1. Introduction
Aquatic ectotherm survival in warm waters is often associated with maintenance of cardiorespiratory capacity and swimming performance, which is supported by cardiorespiratory functions, and is often described as an important trait contributing to the survival of fish [
1,
2,
3,
4,
5,
6]. However, due to the difficulties in empirically assessing relationships between traits and survival in the wild, few data exist to support hypothesized relationships between individual traits and survival [
7]. Triploid (3N) salmonid survival is often low in the wild, and aerobic swimming capacity may be inferior when compared to diploid (2N) conspecifics. Thus, 3N salmonids present themselves as an interesting model with which to investigate the relationships between aerobic swimming performance and survival in warm lakes.
Three swimming modes of fish are typically recognized [
8]: burst speeds (sustainable for <20 s), prolonged speeds (sustainable for more than 20 min, but less than 200 min) and sustained speeds (sustainable for more than 200 min). Only sustainable speeds are thought to be powered entirely by aerobic metabolism, although up to 70% of maximum prolonged swimming speed (most often assessed using critical swimming velocity challenges) may also be aerobically powered [
9,
10].
Though we know of only three attempts to correlate swimming performance with fish survival in a simulated natural ecosystem [
11,
12,
13], substantial evidence exists that burst and prolonged swimming performance can be important to escape predation and thus survival [
5,
11,
14,
15,
16,
17]. For example, prolonged and burst swimming increased the likelihood of sea bass (
Dicentrarchus labrax) surviving an attack by avian piscivores in simulated estuaries [
11], but another study on the same species in the same simulated estuaries found no significant relationship between acceleration ability and growth or survival [
12]. Greater burst swimming speeds were observed in highly predated upon populations of the Trinidadian killifish (
Rivulus hartii) [
5], male mosquitofish (
Gambusia affinis) [
16] and guppies (
Poecilia reticulata) [
17] when compared to populations of the same species experiencing low predation. Additionally, high maximum prolonged and burst swimming speeds increased the likelihood of Atlantic silverside (
Menidia menidia) [
15] and Coho salmon (
Oncorhynchus kisutch) [
2] surviving attacks from piscivorous fish in the lab. Here, to test the hypothesis that aerobic swimming performance influences summer survival at warm temperatures with predation, we relate survival in a high and a low predation lake with endurance swimming of 2N and 3N fish populations. Compared with normal 2N fish, the additional artificially induced set of chromosomes in 3N fish results in enlarged cells throughout the body [
18].
Comparisons of swimming performance for 3N and 2N cohorts for a number of species suggest impaired aerobic swimming capacity in 3N fish. For example, after 3 h of prolonged swimming (1.5 BL s
−1), anaerobic metabolites were elevated in 3N, but not 2N rainbow trout [
19]. The maximum sustained swimming speed of the top 28% of 3N and 40% of 2N Atlantic salmon swimmers, identified through prolonged swimming tests, did not differ with ploidy, but endurance time at speeds above maximum sustained speed was shorter for the 3N than the 2N population subsamples [
20]. Though endurance swimming time at 48 cm s
−1 did not significantly differ between a subsample of 2N and 3N ginbuna (
Carassius auratus) that were able to maintain this speed for 30 min, only 37% of 3N, compared to 53% of 2N individuals, met this 30 min sustained swimming criteria [
21]. On the other hand, critical swimming velocity of 3N rainbow trout, white crappie (
Pomoxis annularis), brook charr (
Salvelinus fontinalis), chinook salmon (
Oncorhynchus tshawytscha) and Atlantic salmon (
Salmo salar) did not differ significantly from that of 2Ns of the same species, despite a tendency of critical swimming velocity of 3N fish to be lower than that of 2N fish [
22,
23,
24,
25,
26]. Whether such small but consistently reduced swimming performance in 3N fish might be amplified into survival consequences in nature is unclear; therefore, knowledge of 3N performance in the wild is useful for industry.
Understanding the mechanisms limiting survival of stocked sport fish in natural environments is important because freshwater recreational fishing forms the basis of a USD 40 billion annual industry in the United States [
27] and CAD 2.5 billion annual industry in Canada [
28] that often depend on hatchery stocking programs to maintain catchable sport fish populations. Many sport fish stocking programs take advantage of reproductive sterility of triploid (3N) fish to control stocked populations. For example, in British Columbia (BC), Canada, the Freshwater Fisheries Society of BC stocks 800 lakes and rivers for sport fishing and conservation and 3N rainbow trout are stocked in approximately 50% of these lakes [
29]. A challenge to stocking 3N fish in lakes lies in frequently reported low 3N fish survival relative to 2N conspecifics [
30,
31,
32,
33,
34]; however, 3N survival has also been observed as higher [
34,
35] or similar [
31,
33,
34,
35,
36,
37,
38,
39] compared with 2N survival, depending on fish size, age and sex and water conditions. However, when reared at chronically low O
2 and/or high temperature in labs/hatcheries [
40,
41] and under suboptimal oxygen and/or thermal conditions in either a lake [
33,
34] (or a marine cage site [
32,
42,
43], 3N survival is consistently low, regardless of size, age or sex. Elevated temperature and environmental hypoxia place burdens on O
2 delivery in fish [
44,
45,
46]; thus, we hypothesized that reduced 3N survival is related to reduced aerobic capacity and therefore aerobic swimming capacity of 3N fish.
Therefore, here we examine the relationship between swimming capacity and survival in 2N and 3N rainbow trout in a natural lake setting to address the question: Does reduced survival of 3N relative to 2N trout in natural ecosystems during periods of high temperature or low oxygen relate to lower aerobic swimming capacity and aerobic scope of 3N compared to 2N conspecifics? The hypothesis that aerobic swimming performance influences survival in the wild was tested in two lakes in which fish were expected to utilize suboptimal oxygen and thermal habitat and experience different levels of avian predation pressure. We predicted that survival would increase in fish with increasing swimming endurance and 2N and 3N fish of similar endurance would have similar survival in both lakes. In order to account for effects of habitat utilization on the swimming endurance–survival relationship, habitat utilization was also monitored using temperature–depth transmitters surgically implanted into a subsample of each population of fish.
2. Materials and Methods
2.1. Hatchery Rearing
In 2007, 2008 and 2009, 2N and 3N Blackwater strain rainbow trout (referred to as rainbow trout from here onwards) were screened for swimming endurance to compare 3N and 2N aerobic swimming performance, then stocked into two lakes to assess survival in the wild and its relationship with swimming endurance. Fish were all female offspring of two to three hormonally masculinized genetic female rainbow trout captive broodstock [
47] and two to three female wild-caught trout from Blackwater River (Cariboo region, BC, Canada) each year. Rainbow trout native to the Blackwater River system inhabit lake and river habitats. All eggs collected from the wild-captured trout were pooled then divided into two batches before fertilization.
