Abstract
This research examined the role of nano curcumin (NC) on growth performances, body composition, and blood parameters of red tilapia (Oreochromis sp.) challenged with Aspergillus flavus. Fish (5.0 g ± 0.30) were randomly distributed in four equal groups (20 fish per pond in triplicates) and fed various concentrations of NC fortified with 0 (Control), 40 mg/kg (NC1), 50 mg/kg (NC2), and 60 mg/kg diet (NC3) of nano curcumin. After eight weeks of the feeding trial, the fish were challenged with A. flavus for 15 days, and the cumulative mortality was recorded. Fish fed with different concentrations of NC improved significantly (p < 0.05) the growth performances, feed utilization, and survival rate. There was no significant (p > 0.05) difference between NC2 and NC3 treatments. However, NC3 exhibited higher performances. Fish feed supplemented with NC decreased the mortality rate when challenged with A. flavus. Hence, dietary supplementation of NC enhanced the growth and health status of Oreochromis sp. and protected it from A. flavus infection. This study suggests the optimum inclusion level of NC is a 50–60 mg/kg diet.
Key Contribution:
Effect of nano curcumin on growth performance and health status of red tilapia challenged with Aspergillus flavus.
1. Introduction
Curcumin [(E, E)-1, 7-bis (4-hydroxy-3-methoxy-phenyl)-1, 6-heptadiene-3, 5-dione], of the ginger family, is a bis-α, β-unsaturated β-diketone [1]. It is a hydrophobic polyphenolic molecule and one of the main active components of turmeric (Curcuma longa) extract, used as a spice and food dye in food preparations [2]. In many nations, the powder of C. longa has long been applied as a folk medicine anti-infective agent [3,4]. Moreover, it has many health-promoting effects on fish health and immunity due to its robust biological actions, including antibacterial, antioxidant, anti-inflammatory, immune-modulating, appetite-inducing, and gastroprotective properties [2,5]. Despite curcumin’s benefits, there are a few drawbacks, including poor water solubility, inferior availability and metabolism, unbalanced molecular assembly, and insufficient absorption by the body that prevents it from being widely used [6,7]. However, curcumins in nanoparticle formula show better performances, such as aqueous medium dispersion and absorption, compared to the powder form [8]. Numerous studies have shown how adding nano-sized food supplements can boost productivity, health, immunity, and resistance to diseases or adverse issues of different fish species, e.g., rainbow trout [9], crucian carp [10], and tilapia fish [11]. The nanoscale resources can continue in the bloodstream for extended periods, with improved bioavailability [12]. Red tilapia is among selfsame few domesticated finfish classes that feedstuff on natural foods at squat trophic doses [13].
Fish suffer various types of diseases that occur by fungi. These fungi are opportunistic pathogens that can produce mycotoxins when the fish suffer stress due to another environmental cause (such as poor water quality, temperature fluctuations, or trauma) or pathogens (bacterial disease or parasites) [14,15,16,17]. For instance, fungi species such as Aspergillus flavus create aflatoxin, a poisonous secondary metabolite from polyketides [18]. Farmers commonly use antibiotics in the feed to avoid these complications such as hepatotoxicity, teratogenicity, and immunotoxicity; however, the overuse of antibiotics creates hazards that lead to resistance of harmful bacteria in the fish body. It is a problem for aquaculture, fish consumers, and the environment [1]. Based on the literature discussed earlier, nano curcumin might be a substitute for antibiotics in fish feed. Consequently, the existing plan was designed to assess the role of different concentrations of nano curcumin on growth indices, feed efficiency, body composition, and biochemical of red tilapia (Oreochromis sp.) challenged with Aspergillus flavus.
2. Materials and Methods
2.1. Curcumin Nanoparticles Synthesis
The materials used for curcumin nanoparticle synthesis were curcumin (purchased from Chemajet Comp., Cairo, Egypt) and Dichloromethane (acquired from Elgomhoreya pharmaceutical company, Cairo, Egypt). The solvent–antisolvent method described by [19], with some minor modifications, was used to synthesize the nano curcumin (NC) particles. Briefly, a syringe pump comprising antisolvent to manufacture NC utilizing dichloromethane as an organic solvent [20]. Firstly, the original curcumin solution was prepared in dichloromethane (5 mg/mL), positioned in a syringe (20 mL), and injected at a proportion of 10 mL/min into the deionized water (antisolvent), and stirred attractively (1000 g) for two hours. At that point, the vacuum-dried manufactured nanoparticles were cleaned. A Zeta sizer (Malvern Instruments, Zeta Potential Analyzer, Malvern, UK) was utilized to assess the NC measurement (Figure 1). Moreover, the EM (electron microscope) was also applied to assess the size of synthesized NC and its distribution (particle size 10–50 nm).

