Effect of Photoperiod and Transfer Time on Atlantic Salmon Smolt Quality and Growth in Freshwater and Seawater Aquaculture Systems
Abstract
1. Introduction
2. Materials and Methods
2.1. Fish Stock and Early Rearing Conditions before the Experiment
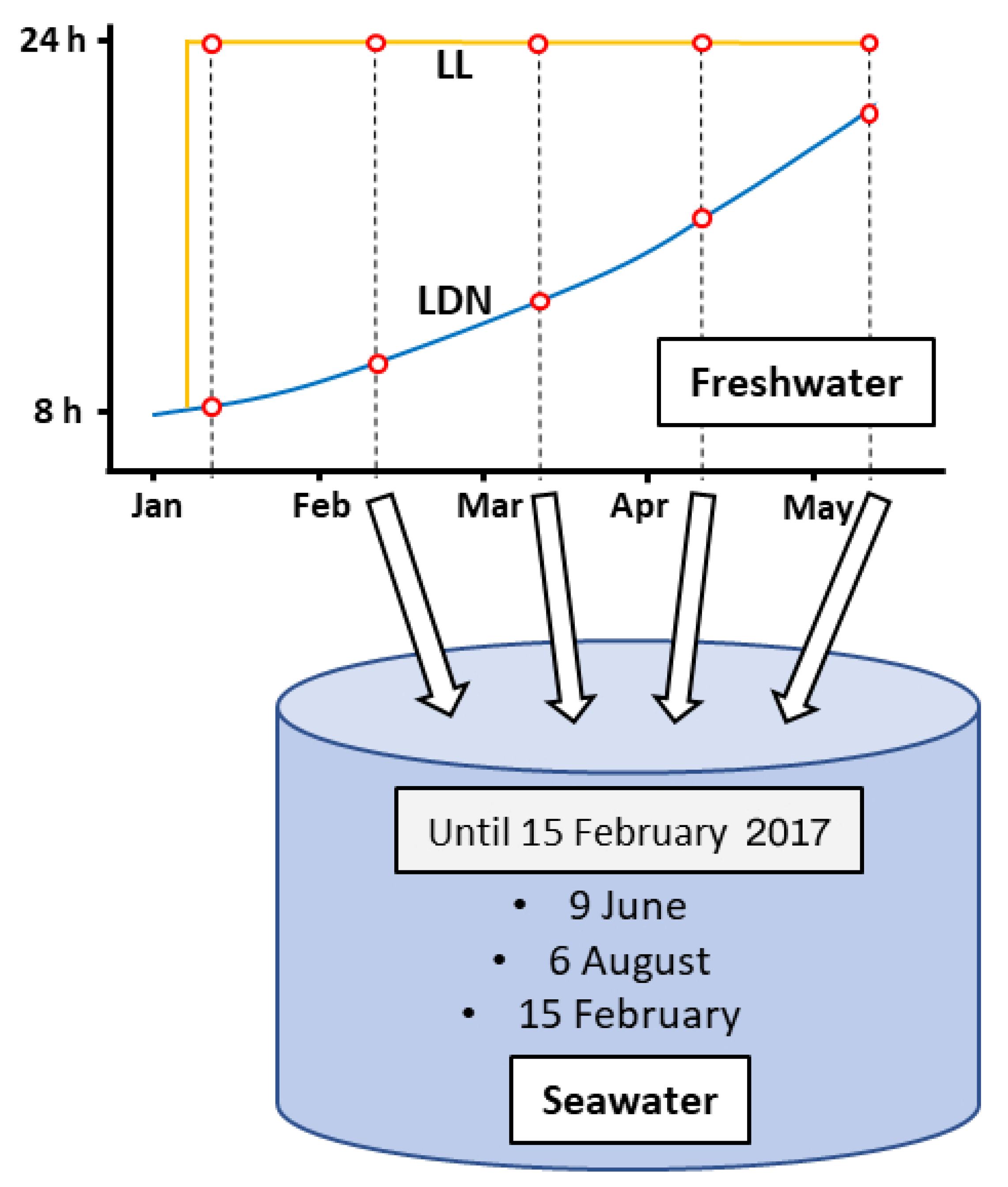
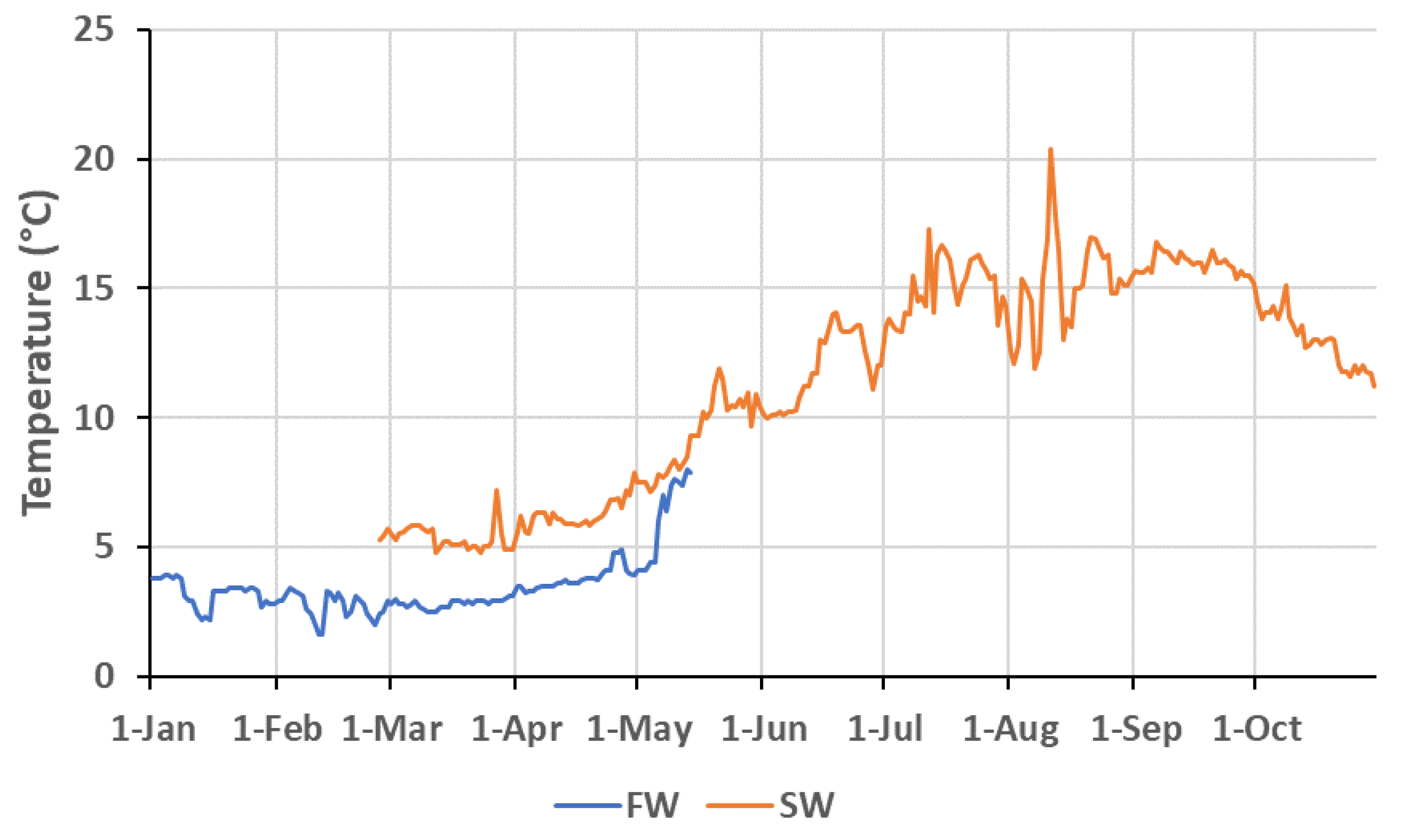
2.2. Experimental Design
2.3. Sampling Procedures and Analyses
2.4. Statistical Analyses
2.5. Ethic Statement
3. Results
3.1. During Smoltification in FW
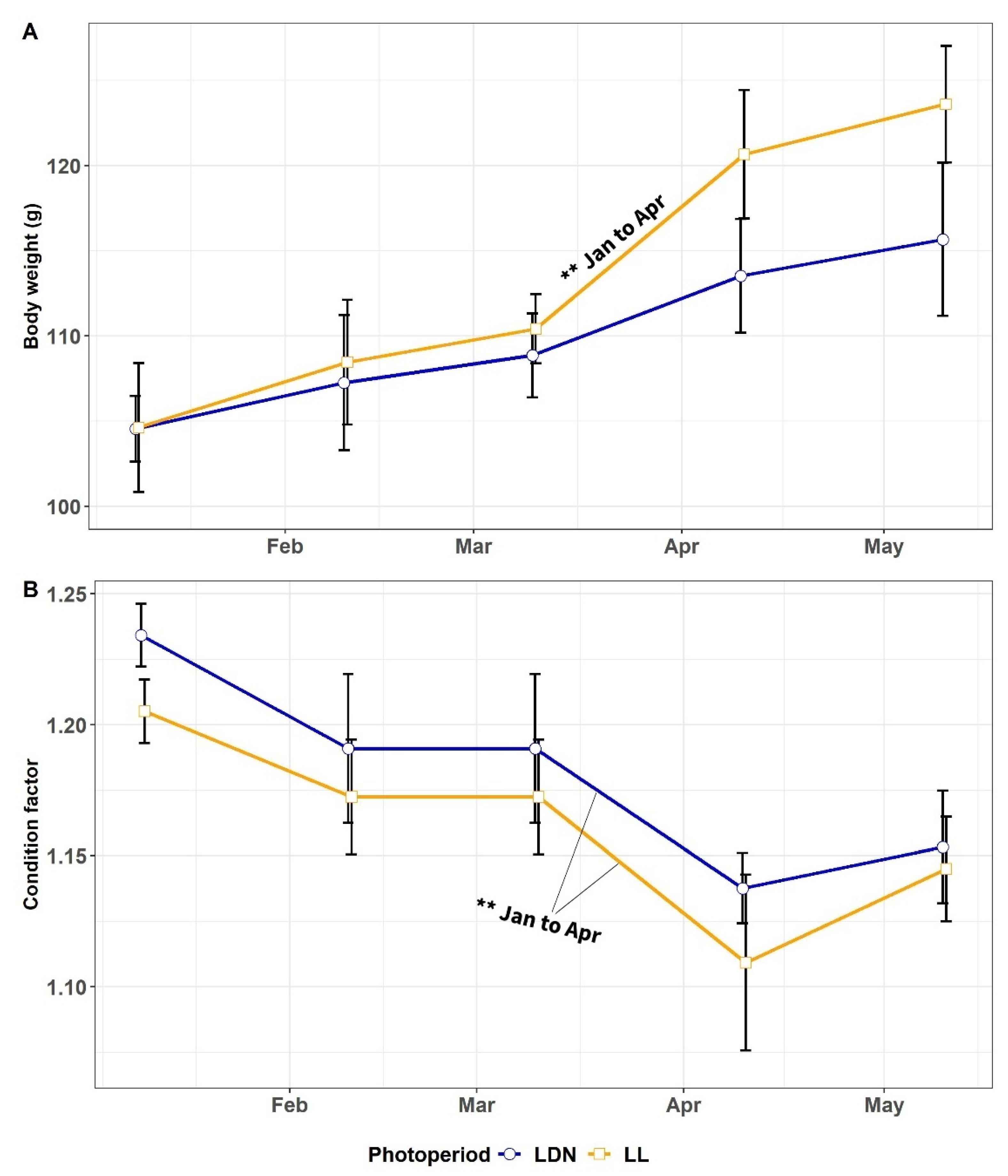
3.1.1. Body Weight and CF
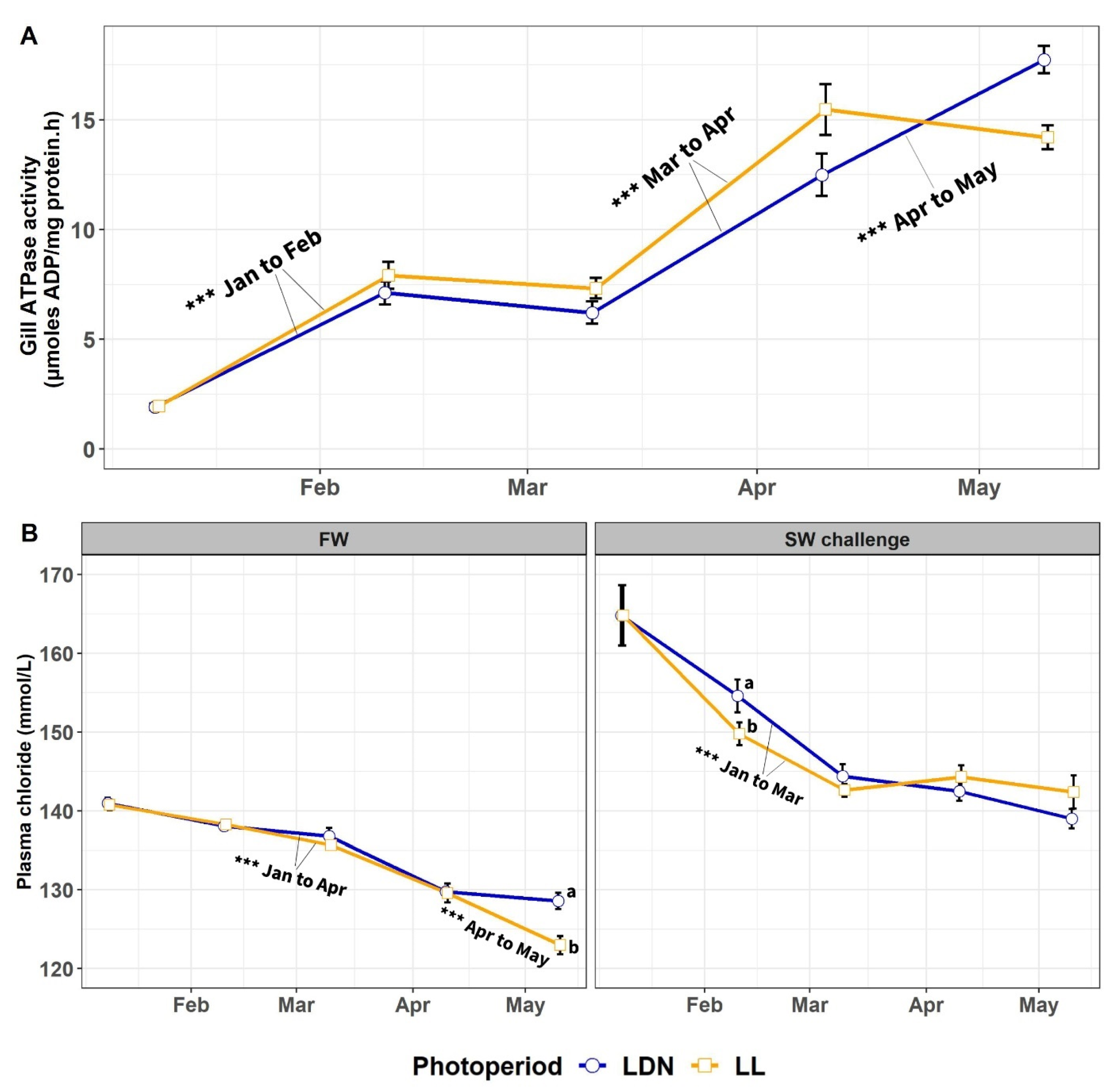
3.1.2. Gill NKA Activity and Plasma Chloride in FW and in 24-h SW Challenge
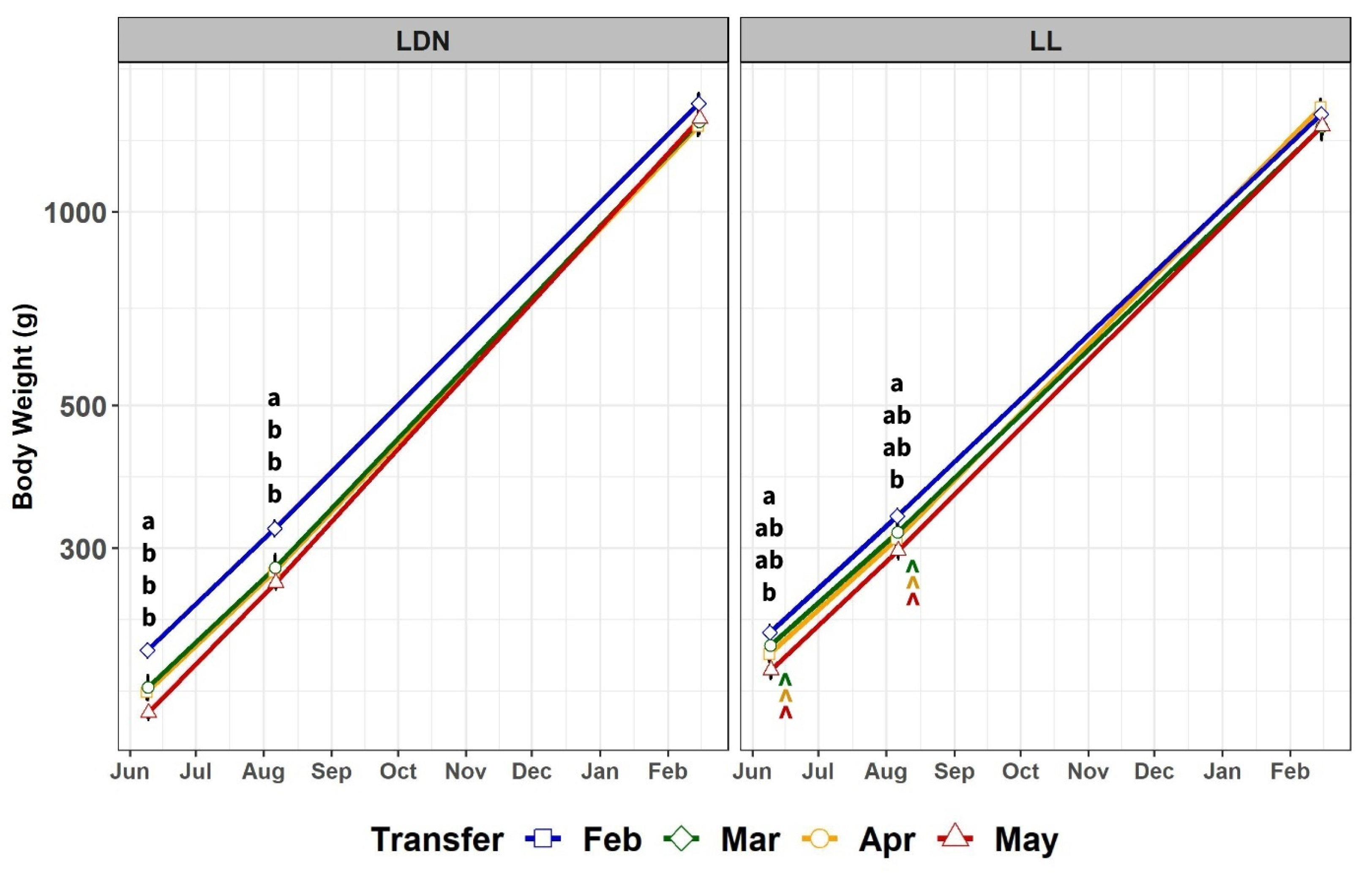
3.2. After Transfer to SW
3.2.1. Body Weight and Mortality
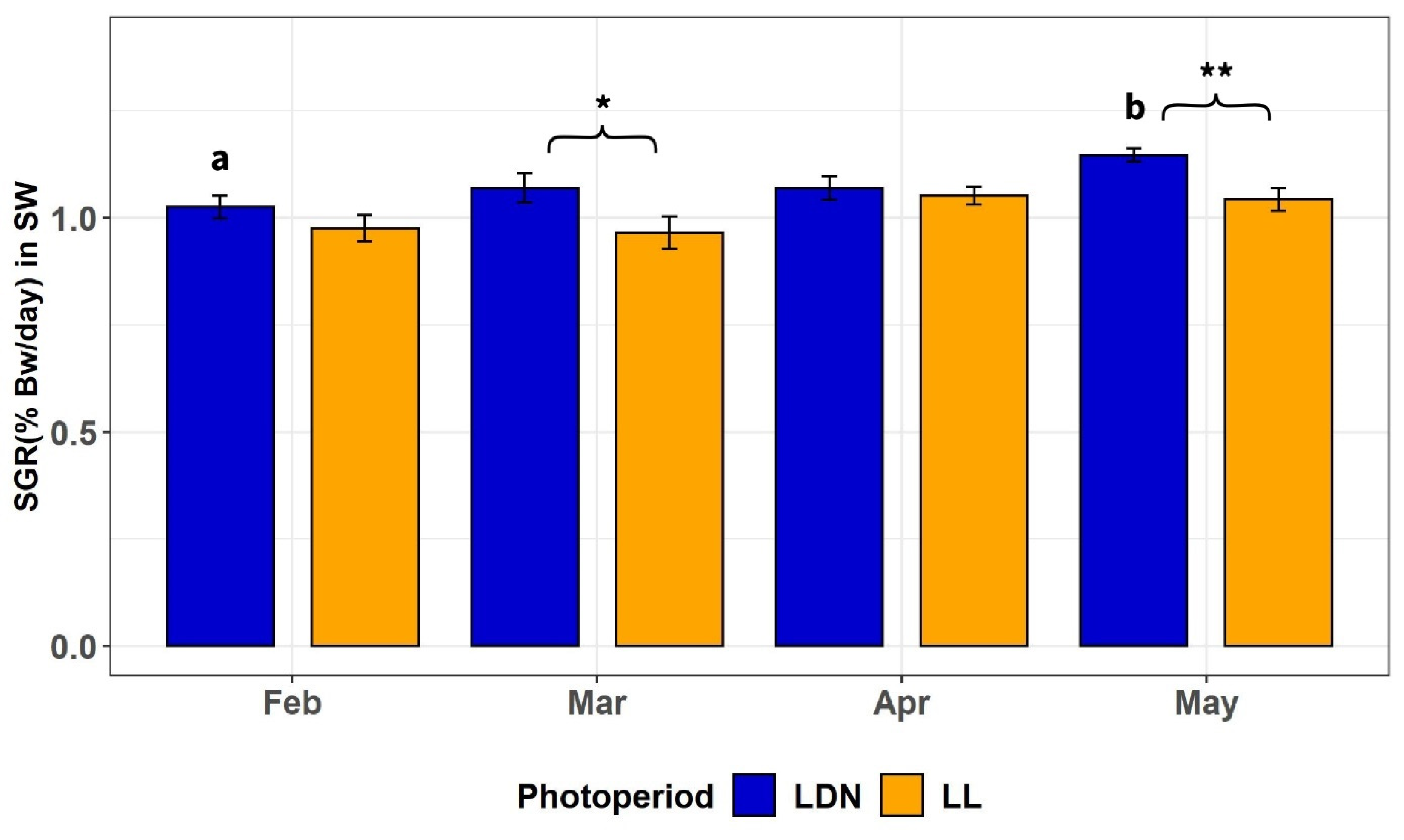
3.2.2. SGR of Individually Tagged Fish after all Periods in SW
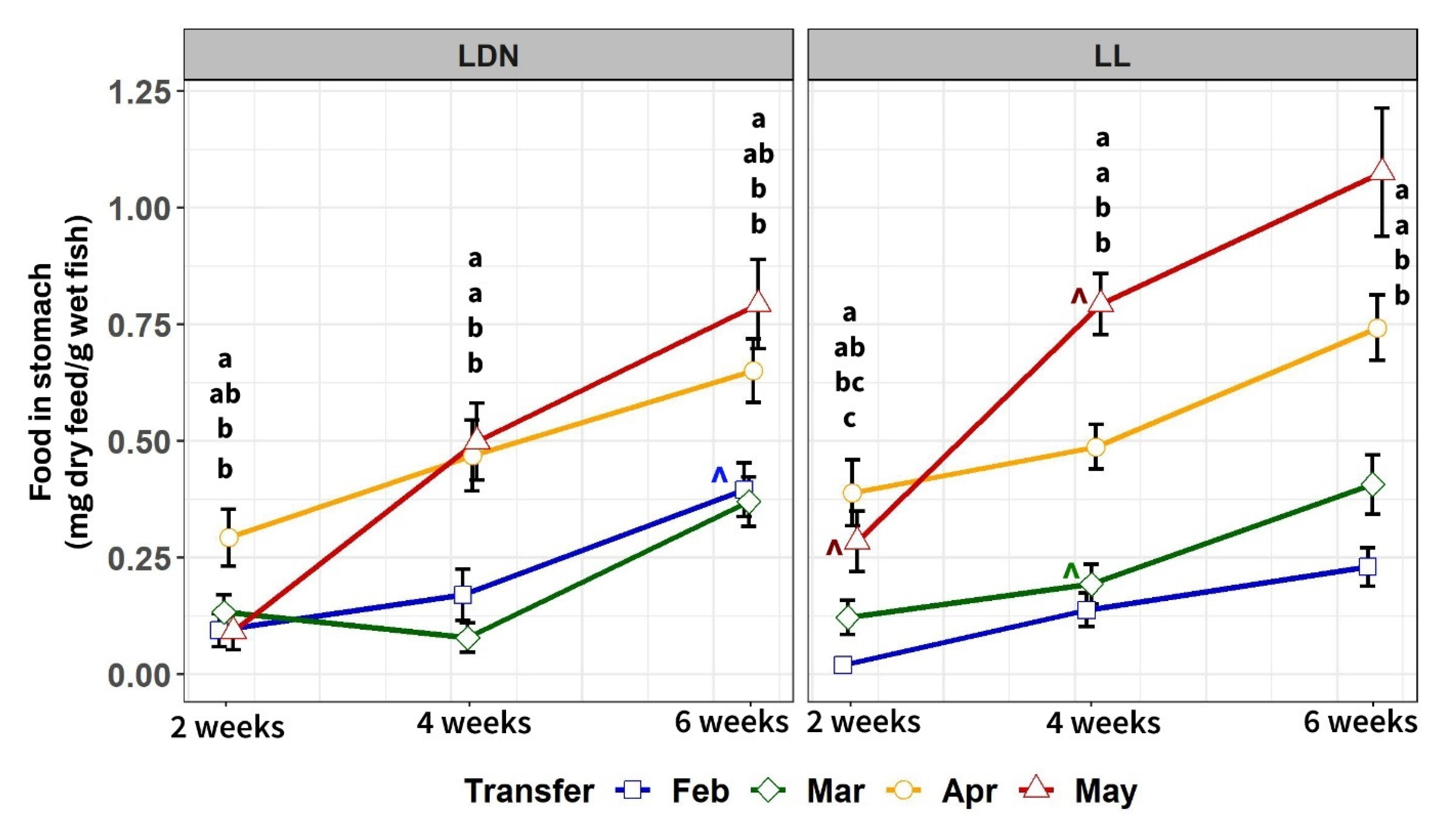
