of five enzymes and mRNA expression of HIF-1α of juvenile P. trituberculatus under nitrite stress. (A) The enzyme activities of HK, (B) the enzyme activities of PK, (C) the enzyme activities of CS, (D) the enzyme activities of SDH, (E) the enzyme activities of FAS, (F) the enzyme activities of CPT-1, (G) mRNA expression of HIF-1α, and (H) the enzyme activities of LDH. At each time point, the letters indicate significant differences between experimental groups (p < 0.05).
CS activity in the LN and HN groups was significantly increased at 24 h and 12 h, respectively, and was significantly decreased at 48 h in both groups (Figure 3C). No significant change in SDH activity in the nitrite-exposed groups was observed at 12 h and 24 h, and the activity in the HN was significantly lower than that in the control group at 48 h (Figure 3D).
FAS activity in both nitrite-treated groups was significantly decreased at all the sample points, except the LN group at 12 h (Figure 3E). CPT-1 activity in the LN group significantly increased at 24 h and decreased at 48 h. For the HN group, CPT-1 activity showed slight increase at 24 h, and it showed significant decrease at 12 h and 48 h (Figure 3F).
The expression of HIF-1α was significantly influenced under nitrite stress (Figure 3G). Both of the nitrite-treated groups showed down-regulated expression at 12 h. The abundance of HIF-1α mRNA in the LN group remained at low levels at 24 h and 48 h; in contrast, the abundance in the HN group increased gradually as nitrite exposure extended, and it became significantly higher than the control group at 48 h. There was no significant difference in LDH activity between the groups, except a significant enhancement at 48 h in the HN group (Figure 3H).
3.3. Antioxidant Defense and Unfolded Protein Response
A significant increase in SOD activity in the LN and HN groups was observed at 12 h and 24 h, and the activity in the HN group decreased to a level significantly lower than the control at 48 h (Figure 4A). The activity of CAT in the nitrite-exposure groups both exhibited an obverse increase at 12 h and a sharp decrease at 24 h and remained at low levels at 48 h (Figure 4B). In general, MDA concentration in the LN and HN groups increased as the exposure duration extended, and its levels in the HN group were significantly higher than the control at 24 h and 48 h (Figure 4C).
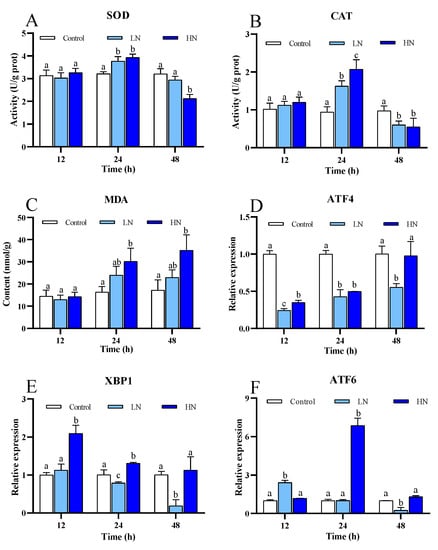
Figure 4.
The enzyme activities of two antioxidant enzymes, the content of MDA and mRNA expression of three genes about ER stress of juvenile P. trituberculatus under nitrite stress. (A) The enzymes activities of SOD, (B) the enzyme activities of CAT, (C) the content of MDA, (D) mRNA expression of ATF4, (E) mRNA expression of ATF6, and (F) mRNA expression of XBP1. At each time point, the letters indicate significant differences between experimental groups (p < 0.05).
The expression of ATF6 was significantly up-regulated in the LN group at 12 h and the HN group at 24 h and showed significant down-regulation in the LN group at 24 h (Figure 4F). ATF4 expression in both the nitrite-exposure groups was significantly down-regulated at almost all time points (Figure 4D). Expression of XBP1 showed significant down-regulation in the HN group at 24 h and 48 h, while it exhibited significant up-regulation in the HN group at 12 h and 24 h (Figure 4E).
3.4. Apoptosis and Autophagy
The expression of Bcl2 and Caspase-3 showed a generally opposite pattern in the nitrite-exposure groups (Figure 5B,C). Bcl2 expression in the LN and HN groups was reduced particularly at early stage after exposure; in contrast, Caspase-3 expression was increased at 12 h and 24 h. AIF expression in both the nitrite-exposure groups was significantly up-regulated at 12 h, and it was substantially increased at 48 h (Figure 5A).