One egg batch was treated with a hydrostatic pressure shock shortly after fertilization to induce triploidy [
48] and the other was allowed to develop into 2N fish. Using sibling 2N and 3N populations allowed for comparisons of populations of identical genetic origin. Though both male and female 3N fish are functionally sterile, only female 3N fish avoid energy investment into gonad production and thus have the potential of superior performance relative to maturing fertile 2N fish. Therefore, using all female 2N and 3N populations not only minimized variability due to gender differences, but also maximized any potential performance advantages of stocking 3N populations.
Fish were reared at Fraser Valley Trout Hatchery (FVTH, Abbotsford, BC, Canada) in 10 °C water and captive rearing mortalities were low for both ploidies across all years. For example, survival from the green egg stage to stocking was 30 and 36%, respectively, for 3N and 2N trout stocked in 2007, and 43 and 48%, respectively, for 3N and 2N trout stocked in 2009. In 2008, to accommodate transmitter implants, fish were transferred from FVTH to Vancouver Island Trout Hatchery (VITH, Duncan, BC, Canada) after screening trials in November, where winter rearing temperature was warmer (14 °C compared to 10 °C at FVTH) and accelerated growth to 100 g.
2.2. Endurance Swimming Screening
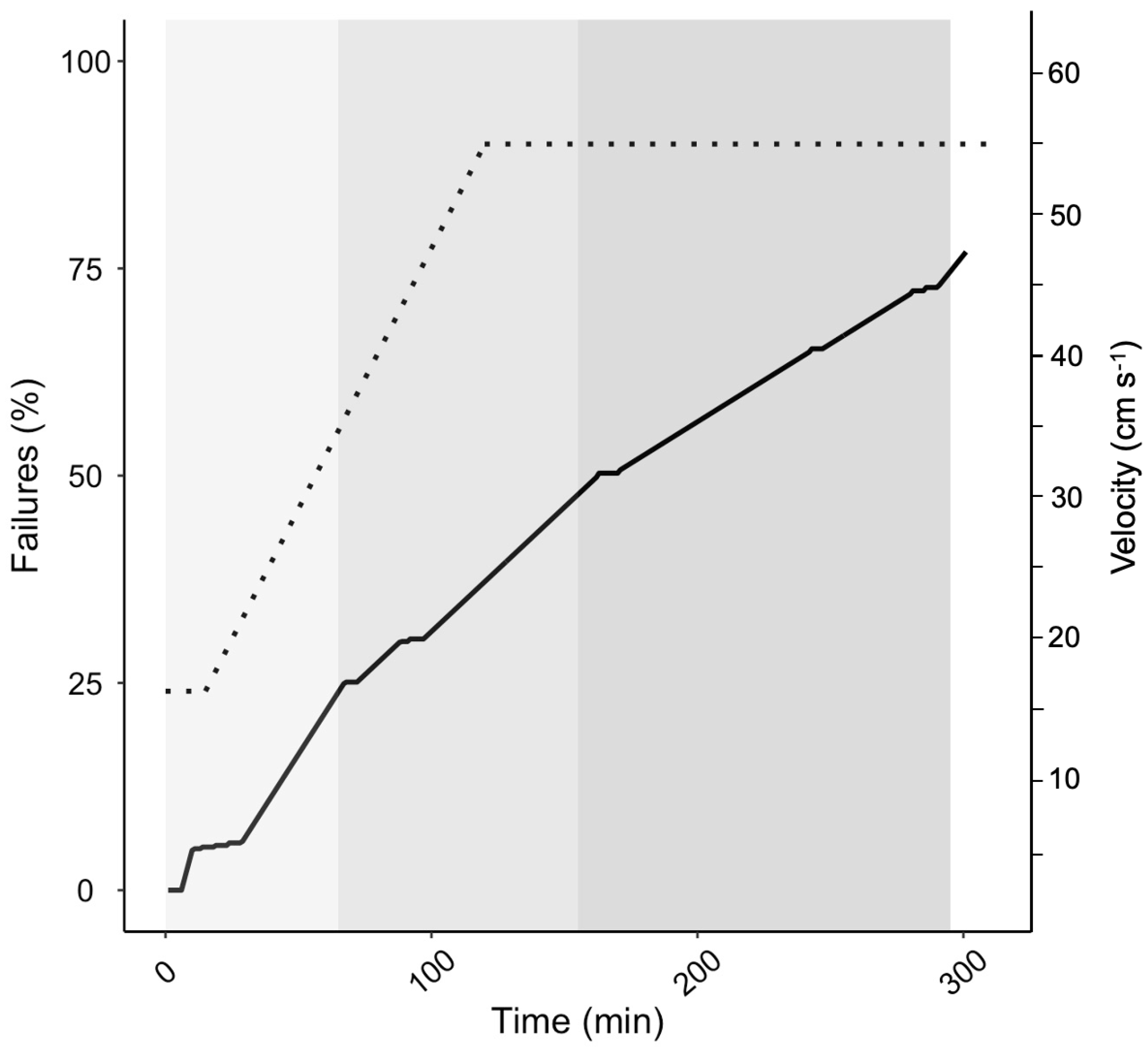
Before lake stocking, fish were screened for endurance swimming either six (October 2007) or nine (January 2008 and 2009) months post-fertilization, which corresponded to seven or four months, respectively, prior to lake stocking). Endurance swimming screens were performed in rounded swimming arenas with a delineated swimming track and a mesh barricade at the downstream end of a ‘failure zone’ to catch failed fish swept downstream. Each swimming endurance trial was performed with 300 to 500 fish in the swimming track. Water velocity through the swimming track was manipulated using a 24 V electric outboard motor. Fish were initially acclimated to 15 cm s
−1 for 15 min before the motor was gradually increased to achieve the maximum water flow velocity (45 to 55 cm s
−1) over 100 to 125 min and then held for another 200 min (
Figure 1). Failure was defined as fish drifting into the delineated ‘failure zone’ and not swimming out when lightly prodded. Failures were netted, measured for weight and length and marked according to failure percentile among the competing fish within the individual swimming endurance trial. Endurance was assessed as total time swum for each fish and percentile rank within the individual trial group. Fish marking entailed a combination of fin clips (a combination of maxilla, ventral and adipose fins), Visible Implant Elastomere tags (Northwest Marine Technology Inc., Washington, DC, USA) and PIT tags (Biomark, Boise, ID, USA). The screening protocol varied slightly each year.