Figure 1.
The characterization of nano curcumin (NC) by the Electron Microscope.
2.2. Experimental Design and Diet
This experiment was carried out in a private fish farm situated in Ismailia Governorate, Egypt. It was conducted upon obtaining animal ethical approval from Suez Canal University (Ref. 68/2022).
After acclimatization, a total of 240 tilapia fingerlings (Av. Wt. 5.00 g ± 0.30) were stocked equally in 12 concrete tanks (1 m3 each), making four groups based on the feeding treatments. These were 40 mg of nanocurcumin/kg (T1), 50 mg of nanocurcumin/kg (T2), 60 mg of nanocurcumin/kg (T3), and the control (no additives, T0). The water quality parameters such as pH, water temperature, and dissolved oxygen were 7.63 ± 0.07 to 8.00 ± 0.07, 25.43 °C ± 0.03 to 26.47 °C ± 0.10, and 6.77 ± 0.11 to 7.03 ± 0.13 respectively during the study.
Table 1 shows the feed constituents and proximate composition of the diets used in this research. The diets were analyzed according to the AOAC protocol described by [21]. The feeding trial was continued for up to eight weeks or 56 days. Fish were fed to the satiation level three times (early morning, mid-afternoon, and late evening) per day.

Table 1.
Constituents and chemical composition of the investigational diets. NC0: control diet; NC1: nanocurcumin 40 mg/kg diet; NC2: nanocurcumin 50 mg/kg diet; NC3: nanocurcumin 60 mg/kg diet.
2.3. Growth and Feed Efficiency
The fish weight was taken fortnightly from the 4th week of the feeding trial. During each sampling, three groups of five fish were collected randomly from each replicate tank. The studied parameters were survival rate (SR), feed intake (FI), body weight gain (WG), specific growth rate (SGR), average daily gain (ADG), and feed conversion ratio (FCR) using the following formula described by [22].
WG (g) = Final weight (g) “−” Initial weight (g).
ADG (g/fish/day) = Final weight “−” Initial weight/Days of feeding trial.
SGR (%): [(ln final weight “−” ln initial weight) nos. of days] “×” 100.
SR (%): {(Final Number of Fish/Initial Number of Fish) “×” 100}.
FI (g/fish): The amounts of feed consumed throughout the investigational period/fish (g).
FCR: (Total Feed Consumption/Weight Gain of Fish).
2.4. Fish Body Analysis
Fish body composition analyses were performed using the standard methods [23] to determine the moisture, protein, lipid, and ash content in percentage. The proximate composition was assessed by oven-drying to a persistent weight at 70 °C and 105 °C, respectively, according to the AOAC guideline described by [21].
2.5. Blood Sampling
From each tank, three fish were anesthetized with tricaine methane sulfonate (MS-222) at a dose of 100 mg/L. Blood was collected via the caudal blood vessel. Heparinized tubes and tubes without heparin were employed to collect blood and serum, respectively. The serum was obtained by centrifugation of the blood at 3500× g for 15 min at 4 °C, then kept at −20 °C until further analysis.
2.6. Hematological and Biochemical Analysis of Blood
The method described by [24] was used to evaluate the RBCs (red blood cells), Hb (hemoglobin), MCV, MCH, and MCHC using an automated hematology analyzer (Hospitex Diagnostics, Sesto Fiorentino, Italy). The hemoglobin was immediately measured after being enriched with Drabkin’s solution by the cyanmethaemoglobin method [25]. Glucose was assessed by colorimetric assay [26]. The serum of total protein (TP) and albumin (ALB) was evaluated spectrophotometrically, while globulin (GLO) was measured by the subscription of total protein /albumin. The standard protocol of [27] was used to determine the liver enzyme activities, including ALP (alkaline phosphatase), AST (aspartate aminotransferase), and ALT (Alanine aminotransferase) of fish blood serum [28].
2.7. Aspergillus Flavus Infection and Sampling
The A. flavus isolated from Oreochromis sp. was provided by the Microbiological Unit of Fish Diseases Department, Animal Health Institute, Giza, Egypt. The method described by [29] prepared the A. flavus spores suspension. Briefly, the pathogen, A. flavus was cultured on SDA (Sabourauds dextrose agar) enriched with antibiotics (Streptomycin 100 ug/mL, and penicillin 100 UI/mL), and the culture was incubated at 25 °C for one week. For collecting conidial bulk, 20 mL of sterile distilled water (SDW) was supplemented to each dish and the suspension was compiled in sterile tubes. Then, the suspension was filtered with sterile gauze, and an erythrocyte counting chamber of a hemocytometer was applied to analyze and modify the conidial suspension (4 × 103 conidia/mL) in SDW. Then, at the end of the feeding trial, 20 tilapia fishes were selected randomly from each treatment that was inoculated with 0.2 mL of A. flavus (4 × 103 conidia/mL) by the I/P (Intraperitoneal injection with a sterile needle). The challenge period was 15 days, and the mortality rate was calculated during the challenge period.
2.8. Histopathological Examinations
At the end of the challenge period, the liver, intestine, spleen, and gills in tilapia fish were isolated for histopathological analysis. The tissues were collected in 10% neutral formalin for 24 h, and an ascending series of ethyl alcohol for dehydration, clearing, mounting, and staining with Hematoxylin and Eosin (H&E) stains, and examined by using the light microscope (Leica, Manchester, UK). It is noted that 10 microscopic fields of five slides obtained from five fish from each experimental group were examined.
According to [30], the histomorphometry for intestinal parameters was measured of five intestinal villi per group in the part of mid intestinal. The histomorphometry for intestinal parameters was explained in the following: villus length (from base to tip), villus width at the villus tip, the muscular fibers layer thickness, and villus width at the junction of the villus/crypt. Accomplished the analytics of computerized quantitative for the photomicrographs evaluated by a digital camera attached to a light microscope.
2.9. Statistical Analysis
Results were statistically explored by the ANOVA test (one-way analysis of variance) employed by SPSS version 26, SPSS Inc., Chicago, IL, USA. Duncan’s test was used to perform multiple comparisons to determine whether the differences between the four feeding treatments were significant at the 95% confidence level. Polynomial regression analysis was recommended for growth and feed indices to explore the linear effects of NC supplementation on the tested variables [31]. Differences were measured significantly at p < 0.05.
3. Results
3.1. Growth and Feed Efficacy
As in Table 2, the specific growth rate, weight gain, and feed conversion ratio were considerably (p < 0.05) greater in fish given various levels of NC compared to the un-supplemented one. The greatest values were noticed in the NC3 treatment. The survival rate of Oreochromis sp. was increased in NC-treated groups 88.33, 96.67, and 96.67% in NC1, NC2, and NC3, respectively, relative to the control (85%).