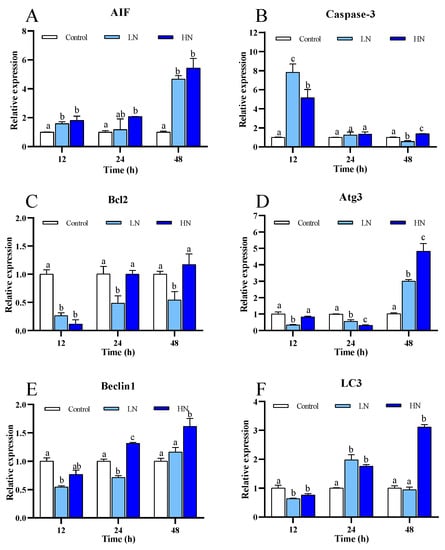
Figure 5.
mRNA expression of six genes about apoptosis and autophagy of juvenile P. trituberculatus under nitrite stress. (A) AIF, (B) Caspase-3, (C) Bcl-2, (D) Atg3, (E) Beclin1, and (F) LC3. At each time point, the letters indicate significant differences between experimental groups (p < 0.05).
The expression of the autophagy related genes, including Beclin1, Atg3 and LC3, in the LN and HN groups, showed a similar trend under nitrite stress (Figure 5D–F). All these genes were down-regulated initially, and then they increased gradually after exposure.
4. Discussion
Nitrite stress is commonly encountered in intensive aquaculture systems, including indoor hatchery systems for crustaceans. However, the toxic effects of nitrite and the underlying mechanisms in brachyura, particularly for the early juveniles at hatchery stages, are still not clearly understood. The results of this study indicated that acute nitrite stress results in disturbance of energy homeostasis, induces oxidative damage and ER stress, and leads to increased levels of apoptosis and autophagy, causing massive mortality of the early juvenile P. trituberculatus. Biochemical and molecular analysis provided further evidence for mechanisms of nitrite toxicity and stress responses that are discussed in detail below.
It has been reported that nitrite stress can significantly influence the survival of crustaceans at different developmental stages [34]. In this study, the survival rate of the C2 juvenile crab in the LN and HN groups at 48 h were 56.67% and 40.00%, respectively. The LD50 of nitrite exposure at 48 h was 27.97 mg/L. Lv et al., 2006 reported that the LD50 of the zoea 1 of P. trituberculatus was 17.884 mg/L at 48 h [35], while our recent study found that the LD50 of sub-adult individuals was 202.86 mg/L at 48 h (unpublished). Similar results were also observed in other decapod species, such as Macrobrachium carcinus [36]. These results confirmed that the decapods at early developmental stages are very sensitive to nitrite stress, highlighting the importance of nitrite control in hatchery management.
Since most cellular stress responses are highly energy-consuming processes, the maintenance of energetic homeostasis plays a key role in survival of the animal under environmental stresses, including nitrite exposure [32,37]. Previous studies in L. vannamei and Eriocheir sinensis reported that nitrite stress can lead to elevated levels of glucose in hemolymph and activity of the glycolytic enzymes and Kreb’s cycle enzymes, suggesting an increased energy demand under nitrite exposure [19,38]. Consistent with the previous studies, enhanced activity of the key enzymes in glycolysis (HK and PK), Kreb’s cycle (CS), and β-oxidation (CPT-1) was observed in the swimming crab at 12 h and 24 h after nitrite stress. In addition, a significant increase in ATP level in both nitrite-exposure groups was observed initially after exposure. These results clearly demonstrated that nitrite stress can result in elevated levels of carbohydrate and lipid catabolism in juvenile P. trituberculatus to meet the energy requirement for cytoprotection against nitrite stress.
AMPK is the primary cellular energy sensor in eukaryotes, and it is critical in maintaining energy homeostasis under stressful conditions [39,40]. Once activated by low energy status, AMPK phosphorylates downstream targets to directly or indirectly promote ATP production by increasing the activity or expression of proteins involved in catabolism while switching off biosynthetic pathways [41]. It was reported that acute nitrite stress can up-regulate AMPKα in fishes [42]. In this study, we found that all the four AMPK subunits showed significant up-regulation at 24 h in the juvenile swimming crab, generally in a dose-dependent manner, accompanied by enhanced activity of the catabolic enzymes (HK, PK, CS, and CPT-1), which indicated that AMPK signaling was activated to restore cellular energy homeostasis under nitrite stress. Unexpectedly, the enhancement of the catabolic enzyme activity occurred earlier than the up-regulation of AMPK after nitrite stress. A previous study in the rock crab Cancer irroratus found that the response of AMPK to thermal stress is characterized by a fast phosphorylation of AMPKα, followed by increased AMPK mRNA after a longer stress, representing an adjustment to varying energy demand [43]. Therefore, the initial increase in the levels of catabolic enzyme activity and ATP production may be attributed to the rapid activation of AMPKα through phosphorylation.