2.3. Lake Stocking
Endurance-screened rainbow trout (1+ year old) were stocked into the two lakes in May of each year (
Table S1). The fish biomass stocked into each lake was 20 kg in 2007 and 2009 and 100 kg in 2008. These biomasses are below the calculated sustainable biomass for Pete’s Pothole (PPH) (200 kg) and Bluey Pothole 2 (BPH) (145 kg) based on total dissolved solids and littoral and pelagic areas of the 2 lakes. Both experimental kettle lakes are small mesotrophic lakes with no natural fish populations and on the southern interior plateau of BC, Canada. The larger and deeper lake (PPH) was expected to have a much higher fish predation pressure from loons and osprey (Beckmann et al., 2006, Biro et al., 2006 and author’s observations;
Table S1). In 2007 and 2008, but not 2009, temperature loggers were moored and positioned at 0.5 m depth and in 1 m intervals from 1 to 6 m depth at the center of PPH and to 4 m depth at the center of BPH. Additionally, 1 m interval depth profiles of temperature and O
2 were measured monthly in both lakes using a handheld dissolved O
2 m (YSI model 550A) with a 20 m probe extension cable.
In 2007 and 2009, fish were transported by road from FVTH to the two lakes (approximately 200 km and 3 h) in 600 L insulated tanks supplied with compressed O2. Temperature and O2 were maintained at 10 to 12 °C and 18 to 12 mg L−1, respectively. At the lakes, fish were released by hand. During fish release, surface temperature ranged from 15 to 16 °C for BPH and 15 to 17 °C for PPH for all stocking years. In 2008, the larger fish used for telemetry were transported from VITH to the lakes (approximately 400 km and 6 h), using a 5-ton truck with an integrated live transport tank. These fish were released into lakes by gravity through hoses from the truck to the lake.
In 2007, while unscreened 2N and 3N trout were stocked into PPH only, endurance-screened 3N rainbow trout were stocked into BPH.
2.4. Fall Depletion Netting
In the second week of October of each year, summer survival was assessed through depletion gill netting in both lakes. In 2007 and 2009, lethal gill netting was performed over four consecutive nights. In 2008, netting was performed over five nights, with an initial three nights followed by a week of no netting and then two additional nights of netting. Gill nets were either 4 or 8 m deep experimental gill nets with panel sizes ranging from 2.25 to 5.62 cm. In an attempt to explain interannual variability in survival, accumulated thermal units (ATU) of air temperature were calculated from the daily mean temperatures reported from the Environment Canada weather station out of Merritt, B.C., which is approximately 40 km from both lakes. Though ATU calculated using air temperature does not allow for determination in inter-lake temperature differences, it was necessary due to failure of lake temperature loggers in 2009 and is expected to be sufficient to predict interannual variability in lake temperature.
2.5. Telemetry
In 2007 and 2008, fish equipped with a VEMCO V9 temperature-depth (TP) transmitter (VEMCO division AMIRIX Systems Inc. Halifax, NS, Canada) were stocked into PPH (in 2007) or both lakes (in 2008). A pilot study was performed in 2007 using 20 fish (10 each of 2N and 3N) that had surgically implanted transmitters in their abdomens. There were no mortalities during the 2-week recovery period following surgery and fish were released into PPH in September. Transmitters were 39 mm long and 9 mm wide and weighed 2.2 g in water and 4.6 g in air, which averaged 1.5% of fish body mass in air. Transmitters were programmed to ping temperature and depth measurements every 30 min (estimated battery life 615 days) to a VEMCO VR2W Acoustic Monitoring Receiver moored at the center of the lake. Previously, radio tags surgically implanted into smaller chinook salmon (16 to 54 g) and adding 2.2 to 5.6% to body mass compared with 1.5% in the present telemetry study, caused a 2% mortality rate (i.e., 1 mortality 36 days after tag insertion) and small reductions in growth rate, which returned to control rates within 58 days post-surgery [
49]. In this study, fish were stocked into lakes 14 days after surgeries and depletion netting was performed approximately 150 days post-surgery. Critical swimming velocity of chinook salmon tagged at similar size with similar radio tags did not differ from that of controls by 19 to 23 days post-surgery [
50]. However, in the same study, though no direct tag-related mortality was observed, tagged fish were more likely than controls to be eaten by smallmouth bass (
Micropterus dolomieu).
Surgery, which took 7 min on average, involved anaesthetizing fish with an MS222 solution (75 mg L−1 MS222 buffered with 75 mg L−1 sodium bicarbonate dissolved in distilled water) until they were refractory to a caudal pinch. Then, aerated water at 12 °C containing a maintenance dosage of buffered MS222 (50 mg L−1 MS222) was pumped over the gills while a 1 cm incision through the peritoneum was made approximately 1 cm to the side of and parallel to the linea alba, immediately anterior to the cartilage of the ventral fins for insertion of the transmitter. The incision was then closed with three or four discontinuous #2 silk sutures. Fish were revived in a flow-through, aerated recovery tank before being returned to their stock tank. Transmitters were tested for 24 h in the stock tank and temperature logs of individual transmitters were calibrated from these test data. No fish mortality or transmitter loss occurred before lake stocking 2 weeks later.
A similar procedure was used in 2008, using a subsample of four to five fish randomly chosen from each screened quartile destined for each lake for transmitter implants (N = 20 fish in total for each ploidy). These fish were stocked into lakes with the rest of the fish in May 2008.
2.6. Temperature and Depth Log Data Analysis
Recordings from transmitters were analyzed for seasonal, diurnal, ploidy and lake differences in temperature and depth habitat utilization of fish. A small campground is located on the edge of PPH, raising concern over human activities on the lake affecting fish behavior/habitat utilization. As use of this campground and recreational activities on the lakes were most dense on weekends and holidays, these periods were filtered out of the temperature and depth transmitter logs before analyses.
Fish were determined to be alive when transmitter depth recordings continued to fluctuate and dead when recordings either disappeared or remained constant across a 24 h period, though some error in terms of transmitter loss instead of fish death may have arisen using this criterion. Even so, no transmitter loss occurred in the hatchery during the weeks before lake stocking, only one transmitter was lost during fish transport, and no transmitter loss occurred during the two-week pilot study in 2007, so this error is expected to be small.