3.2.3. Food in Stomach after 2, 4 and 6 Weeks in SW
3.2.4. Size at Transfer to SW vs. Final Size at Slaughter
4. Discussion
4.1. Growth and Smoltification in Freshwater
4.2. Body Weight, Growth Rate, Mortality, and Feed Intake after Transfer to Seawater
4.3. Relationship between Weight at Transfer and Weight at Slaughter
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCormick, S.; Saunders, R.L. Preparatory physiological adaptations for marine life in salmonids: Osmoregulation, growth and metabolism. Am. Fish. Soc. Symp. 1987, 1, 211–229. [Google Scholar]
- Stefansson, S.O.; Björnsson, B.T.; Ebbesson, L.O.; McCormick, S.D. Smoltification. In Fish Larval Physiology; Finn, R., Kapoor, B.E., Eds.; Science Publishers: Enfield, NH, USA, 2008; pp. 639–681. [Google Scholar] [CrossRef]
- McCormick, S.D. Smolt Physiology and Endocrinology. Fish Physiol. 2012, 32, 199–251. [Google Scholar] [CrossRef]
- Björnsson, B.T.; Stefansson, S.O.; McCormick, S.D. Environmental endocrinology of salmon smoltification. Gen. Comp. Endocrinol. 2011, 170, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Stefansson, S.O.; Nilsen, T.O.; Ebbesson, L.O.E.; Wargelius, A.; Madsen, S.S.; Björnsson, B.T.; McCormick, S.D. Molecular mechanisms of continuous light inhibition of Atlantic salmon parr-smolt transformation. Aquaculture 2007, 273, 235–245. [Google Scholar] [CrossRef]
- Duston, J.; Saunders, R.L. The entrainment role of photoperiod on hypoosmoregulatory and growth-related aspects of smolting in Atlantic salmon (Salmo salar). Can. J. Zool. 1990, 68, 707–715. [Google Scholar] [CrossRef]
- McCormick, S.D.; Saunders, R.L.; Henderson, E.B.; Harmon, P.R. Photoperiod Control of Parr-Smolt Transformation in Atlantic Salmon (Salmo salar): Changes in salininity tolerance, Gill ATPase Activity, and Plasma Thyroid Hormones. Can. J. Fish. Aquat. Sci. 1987, 44, 1462–1468. [Google Scholar] [CrossRef]