Although AMPKβ1, AMPKβ2, and AMPKγ remained up-regulated in the HN group at the end of the exposure, we observed decreased activity of HK, CS, PK, SDH, CPT-1, as well as the levels of ATP and AEC, indicating that prolonged nitrite disrupted energy homeostasis in the juvenile crabs. The mismatch between the increased energy demand, as indicated by up-regulation of AMPKs, and reduced ATP generation, is possibly due to that continuous nitrite stress impaired AMPK signaling. As many studies in crustaceans have reported that nitrite can cause a reduction of oxyhemocyanin and oxygen carrying capacity [44,45], another possibility was that nitrite exposure resulted in hypoxia, and this thereby inhibited aerobic metabolism and enhanced anaerobic metabolism, which were supported by decreased activity of oxidative enzymes and increased activity of LDH, a key enzyme in the anaerobic glycolytic pathway, as well as being evidenced by HIF-1α expression, a reliable indicator of hypoxia.
It has been shown that nitrite can lead to over-production of ROS in crustaceans, causing oxidative stress, and consequently trigger the antioxidant system to minimize and repair oxidative damage [8,9]. In a recent study of mud crab S. paramamosain, activities of the key enzymes in antioxidant defense, SOD, and CAT showed no enhancement after nitrite stress, and they decreased gradually as the exposure prolonged, while the levels of MDA, the end product of lipid oxidation, which is widely considered as a marker of oxidative damage, showed a gradually increase following nitrite exposure [13]. In contrast, significant enhancement of SOD and CAT was observed at 24 h in both nitrite-exposure groups of this study, indicating that nitrite induced oxidative stress in the juvenile crabs, and antioxidant defense was activated to alleviate the oxidative damage. However, it is noteworthy that activity of SOD and CAT show significant decrease at 48 h, and the levels of MDA built up in a dose- and time-dependent manner. These results indicate that, though the responses of antioxidant systems to nitrite may be of difference between different brachyuran species, prolonged exposure can depress antioxidant defense and result in accumulation of oxidative damage in different species.
Oxidative stress is often accompanied by ER stress, as well as the accumulation of unfolded and misfolded proteins in the ER [46,47]. To reduce ER stress and to restore ER homeostasis, cells initiate a cytoprotective ER quality control system called UPR [48]. A recent study in L. vannamei showed that the expression of the genes involved in UPR in the nitrite-tolerant family were higher than those in the nitrite-sensitive family [17]. The results of the present study found that the important genes in UPR pathways, namely, ATF6 and XBP1, showed significant up-regulation under nitrite stress, which demonstrated that nitrite can induce ER stress and activate UPR in decapod species. As the exposure extended, expression of all the tested genes involved in UPR decreased to the levels similar to the control or lower than the control at 48 h, indicating that nitrite stress for 48 h can compromise UPR in the juvenile crabs, which may result in aggravation of ER stress.
When cellular stress, such as oxidative stress and ER stress, overwhelms cellular homeostasis, cells can undergo diverse forms of death [49,50]. It is known that nitrite can elicit apoptotic cell death through a caspase-dependent pathway in crustaceans [13,51]. In agreement with previous reports, our results showed that Caspase-3, the executioner of apoptosis, was significantly up-regulated, while Bcl2, the anti-apoptotic gene, exhibited significant down-regulation after nitrite stress. Furthermore, we found that nitrite may induce apoptosis via caspase-independent pathway in juvenile P. trituberculatus, as suggested by the up-regulation of AIF. AIF is a mitochondrial intermembrane flavoprotein, which processes the capacity to induce caspase-independent peripheral chromatin condensation and large-scale DNA fragmentation [52]. In this study, Caspase-3 and AIF generally showed opposite patterns after exposure, indicating the time-dependent activation of different apoptotic pathways.