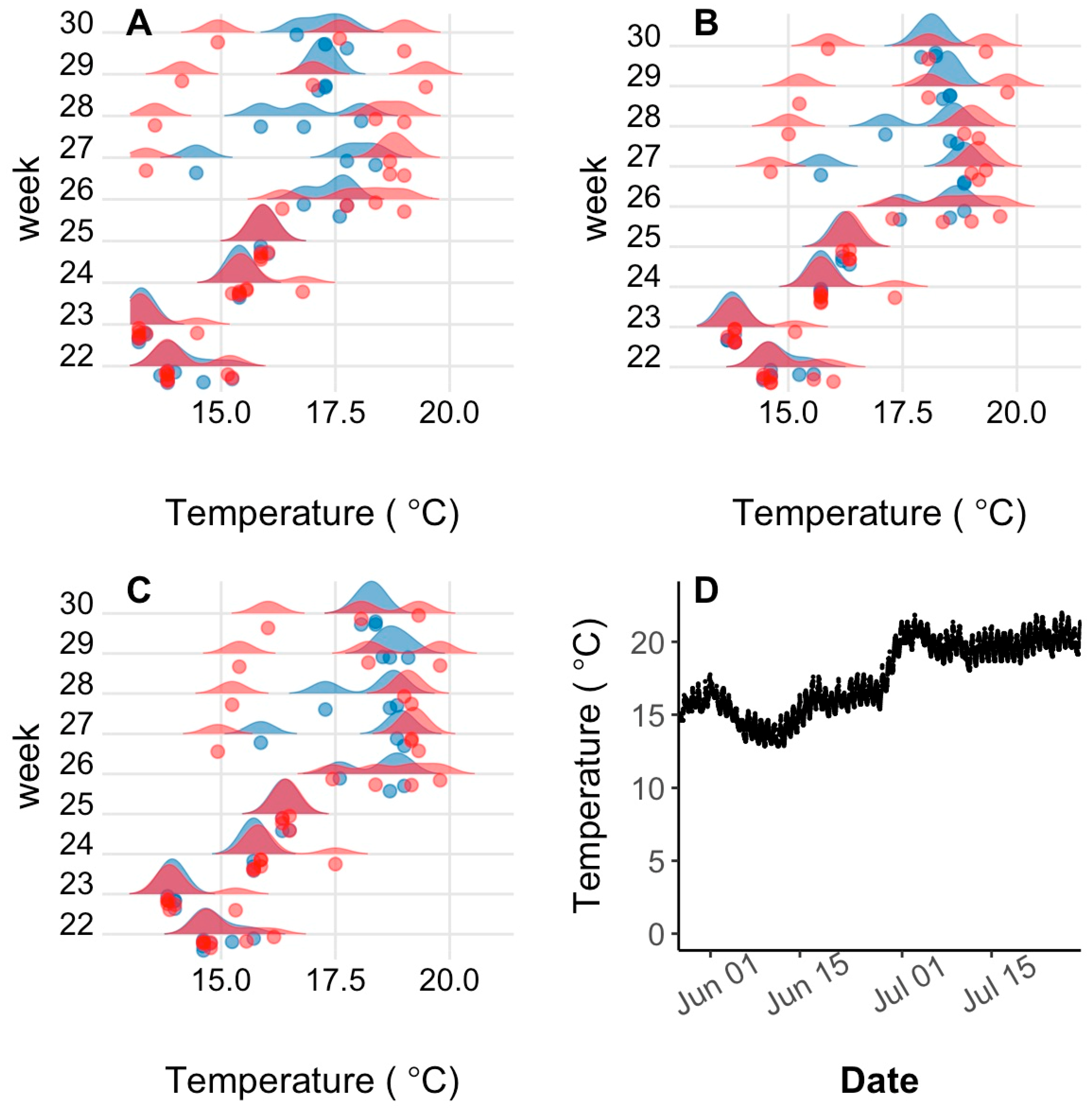
Seasonal and lake effects on habitat utilization were assessed by creating weekly summer temperature and depth ploidy-specific density plots of weekly quartiles determined for each individual transmitter with surviving fish. After weekend and holiday logs were filtered from the logger dataset, weekly quartiles (25, 50, and 75%) of individual surviving fish half-hourly temperature and depth logs were quantified using the base R quantile () function [
51]. Ploidy-specific kernel density plots of the three quantiles were created for each week after lake stocking in late May until late July (27 May to 2 June; 5 to 10 June; 14 to 20 June; 27 June to 2 July; 8 to 14 July; and 14 to 20 July) when water temperature had peaked, with ploidies separate. Although fish were not recaptured until October, low sample sizes (i.e., only one moving transmitter in October) precluded useful analysis post-July. The kernel density plot is a non-parametric technique for estimating the probability density function in which each data point is represented by a smoothing kernel of predetermined variance and the area under the probability density function between two temperatures is the probability of the fish being found between those two temperatures. Density plots were made using the geom_density_ridges () function of the ggridges package [
52] using a bandwidth of 0.3. Because kernel density plots add a variance to each data point plotted and surface measurements were obtained from temperature loggers at 0.5 m below the water surface, the fish transmitter temperature recordings and density plot tails sometimes conflicted slightly with reported lake conditions.