- McCormick, S.D.; Björnsson, B.T.; Sheridan, M.; Eilerlson, C.; Carey, J.B.; O’Dea, M. Increased daylength stimulates plasma growth hormone and gill Na+, K+-ATPase in Atlantic salmon (Salmo salar). J. Comp. Physiol. B 1995, 165, 245–254. [Google Scholar] [CrossRef]
- McCormick, S.D.; Shrimpton, J.M.; Moriyama, S.; Björnsson, B.T. Effects of an advanced temperature cycle on smolt development and endocrinology indicate that temperature is not a zeitgeber for smolting in Atlantic salmon. J. Exp. Biol. 2002, 205, 3553–3560. [Google Scholar] [CrossRef]
- Shepherd, C.J.; Bromage, N.R. Intensive Fish Farming; Blackwell Scientific Publications: Oxford, UK, 1992; 404p. [Google Scholar]
- Handeland, S.O.; Stefansson, S.O. Photoperiod control and influence of body size on off-season parr-smolt transformation and post-smolt growth. Aquaculture 2001, 192, 291–307. [Google Scholar] [CrossRef]
- Arnesen, A.M.; Toften, H.; Agustsson, T.; Stefansson, S.O.; Handeland, S.O.; Björnsson, B.T. Osmoregulation, feed intake, growth and growth hormone levels in 0 + Atlantic salmon (Salmo salar L.) transferred to seawater at different stages of smolt development. Aquaculture 2003, 222, 167–187. [Google Scholar] [CrossRef]
- Björnsson, B.T.; Bradley, T.M. Epilogue: Past successes, present misconceptions and future milestones in salmon smoltification research. Aquaculture 2007, 273, 384–391. [Google Scholar] [CrossRef]
- Handeland, S.O.; Wilkinson, E.; Sveinsbø, B.; McCormick, S.D.; Stefansson, S.O. Temperature influence on the development and loss of seawater tolerance in two fast-growing strains of Atlantic salmon. Aquaculture 2004, 233, 513–529. [Google Scholar] [CrossRef]
- McCormick, S.D.; Cunjak, R.A.; Dempson, B.; O’Dea, M.F.; Carey, J.B. Temperature-related loss of smolt characteristics in Atlantic salmon (Salmo salar) in the wild. Can. J. Fish. Aquat. Sci. 1999, 56, 1649–1658. [Google Scholar] [CrossRef]