Autophagy is an intracellular catabolic process of self-digestion by the action of enzymes within the lysosome, and it can not only play a protective role by removing and degrading unfolded proteins and damaged organelle, but it also can act as a cell death mechanism under stress conditions, such as nutrient stress, oxidative stress, and ER stress [52]. A recent study in the shrimp L. vannamei reported significant up-regulation of autophagy related genes at early stage of nitrite exposure [24]. In this study, we found that expression of all the tested autophagy related genes, including Beclin1, LC3, and Atg3, which were involved in the phagophore nucleation, extension, and maturation of autophagosomes [53,54], were up-regulated at the late stage of nitrite exposure. These results demonstrate that nitrite promotes autophagy in decapods. However, considering the accumulation of lipid peroxidation and depressed UPR at the late stage of exposure when the autophagy related genes showed significant up-regulation, nitrite-induced autophagy might be a cell death machinery in the juvenile swimming crab. However, further research is required to confirm this speculation.
5. Conclusions
In summary, this study investigated the survival, energy metabolism, and cellular stress responses in the juvenile swimming crab after acute nitrite stress. The results revealed that nitrite exposure can perturb energy homeostasis, possibly by impairing AMPK signaling and enhancing anaerobic metabolism. In addition, nitrite stress resulted in oxidative damage and ER stress, eliciting apoptosis through both caspase-dependent and caspase-independent pathways, as well as autophagy. The results of this study improved our understanding of toxic effects of nitrite in P. trituberculatus and provided useful information for hatchery management.
Author Contributions
Conceptualization, X.L. and X.M.; Data curation, X.L. and D.W.; Formal analysis, X.L.; Funding acquisition, X.M.; Investigation, D.W., Y.S., X.Y., B.G. and J.L. (Jitao Li); Methodology, X.L., D.W., Y.S., X.Y. and J.L. (Jianjian Lv); Project administration, P.L. and J.L. (Jian Li); Resources, B.G., J.L. (Jianjian Lv), J.L. (Jitao Li) and P.L.; Software, X.L., D.W. and J.L. (Jitao Li); Supervision, J.L. (Jian Li) and X.M.; Writing—original draft, X.L.; Writing—review and editing, X.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Chinese National Science Foundation (42276122, 41976106), National Key R&D Program of China (2019YFD0900402), Earmarked Fund (CARS48), Central Public-interest Scientific Institution Basal Research Fund, CAFS (2020TD46).
Institutional Review Board Statement
The study was conducted approved by Institutional Animal Care and Use Committee (IACUC) (Approval Code: YSFRI-2022040 Approval Date: 26 March 2022).
Data Availability Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
References
- Fishery Department of Ministry of Agriculture and Rural Affairs of China. China Fishery Statistical Yearbook; China Agriculture Press: Beijing, China, 2021. (In Chinese) [Google Scholar]
- Shi, C.; Zeng, T.; Li, R.; Wang, C.; Ye, Y.; Mu, C. Dynamic metabolite alterations of Portunus trituberculatus during larval development. J. Oceanol. Limnol. 2019, 37, 361–372. [Google Scholar] [CrossRef]
- Sun, P.; Jin, M.; Ding, L.; Lu, Y.; Ma, H.; Yuan, Y.; Zhou, Q. Dietary lipid levels could improve growth and intestinal microbiota of juvenile swimming crab, Portunus trituberculatus. Aquaculture 2018, 490, 208–216. [Google Scholar] [CrossRef]
- Liao, Y.Y.; Wang, H.H.; Lin, Z.G. Effect of ammonia and nitrite on vigour, survival rate, moulting rate of the blue swimming crab Portunus pelagicus zoea. Aquacult. Int. 2011, 19, 339–350. [Google Scholar] [CrossRef]