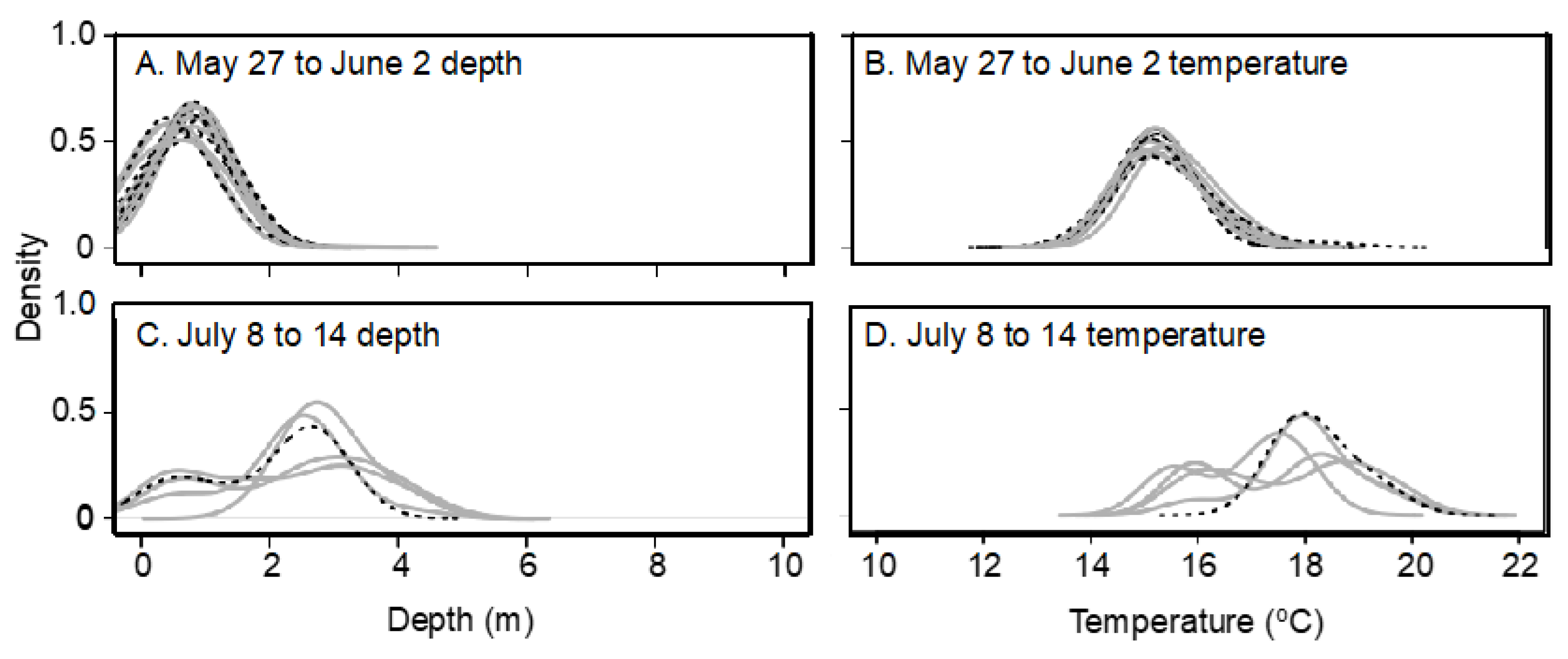
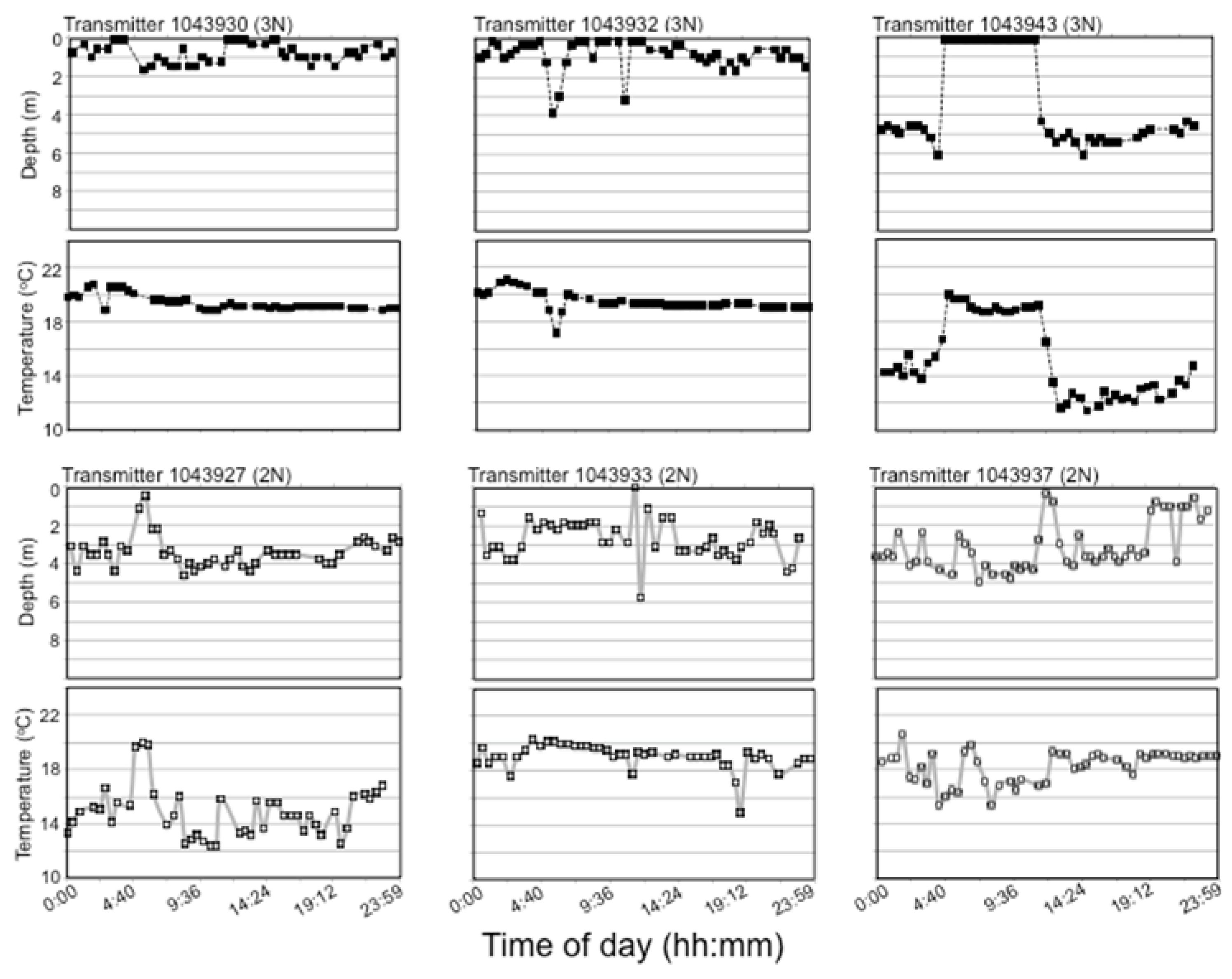
Plots of 24 h temperature and depth utilization were also analyzed to investigate interindividual and interploidy variability during warm summer temperatures. As all fish spent the entire day at the lake surface at the beginning of the summer and individual differences did not appear until surface temperature exceeded 18 °C, 24 h habitat utilization was compared for July 10, and only for the 6-transmitter fish (three each of 2N and 3N fish) in PPH that survived until the end of July. Only one 3N fish remained alive in BPH by July 10, so these comparisons were note performed for BPH.
2.7. Recapture Analyses
Effects of lake, ploidy, year and ATU on summer survival were assessed using a generalized linear mixed model with a binomial distribution to predict probability of recapture as a function of ploidy, lake and temperature as fixed factors and year as a random factor. The best-fit model predicting recapture was determined using a top-down approach [
53], beginning with the complete model, which included the fixed terms (ploidy, temperature and lake), the single random term (year) and all possible interaction terms. Then, the importance of specific terms was tested by sequentially and individually eliminating terms (beginning with the random term) and testing if the reduced model significantly differed from the complete model using likelihood ratio tests. The significance cutoff applied was
p < 0.05. This was performed using the ‘glmer’ function of the ‘lme4’ package [
54] in R.