- Handeland, S.O.; Järvi, T.; Fernö, A.; Stefansson, S.O. Osmotic stress, antipredator behaviour, and mortality of Atlantic salmon (Salmo salar) smolts. Can. J. Fish. Aquat. Sci. 1996, 53, 2673–2680. [Google Scholar] [CrossRef]
- Handeland, S.O.; Imsland, A.K.; Nilsen, T.O.; Ebbesson, L.O.E.; Hosfeld, C.D.; Pedrosa, C.; Toften, H.; Stefansson, S.O. Osmoregulation in Atlantic salmon Salmo salar smolts transferred to seawater at different temperatures. J. Fish Biol. 2014, 85, 1163–1176. [Google Scholar] [CrossRef]
- McCormick, S.D. Methods for Nonlethal Gill Biopsy and Measurement of Na+, K+-ATPase Activity. Can. J. Fish. Aquat. Sci. 1993, 50, 656–658. [Google Scholar] [CrossRef]
- Talbot, C.; Higgins, P.J. A radiographic method for feeding studies on fish using metallic iron powder as a marker. J. Fish Biol. 1983, 23, 211–220. [Google Scholar] [CrossRef]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; SAGE: London, UK, 2019. [Google Scholar]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Kassambara, A. Ggpubr:“ggplot2” Based Publication Ready Plots. Available online: https://cran.r-project.org/web/packages/ggpubr/index.html (accessed on 14 April 2023).
- Hope, R.M. Rmisc: Ryan Miscellaneous. 2013. Available online: https://CRAN.R-project.org/package=Rmisc (accessed on 14 April 2023).
- Lenth, R.; Singmann, H.; Love, J. Emmeans: Estimated Marginal Means, Aka Least-Squares Means. 2018. Available online: https://github.com/rvlenth/emmeans (accessed on 14 April 2023).
- Pinheiro, J.; Bates, D.; DebRoy, S.; Sarkar, D.; Heisterkamp, S.; Van Willigen, B.; Maintainer, R. Package ‘Nlme.’ Linear and Nonlinear Mixed Effects Models. 2017. Available online: https://cran.r-project.org/web/packages/nlme (accessed on 14 April 2023).