- Romano, N.; Zeng, C. Toxic Effects of Ammonia, Nitrite, and Nitrate to Decapod Crustaceans, A Review on Factors Influencing their Toxicity, Physiological Consequences, and Coping Mechanisms. Rev. Fish. Sci. 2013, 21, 1–21. [Google Scholar]
- Meng, X.; Jayasundara, N.; Zhang, J.; Ren, X.; Gao, B.; Li, J.; Liu, P. Integrated physiological, transcriptome and metabolome analyses of the hepatopancreas of the female swimming crab Portunus trituberculatus under ammonia exposure. Ecotoxicol. Environ. Saf. 2021, 228, 113026. [Google Scholar] [CrossRef]
- Kültz, D. Molecular and evolutionary basis of the cellular stress response. Annu. Rev. Physiol. 2005, 67, 225–257. [Google Scholar] [CrossRef]
- Guo, H.; Xian, J.A.; Li, B.; Ye, C.X.; Wang, A.L.; Miao, Y.T.; Liao, S.A. Gene expression of apoptosis-related genes, stress protein and antioxidant enzymes in hemocytes of white shrimp Litopenaeus vannamei under nitrite stress. Comp. Biochem. Physiol. C 2013, 157, 366–371. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, W.; Tan, H.; Pan, D.; Yang, Y.; Ren, Q.; Yang, G. Analysis of gene expression changes, caused by exposure to nitrite, in metabolic and antioxidant enzymes in the red claw crayfish, Cherax quadricarinatus. Ecotox. Environ. Saf. 2014, 104, 423–428. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Jayasundara, N.; Ren, X.; Gao, B.; Liu, P.; Li, J.; Meng, X. Physiological and molecular responses in the gill of the swimming crab portunus trituberculatus during long-term ammonia stress. Front. Mar. Sci. 2021, 8, 1849. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Y.; Li, S.; Guo, X.; Fu, Y.; He, N.; Ruan, G.; Wang, Q.; Gao, W.; Liu, F. Impact of nitrite exposure on oxidative stress and antioxidative-related genes responses in the gills of Procambarus clarkii. Fish Shellfish Immunol. 2022, 131, 624–630. [Google Scholar] [CrossRef]
- Cheng, C.H.; Su, Y.L.; Ma, H.L.; Deng, Y.Q.; Feng, J.; Chen, X.L.; Guo, Z.X. Nitrite-Induced Oxidative Stress, Histopathology, and Transcriptome Changes in the Mud Crab (Scylla paramamosain). Isr. J. Aquacult.-Bamid. IJA 2019, 71, 1626. [Google Scholar]
- Cheng, C.H.; Su, Y.L.; Ma, H.L.; Deng, Y.Q.; Feng, Y.Q.; Guo, Z.X. Effect of nitrite exposure on oxidative stress, DNA damage and apoptosis in mud crab (Scylla paramamosain). Chemosphere 2019, 239, 124668. [Google Scholar] [CrossRef] [PubMed]
- Logue, S.E.; Cleary, P.; Saveljeva, S.; Samali, A. New directions in ER stress-induced cell death. Apoptosis 2013, 18, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Zhang, J.; Cao, J.; Liu, P.; Li, J.; Meng, X. Long-term Ammonia Toxicity in the Hepatopancreas of Swimming Crab Portunus Trituberculatus: Cellular Stress Response and Tissue Damage. Front. Mar. Sci. 2022, 8, 2105. [Google Scholar] [CrossRef]
- Ron, D. Translational control in the endoplasmic reticulum stress response. J. Clin. Investig. 2002, 110, 1383–1388. [Google Scholar] [CrossRef]
- Xiao, J.; Luo, S.S.; Du, J.H.; Liu, Q.Y.; Huang, Y.; Wang, W.F.; Wang, H.L. Transcriptomic analysis of gills in nitrite-tolerant and -sensitive families of Litopenaeus vannamei. Comp. Biochem. Physiol. C 2021, 253, 109212. [Google Scholar] [CrossRef]
- Fu, L.; Gao, T.; Jiang, H.; Zhang, Y.; Pan, J.L. Integrated miRNA-mRNA transcriptomic analysis of hepatopancreas reveals molecular strategies in Chinese mitten crab (Eriocheir sinensis) under acute nitrite stress. Aquacult. Int. 2021, 29, 1015–1030. [Google Scholar] [CrossRef]
- Li, Z.S.; Ma, S.; Shan, H.W.; Wang, T.; Xiao, W. Responses of hemocyanin and energy metabolism to acute nitrite stress in juveniles of the shrimp Litopenaeus vannamei. Ecotox. Environ. Saf. 2019, 186, 109753. [Google Scholar] [CrossRef]
- D’Arcy, M.S. Cell death: A review of the major forms of apoptosis, necrosis and autophagy. Cell Biol. Int. 2019, 43, 582–592. [Google Scholar] [CrossRef]