The effect of ploidy on fish endurance was tested using a linear model with the ‘lm’ function in base R. The significance cutoff applied was p < 0.01.
The relationship between endurance and recapture was tested using a generalized linear model with a binomial distribution to model probability of recapture as a function of ploidy, endurance and lake. This was performed using the ‘glm’ function in R. As with the mixed effects models, the importance of specific terms was tested by sequentially and individually eliminating terms and testing if the reduced model significantly differed from the complete model using likelihood ratio testing and a significance cutoff of p < 0.05). To include endurance as a quantitative variable in the model, the fourth quartile was not included in the analysis because the endurance of this quartile was not quantified.
4. Discussion
As predicted, aerobic swimming performance was lower for 3N compared to 2N salmonids and endurance swimming performance related to summer survival in a natural system. An interaction between ploidy, lake and endurance in the endurance–recapture relationship reflected differences in survival between the two lakes and survival and endurance between the two ploidies. Ploidy effects on survival and endurance swimming likely reflect limitations of 3N cardiorespiratory capacity, and variability in survival between lakes and years was at least partly explained by lake temperature, but also lake-specific characteristics, such as lake morphology or avian predation on the stocked fish.
4.1. Summer Lake Habitat Utilization
Summer fish survival and habitat utilization was monitored in two dissimilar lakes. PPH is a deep lake with warmer surface water temperatures and greater potential for avian predation on fish than the shallower, slightly cooler BPH. Pete’s Pothole (authors’ observations) [
55], but not BPH [
55,
56], is frequented by common loons (
Gavia immer), which are voracious fish predators. Additionally, O
2 and temperature depth profiles of the two lakes show PPH to be a warmer lake than BPH, but due to its greater depth, it has several meters depth of cool high O
2, which is not present in BPH.
Reflecting the differences in lake characteristics, rainbow trout habitat utilization differed between BPH and PPH. Previous lab studies that tracked heart rate response to warming for the same population of fish found an optimal temperature of 14 °C and that mortalities likely arise for a significant portion of the population as temperature approaches and exceeds 18 °C [
57]. In BPH, after mid-June, rainbow trout spent no time at the lab-determined optimal temperature of 14 °C, and actually spent the majority of their time at temperatures approaching 18 °C, such that they were spending the entire month of July at supra-optimal temperatures. At 18 °C, the lab study showed onset of arrhythmic heartbeats in 25% of 2N and 3N fish tested when temperature reached 18 °C, which is likely a prelude to cardiac collapse and thus imminent death. With increasing acclimation temperature, high temperature tolerance increases [
58], so high temperature tolerance of the fish in BPH in July was most likely greater than for the fish tested in the lab, which were acclimated to 10 °C. However, salmonid fish are limited in their thermal tolerance plasticity. When acclimation temperature of sockeye salmon was increased from 10 to 20 °C, the upper lethal limit increased by only 1 °C from 23 to 24 °C [
59]. Furthermore, the critical thermal maxima of redband trout (
Oncorhynchus mykiss gairdneri) originating from creeks of different temperatures (mean temperatures ranging from 15 to 23 °C, and maximum temperatures ranging from 18 to 29 °C) only differed by 0.7 °C [
60]. Thus, high temperature tolerance of the fish in BPH in July was likely slightly higher than that of the fish tested in the lab. However, this increase in tolerance was unlikely to exceed 1 °C, suggesting fish were at the limits of their thermal tolerance in both lakes in July.
Despite the near lethal temperatures utilized in BPH, fish even hotter temperatures in PPH. After mid-June, fish in PPH similarly spent no time at 14 °C but spent the majority of their time at 19 to 20 °C, which was 1 to 2 °C warmer than those in BPH and expected to result in arrhythmia in more than 50% of the stocked populations [
57]. Additionally, maximum heart rate of rainbow trout in the lab was reached at 20.0 ± 1.1 °C for 2Ns and 19.5 ± 0.5 °C for 3Ns [
57], suggesting the resting metabolic requirements at this temperature range required maximum capacity of the cardiovascular system. Thus, any metabolic challenges at these temperatures would only be transiently sustainable and only through anaerobic metabolism. The proximity of these lab-determined lethal temperatures to the thermal habitat of fish in PPH in July potentially reflects an inability of fish in PPH to meet the day-to-day metabolic challenges (e.g., foraging, competitive interactions and predator avoidance) required to survive in the wild.
Lake habitat utilization also differed between 3N and 2N rainbow trout. Early in the summer, when lake temperatures were cool, habitat utilization of 2N and 3N trout was similar and surface-oriented, but as the lake warmed during summer, 3N trout spent most of their time at the surface while 2N trout preferred slightly deeper and cooler water. Considering the summer lake surface temperatures exceeded lab-determined thermal tolerance limits, it is surprising that 3N trout spent more time at the surface in slightly warmer temperatures than their 2N cohorts. This apparently detrimental 3N behavior may be a result of competitive interactions with 2N trout over the safer, deeper, and slightly cooler, but still well oxygenated water. Previous behavioral comparisons suggest that 3N fish are less aggressive/dominant than 2N fish [
61,
62,
63], but 3N behavior in the wild has never been assessed. Even though all the 3N fish with transmitters in PPH displayed different behavior compared to their 2N siblings, these trends must be interpreted cautiously because of the small sample sizes of surviving fish with transmitters by the time these differences had appeared. Of course, as most 3N fish with transmitters had died at this point, it is possible that these behavioral differences reflect only those 3Ns able to survive the warm summer water.
4.2. Survival in Summer Lakes
Survival of Blackwater rainbow trout was influenced by lake, temperature and swimming endurance. Summer survival in PPH was consistently lower compared to that in BPH across three years of observations. A study comparing lakes from the same region with and without loon predation showed loons can remove 50% of all stocked 1-year-old (26 g) rainbow trout, as similar size to fish stocked in PPH and BPH in this study, from a lake [
56]. When cannibalism occurred in BPH in 2000, due to stocking large 1+ year old with small 0+ year old rainbow trout, survival of 0+ year olds decreased to 10 to 20% [
64]. Cannibalism was unlikely to have occurred with the single year class stocking used here, and so generally greater survival was observed compared with earlier studies in similar lakes.