- Endal, H.P.; Taranger, G.L.; Stefansson, S.O.; Hansen, T. Effects of continuous additional light on growth and sexual maturity in Atlantic salmon, Salmo salar, reared in sea cages. Aquaculture 2000, 191, 337–349. [Google Scholar] [CrossRef]
- Kråkenes, R.; Hansen, T.; Stefansson, S.O.; Taranger, G.L. Continuous light increases growth rate of Atlantic salmon (Salmo salar L.) postsmolts in sea cages. Aquaculture 1991, 95, 281–287. [Google Scholar] [CrossRef]
- Imsland, A.K.; Handeland, S.O.; Stefansson, S.O. Photoperiod and temperature effects on growth and maturation of pre- and post-smolt Atlantic salmon. Aquac. Int. 2017, 22, 1331–1345. [Google Scholar] [CrossRef]
- Stefansson, S.O.; Bjömsson, B.T.; Hansen, T.; Haux, C.; Taranger, G.L.; Saunders, R.L. Growth, parr–smolt transformation, and changes in growth hormone of Atlantic salmon (Salmo salar) reared under different photoperiods. Can. J. Fish. Aquat. Sci. 1991, 48, 2100–2108. [Google Scholar] [CrossRef]
- Björnsson, B.T.; Hemre, G.I.; Bjornevik, M.; Hansen, T. Photoperiod regulation of plasma growth hormone levels during induced smoltification of underyearling Atlantic salmon. Gen. Comp. Endocrinol. 2000, 119, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Imsland, A.K.D.; Roth, B.; Fjelldal, P.G.; Stefansson, S.O.; Handeland, S.; Mikalsen, B. The effect of continuous light at low temperatures on growth in Atlantic salmon reared in commercial size sea pens. Aquaculture 2017, 479, 645–651. [Google Scholar] [CrossRef]
- McCormick, S.D.; Moriyama, S.; Björnsson, B.T. Low temperature limits photoperiod control of smolting in Atlantic salmon through endocrine mechanisms. Am. J. Physiol. Integr. Comp. Physiol. 2000, 278, 1352–1361. [Google Scholar] [CrossRef]
- Handeland, S.O.; Imsland, A.K.; Stefansson, S.O. The effect of temperature and fish size on growth, feed intake, food conversion efficiency and stomach evacuation rate of Atlantic salmon post-smolts. Aquaculture 2008, 283, 36–42. [Google Scholar] [CrossRef]
- Fjelldal, P.G.; Schulz, R.; Nilsen, T.O.; Andersson, E.; Norberg, B.; Hansen, T.J. Sexual maturation and smoltification in domesticated Atlantic salmon (Salmo salar L.)–is there a developmental conflict? Physiol. Rep. 2018, 6, 1–18. [Google Scholar] [CrossRef]
- Pino Martinez, E.; Balseiro, P.; Pedrosa, C.; Haugen, T.S.; Fleming, M.S.; Handeland, S.O. The effect of photoperiod manipulation on Atlantic salmon growth, smoltification and sexual maturation: A case study of a commercial RAS. Aquac. Res. 2021, 52, 2593–2608. [Google Scholar] [CrossRef]
- Staurnes, M.; Sigholt, T.; Gulseth, O.A. Effects of seasonal changes in water temperature on the parr–smolt transformation of Atlantic salmon and anadromous Arctic char. Trans. Am. Fish. Soc. 1994, 123, 408–415. [Google Scholar] [CrossRef]
- Berge, Å.I.; Berg, A.; Fyhn, H.J.; Barnung, T.; Hansen, T.; Stefansson, S.O. Development of salinity tolerance in underyearling smolts of Atlantic salmon (Salmo salar) reared under different photoperiods. Can. J. Fish. Aquat. Sci. 1995, 52, 243–251. [Google Scholar] [CrossRef]
- Morgan, I.J.; Metcalfe, N.B. Deferred costs of compensatory growth after autumnal food shortage in juvenile salmon. Proc. R. Soc. B Biol. Sci. 2001, 268, 295–301. [Google Scholar] [CrossRef]
- Mortensen, A.; Damsgård, B. Compensatory growth and weight segregation following light and temperature manipulation of juvenile Atlantic salmon (Salmo salar L.) and Arctic charr (Salvelinus alpinus L.). Aquaculture 1993, 114, 261–272. [Google Scholar] [CrossRef]
- Stefansson, S.O.; Imsland, A.K.; Handeland, S.O. Food-deprivation, compensatory growth and hydro-mineral balance in Atlantic salmon (Salmo salar) post-smolts in sea water. Aquaculture 2009, 290, 243–249. [Google Scholar] [CrossRef]
- Handeland, S.O.; Berge, Å.I.; Björnsson, B.T.; Lie, Ø.; Stefansson, S.O. Seawater adaptation by out-of-season Atlantic salmon (Salmo salar L.) smolts at different temperatures. Aquaculture 2000, 181, 377–396. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pino Martinez, E.; Imsland, A.K.D.; Hosfeld, A.-C.D.; Handeland, S.O. Effect of Photoperiod and Transfer Time on Atlantic Salmon Smolt Quality and Growth in Freshwater and Seawater Aquaculture Systems. Fishes 2023, 8, 212. https://doi.org/10.3390/fishes8040212
Pino Martinez E, Imsland AKD, Hosfeld A-CD, Handeland SO. Effect of Photoperiod and Transfer Time on Atlantic Salmon Smolt Quality and Growth in Freshwater and Seawater Aquaculture Systems. Fishes. 2023; 8(4):212. https://doi.org/10.3390/fishes8040212
Chicago/Turabian StylePino Martinez, Enrique, Albert Kjartan Dagbjartarson Imsland, Anne-Camilla Diesen Hosfeld, and Sigurd Olav Handeland. 2023. "Effect of Photoperiod and Transfer Time on Atlantic Salmon Smolt Quality and Growth in Freshwater and Seawater Aquaculture Systems" Fishes 8, no. 4: 212. https://doi.org/10.3390/fishes8040212
APA StylePino Martinez, E., Imsland, A. K. D., Hosfeld, A.-C. D., & Handeland, S. O. (2023). Effect of Photoperiod and Transfer Time on Atlantic Salmon Smolt Quality and Growth in Freshwater and Seawater Aquaculture Systems. Fishes, 8(4), 212. https://doi.org/10.3390/fishes8040212