- Xian, J.A.; Wang, A.L.; Hao, X.M.; Miao, Y.T.; Li, B.; Ye, C.X.; Liao, S.A. In vitro toxicity of nitrite on haemocytes of the tiger shrimp, Penaeus monodon, using flow cytometric analysis. Comp. Biochem. Physiol. C 2012, 156, 75–79. [Google Scholar] [CrossRef]
- Luzio, A.; Monteiro, S.M.; Fontaínhas-Fernandes, A.A.; Pinto-Carnide, O.; Matos, M.; Coimbra, A.M. Copper induced upregulation of apoptosis related genes in zebrafish (Danio rerio) gill. Aquat. Toxicol. 2013, 128–129, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Ji, X.; Wang, X.; Li, T.; Wang, H.; Zeng, Q. Identification and characterization of differentially expressed genes in hepatopancreas of oriental river prawn Macrobrachium nipponense under nitrite stress. Fish. Shellfish Immunol. 2019, 87, 144–154. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Luo, J.; Huang, Y.; Yuan, Y.; Cai, S. Effect of sub-lethal ammonia and nitrite stress on autophagy and apoptosis in hepatopancreas of Pacific whiteleg shrimp Litopenaeus vannamei. Fish. Shellfish Immunol. 2022, 130, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Shintani, T.; Klionsky, D.J. Autophagy in Health and Disease: A Double-Edged Sword. Science 2004, 306, 990–995. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Levine, B.; Cuervo, A.M.; Klionsky, D.J. Autophagy fights disease through cellular self-digestion. Nature 2008, 451, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Dong, B.; Li, A.; Wu, L.; Zhang, Y.; Han, T.; Liu, X. scRNA-seq analysis reveals toxicity mechanisms in shrimp hemocytes subjected to nitrite stress. Chemosphere 2023, 316, 137853. [Google Scholar] [CrossRef]
- Chen, J.C.; Chin, T.S. Acute Toxicity of Nitrite to Tiger Prawn, Penaeus monodon, Larvae. Aquaculture 1988, 69, 253–262. [Google Scholar] [CrossRef]
- Chand, R.K.; Sahoo, P.K. Effect of nitrite on the immune response of freshwater prawn Macrobrachium malcolmsonii and its susceptibility to Aeromonas hydrophila. Aquaculture 2006, 258, 150–156. [Google Scholar] [CrossRef]
- Guo, H.; Xian, J.A.; Wang, A.L. Analysis of digital gene expression profiling in hemocytes of white shrimp Litopenaeus vannamei under nitrite stress. Fish. Shellfish Immunol. 2016, 56, 1–11. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, D.; Wang, F.; Dong, S. Hypothermal effects on survival, energy homeostasis and expression of energy-related genes of swimming crabs Portunus trituberculatus during air exposure. J. Therm. Biol. 2016, 60, 33–40. [Google Scholar] [CrossRef]
- Tian, W.X.; Hsu, R.Y.; Wang, Y.S. Studies on the reactivity of the essential sulfhydryl groups as a conformational probe for the fatty acid synthetase of chicken liver. Inactivation by 5,5’-dithiobis-(2-nitrobenzoic acid) and intersubunit cross-linking of the inactivated enzyme. J. Biol. Chem. 1985, 260, 11375–11387. [Google Scholar] [CrossRef] [PubMed]
- Bieber, L.L.; Fiol, C. Purification and assay of carnitine acyltransferases. Method. Enzynol. 1986, 123, 276–284. [Google Scholar]
- Mallasen, M.; Valenti, W.C. Effect of nitrite on larval development of giant river prawn Macrobrachium rosenbergii. Aquaculture 2006, 261, 1292–1298. [Google Scholar] [CrossRef]
- Lv, G.T.; Wang, Z.Z.; Wang, H.P.; Yuan, J.Y. Acute toxic efects of NaNO2 on Portunus trituberculatus larvas. J. Zhejiang Ocean Univ. (Nat. Sci. Ed.) 2006, 25, 244–248. [Google Scholar]
- Gomes, R.S.; De Lima, J.P.V.; Cavalli, O.; Correia, E.S. Acute Toxicity of Ammonia and Nitrite to Painted River Prawn, Macrobrachium carcinus, Larvae. J. World Aquacult. Soc. 2016, 47, 239–247. [Google Scholar] [CrossRef]