In addition to predation, high water temperature also correlated with mortality in PPH compared with BPH. Both surface temperature and the temperatures fish frequented in PPH were consistently 1 to 2 °C higher than in BPH throughout the entire summer. The near maximum heart rates required of rainbow trout to maintain corporeal O2 supply at these high temperatures reflect a severely reduced aerobic scope, which is the difference between resting and maximum metabolic rates.
Aerobic scope can be thought of as the metabolic reserves available to a fish for responding to the many, usually cumulative, metabolic challenges a fish must successfully navigate to survive in the wild. It has been hypothesized that the breakdown in aerobic scope, due to thermodynamic increases in resting but decreases to maximum metabolic rate, is an important mechanism underlying fish failure at high temperature [
44,
46], but it is important to note that some groups challenge this hypothesis [
65,
66]. Thus, aerobic scope may be especially important in a high-temperature lake like PPH.
The metabolic demands of escaping and recovering from predation attempts in a high predation lake like PPH may compound upon potential limitations in available aerobic scope within the high temperatures fish inhabit in PPH. Lake trout (
Salvelinus namaycush) required 75% of their aerobic scope for chronic maintenance of life [
67]. On the other hand, EMG studies on rainbow trout suggested metabolic costs in the wild rarely exceed 20% of the aerobic scope for activity [
68]. However, calibration of EMG readings to metabolic rate was performed on different fish populations than the field EMG recordings were on, and interpopulation differences in the EMG–metabolic rate relationship may have introduced error in this estimate. Additionally, the field EMG measurements were performed in experimental ponds with no predators. In PPH, predator escape attempts from loons, which can chase their prey for as much as 30 m [
69], would almost certainly increase metabolic costs in the wild. Thus, in the heat of the summer, the aerobic scope of fish in PPH likely approaches zero but fish are being forced to either expend metabolic energy in escape attempts or become easy prey and die. Thus, the elevated mortality in PPH compared with BPH is likely related to reduced aerobic scope with preference for supra-optimal surface temperatures as a significant contributing factor. Indeed, in both lakes, there was a sudden loss of fish equipped with transmitters coincident with surface temperature first reaching 18 °C, which occurred a week later in BPH than PPH. Similarly, when reared at 18 °C, 3N brook charr died after a chase to exhaustion, while 2N charr were able to recover with no mortalities [
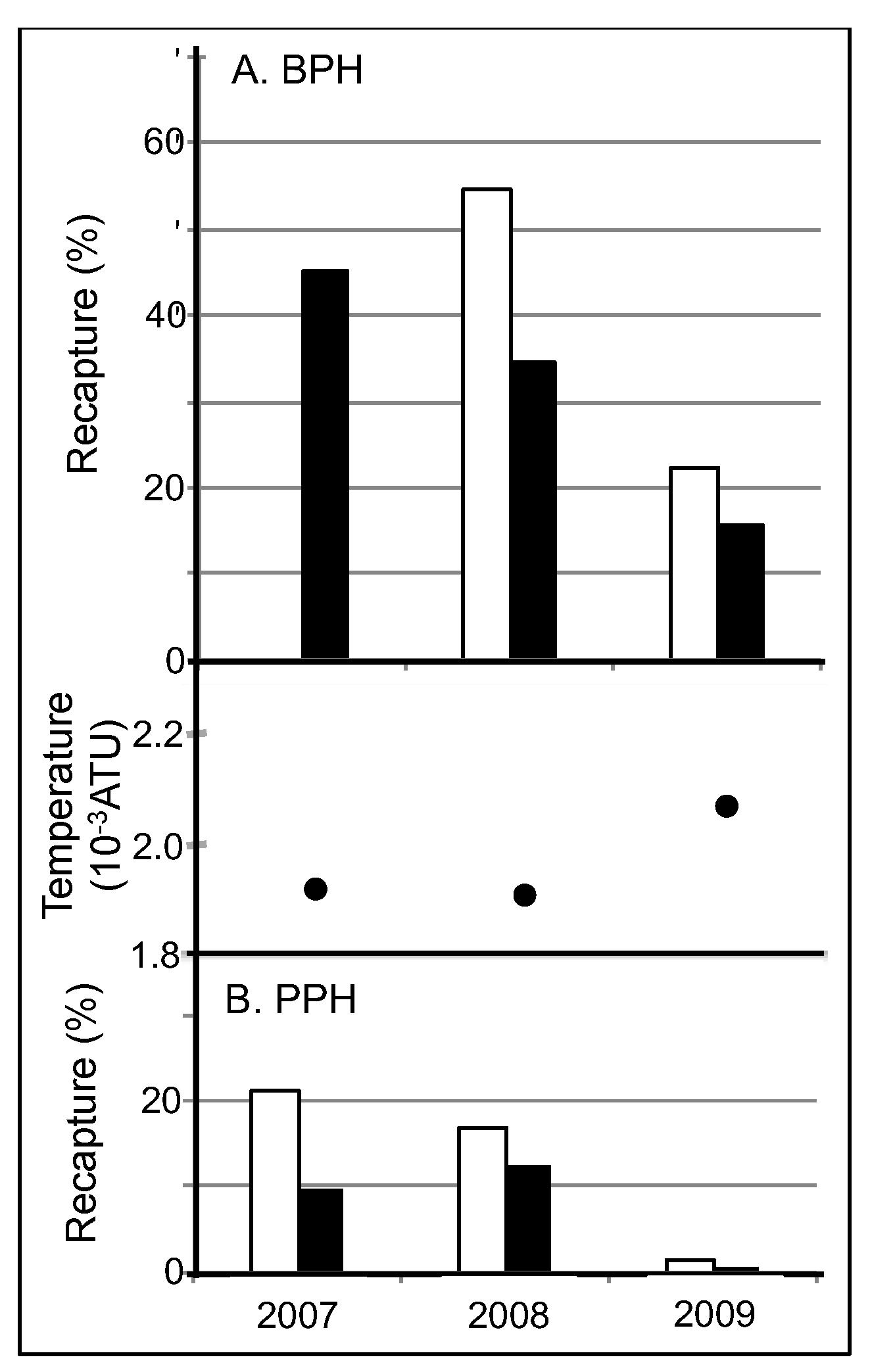
70]. Thus, a similar explanation can account for the ploidy differences in survival. In fact, 3N survival was consistently nearly 50% lower than 2N survival in both lakes across all 3 years whenever both ploidies were stocked together.
Comparison of lab-determined lethal temperatures and utilized thermal habitats in lakes relative to available oxy-thermal habitat raises a rather obvious question: Why did Blackwater rainbow trout prefer near lethal temperatures during the summer? The answer may lie in food availability and field metabolic rates. Food availability is likely tied to photosynthesis and aquatic invertebrates, which are greatest in the surface water of these lakes [
71]. Lower aerobic scope requirements due to cooler temperatures and less predation would allow fish in BPH to survive well at surface temperatures above optimal. However, in PPH, where a greater aerobic scope was required for both predator avoidance and the metabolic consequences of enduring 1 to 2 °C higher temperatures, survival was predictably lower than in BPH. Furthermore, the uncharacteristically warm weather in 2009 decreased survival in both PPH and BPH compared with both 2007 and 2008. Thus, trends in summer mortality between lakes, across the summer season and from year to year strongly suggest preference for temperatures above optimal caused significant mortality in both lakes, with predation almost doubling mortality in PPH relative to BPH.
4.3. Reduced 3N Swimming Endurance
As predicted, 3N trout had reduced endurance swimming ability compared to their 2N cohorts, potentially reflecting lower aerobic scope in 3Ns relative to 2Ns. Lab studies have shown that within a 2N rainbow trout population, the fish with the highest prolonged swimming capacity (which, like endurance swimming is primarily aerobically fueled) had the greatest aerobic scope, maximum O
2 consumption rate and cardiac output [
72]. While previous comparisons of fish swimming ability for 2N and 3N cohorts have been equivocal, reduced aerobic swimming performance has been suggested for 3N Atlantic salmon [
20], ginbuna [
21], rainbow trout [
22], white crappie [
23], brook charr [
24] and chinook salmon [
25]. Elevation of anaerobic metabolites in 3N, but not 2N rainbow trout, after 3 h of prolonged swimming [
19] suggests 3N trout lack aerobic capacity and switch sooner to anaerobic energy production to fuel their swimming. Similarly, aerobic scope of 3N chinook salmon was 70% of 2N siblings’ [
25]. Thus, reduced swimming endurance of 3N Blackwater rainbow trout in this study was likely due to ploidy effects on aerobic scope and could have contributed to reduced 3N fish survival in the natural lakes.
4.4. Endurance and Survival
As initially predicted, we showed a significant relationship between endurance swimming and the probability of recapture for individual fish and a dependence of this relationship on lake and ploidy. This significant relationship was further supported with an increase in the average endurance of surviving 2N and 3N populations after a summer in lakes compared to the stocked populations. The interactions between the survival–endurance relationship and lake and ploidy were likely due to biotic and abiotic characteristics of the lakes and behavioral and cardiorespiratory differences between 2N and 3N trout, respectively.
The interaction between lake and the endurance–survival relationship raises the concern of pseudo replication in this study. This relationship was tested in two dissimilar lakes, in one of which (BPH) recapture rates were high, and the endurance–survival relationship was not evident. Though it appears that the potential predation and high temperatures of PPH explain reduced survival and manifestation of the endurance–survival relationship, replication across many lakes similar to PPH and BPH is necessary in order to confirm this conclusion.
The effect of lake on the endurance–survival relationship is not surprising considering the differences between the two stocked lakes. Reduced survival in PPH was potentially due to predation and high temperature, presenting the possibility that strong selection had occurred for high-temperature-tolerant fish with predator avoidance skills. Previous research has established a link between high temperature tolerance and aerobic scope [
44,
46,
73]. In captivity, rainbow trout with high aerobic swimming capacity had greater aerobic scope and maximum O
2 consumption rate and cardiac output than poor swimmers [
72]. Therefore, high-endurance fish are likely more high-temperature-tolerant as a consequence of their large aerobic scope.
Though the relationship between sustained swimming capacity and predation escape has never been assessed, some evidence exists that fish with high burst [
2,
5,
11,
15,
16,
17] and prolonged swimming capacity are better at escaping or avoiding predation [
2,
11,
15]. Extending these findings to PPH suggests that fish with high swimming endurance were better able to escape predation by loons or recover metabolically from escape attempts and therefore had improved chances of survival.
Therefore, due to the importance of aerobic scope to both high temperature tolerance and endurance swimming capacity, selection for high aerobic scope may be even greater than that for endurance swimming. Furthermore, although endurance swimming was important for survival in a high predation warm lake (PPH), it was not important to survival in a cooler low predation lake (BPH) where aerobic scope requirements are not expected to exceed the available reserves.
The ploidy effect on the lake survival–endurance relationship is likely due to behavioral and or aerobic metabolic differences between the ploidies. The 30 to 50% greater survival of 2N relative 3N populations in PPH across 3 years corresponded with a 40% greater endurance in 2008. Furthermore, a 3-fold increase in endurance doubled survival for 3N fish. Reduced endurance in 3N relative to 2N rainbow trout most likely reflects a reduced aerobic scope, which has been shown in 3N chinook salmon [
25]. Thus, the endurance–survival relationship within the 3N population in PPH further supports the importance of aerobic scope to survival.
Considering 3N salmonids appear to have reduced aerobic scope compared to their 2N cohorts, the relationship between swimming endurance and aerobic scope, and therefore survival, may differ between ploidies. It is important to note that here, aerobic scope was not directly quantified for 2N and 3N rainbow trout. In fact, no studies have shown a connection between directly measured AS and survival in a warm natural ecosystem. Unfortunately, the time requirements to measure aerobic scope make it challenging to test its influence on survival at the population level, but these studies will be important to confirming hypothesized relationships between aerobic scope and survival of ectotherms in warm aquatic systems.
Alternatively, differences between the behavior of 2N and 3N trout may explain variability in the endurance–survival relationship between ploidies. Previously reported altered 3N performance and behavior in the presence of 2N fish [
47,
62] may have confounded the endurance ranking of fish in this study, because fish were endurance screened with ploidies separate, but stocked into lakes together with ploidy cohorts combined. The potential of interploidy behavioral differences influencing the endurance survival relationship is further supported by evidence of differential habitat utilization by 2N and 3N rainbow trout in our study system during the hottest part of the summer. Considering 3N preference of the high temperature surface waters of PPH, it appears that the 3N trout spent their time in inferior habitat to the 2Ns, which were more frequently in cooler, well oxygenated water. However, the 3N fish may have been utilizing the littoral zones where predation risk was low or the surface of the pelagic zone where Daphnoid (an important food source for rainbow trout in these lakes) density was high [
71,
74], but so was predation [
75]. For example, previous studies in lakes showed fish spending more time in the pelagic zone tended to have reduced survival in predator-present but not predator-free lakes [
55,
75]. Thus, differences in the aerobic scope, endurance swimming capacity and behavior between 2N and 3N Blackwater rainbow trout probably result in different selection pressures which could have profound effects on the endurance–survival relationship.