- Sokolova, I.M.; Frederich, M.; Bagwe, R.; Lannig, G.; Alexey, A.; Sukhotin, D. Energy homeostasis as an integrative tool for assessing limits of environmental stress tolerance in aquatic invertebrates. Mar. Environ. Res. 2012, 79, 1–15. [Google Scholar] [CrossRef]
- Hong, M.; Chen, L.; Qin, J.G.; Sun, X.; Li, E.; Gu, S.; Yu, N. Acute tolerance and metabolic responses of Chinese mitten crab (Eriocheir sinensis) juveniles to ambient nitrite. Comp. Biochem. Physiol. C 2009, 149, 419–426. [Google Scholar] [CrossRef]
- Hardie, D.G.; Scott, J.W.; Pan, D.A.; Hudson, E.R. Management of cellular energy by the AMP-activated protein kinase system. FEBS Lett. 2003, 546, 113–120. [Google Scholar] [CrossRef]
- Garcia, D.; Shaw, R.J. AMPK: Mechanisms of Cellular Energy Sensing and Restoration of Metabolic Balance. Mol. Cell 2017, 66, 789–800. [Google Scholar] [CrossRef]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef]
- Xu, Z.; Li, E.; Xu, C.; Gan, L.; Qin, J.G. Response of AMP-activated protein kinase and energy metabolism to acute nitrite exposure in the Nile tilapia Oreochromis niloticus. Aquat. Toxicol. 2016, 177, 86–97. [Google Scholar] [CrossRef] [PubMed]
- Frederich, M.; O’Rourke, M.R.; Furey, N.B.; Jost, J.A. AMP-activated protein kinase (AMPK) in the rock crab, Cancer irroratus: An early indicator of temperature stress. J. Exp. Biol. 2009, 212, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.Y.; Chen, J.C. Hemocyanin oxygen affinity, and the fractionation of oxyhemocyanin and deoxyhemocyanin for Penaeus monodon exposed to elevated nitrite. Aquat. Toxicol. 1999, 45, 35–46. [Google Scholar] [CrossRef]
- Xiao, J.; Liu, Q.Y.; Du, J.H.; Zhu, W.L.; Wang, H.L. Integrated analysis of physiological, transcriptomic and metabolomic responses and tolerance mechanism of nitrite exposure in Litopenaeus vannamei. Sci. Total. Environ. 2019, 711, 134416. [Google Scholar] [CrossRef] [PubMed]
- Borgese, N.; Francolini, M.; Snapp, E. Endoplasmic reticulum architecture: Structures in flux. Curr. Opin. Cell Biol. 2006, 18, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Su, S.; Song, C.; Yu, F.; Zhou, J.; Li, J.; Jia, R.; Xu, P.; Tang, Y. Effects of Copper Exposure on Oxidative Stress, Apoptosis, Endoplasmic Reticulum Stress, Autophagy and Immune Response in Different Tissues of Chinese Mitten Crab (Eriocheir sinensis). Antioxidants 2022, 11, 2029. [Google Scholar] [CrossRef]
- Liu, H.J.; Dong, M.; Jiang, W.D.; Wu, P.; Liu, Y.; Jin, X.W.; Kuang, S.Y.; Tang, L.; Zhang, L.; Feng, L.; et al. Acute nitrite exposure-induced oxidative damage, endoplasmic reticulum stress, autophagy and apoptosis caused gill tissue damage of grass carp (Ctenopharyngodon idella): Relieved by dietary protein. Ecotoxicol. Environ. Saf. 2022, 243, 113994. [Google Scholar] [CrossRef]
- Sano, R.; Reed, J.C. ER stress-induced cell death mechanisms. Biochim. Biophys. Acta 2013, 1833, 3460–3470. [Google Scholar] [CrossRef]
- Cao, S.S.; Kaufman, R.J. Endoplasmic reticulum stress and oxidative stress in cell fate decision and human disease. Antioxid. Redox Sign. 2014, 21, 396–413. [Google Scholar] [CrossRef]
- Candé, C.; Cohen, I.; Daugas, E.; Ravagnan, L.; Larochette, N.; Zamzami, N.; Kroemer, G. Apoptosis-inducing factor (AIF): A novel caspase-independent death effector released from mitochondria. Biochimie 2002, 84, 215–222. [Google Scholar] [CrossRef]
- Denton, D.; Nicolson, S.; Kumar, S. Cell death by autophagy: Facts and apparent artefacts. Cell Death. Differ. 2012, 19, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Zeh, H.J.; Lotze, M.T.; Tang, D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death. Differ. 2011, 18, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Menon, M.B.; Dhamija, S. Beclin 1 Phosphorylation—At the Center of Autophagy Regulation. Front. Cell. Dev. Biol. 2018, 6, 137. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).