Table 2.
Growth parameters and survival rate of Red Tilapia (Oreochromis sp.) after 56 day-feeding trials.
3.2. Chemical Composition of Fish Body
The entire body composition of the tilapia fingerlings was affected by the addition of NC in terms of lipid, protein, and ash contents. Protein and ash contents were significantly (p < 0.05) greater in fish feed with NC-supplemented diets compared to the control. In contrast, the opposite was observed in lipid content that was significantly (p < 0.05) lower in NC-supplemented groups as relative to standard one (Table 3).

Table 3.
Body composition of Red Tilapia (Oreochromis sp.) after 56-day-feeding trial.
3.3. Hematological and Biochemical Analysis of Blood
The hemoglobin and RBCs disclosed (p < 0.05) augmented values significantly in fish given NC (Table 4). The ALT, AST, and glucose activities (p < 0.05) decreased significantly in all supplemented NC groups compared to the NC0 group. Meanwhile, all groups had similar ALP levels (p > 0.05). After 56 days of fish NC treatment, the supplementation of NC significantly (p < 0.05) augmented the serum protein fraction, including albumin, globulin, and total protein, relative to the control one. Fish given NC at different levels had inferior glucose levels (p < 0.05) than the control group. The MCH and MCHC were significantly (p < 0.05) increased by dietary NC inclusion, while MCV was increased significantly in all treated groups compared with the control one.

Table 4.
Biochemical indices of red tilapia (Oreochromis sp.) after 56-day-feeding trial.
3.4. The Survival Rate after the A. flavus Challenge
As depicted in Figure 2, fish changed with A. flavus began to die on the 3rd day post-challenge. The infected fish displayed marks of hemorrhages in various portions of the exterior body surface with a detachment of scales and enlarged mucus secretions. A. flavus was re-isolated from the interior tissues of these diseased fish. Feeding infected fish with NC in their diets demonstrated greater dose-dependent (p < 0.05) survival rates than the uninfected group.
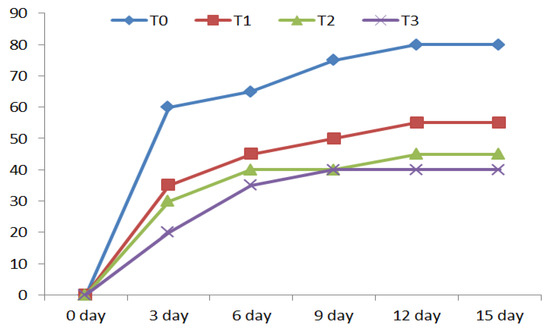
Figure 2.
Cumulative mortality (%) of Red Tilapia (Oreochromis sp.) at days post-challenge.
3.5. Histopathological Examination
3.5.1. Hepatic Tissues
Photomicrography of liver tissues in Red Tilapia (Oreochromis sp.) (Figure 3a–d); NC0 group was fish exposed to A. flavus and not fed on NC, show the inflammatory cells, and many vacuoles in the structure of hepatic tissues, endothelium which around the hepatic central had some lymphatic distortion that caused by A. flavus challenge (Figure 3a). The liver tissue from the NC1 group represents moderately conjugated blood within the central hepatic vein with the endothelial membrane. These tissue of hepatocytes, vacuoles, and moderate inflammatory cells appeared within the hepatic sinusoids (Figure 3b). The hepatic tilapia tissues from the NC2 group show moderate amelioration of the hepatocytes around the central vein, lining with the normal endothelium, blood sinusoid, inflammatory cells, and absence of the vacuoles (Figure 3c). The liver tissue from the NC3 group represents highly marked amelioration of the central hepatic vein and normal inflammatory cells that appeared between hepatocytes and hepatic blood sinusoids (Figure 3d).
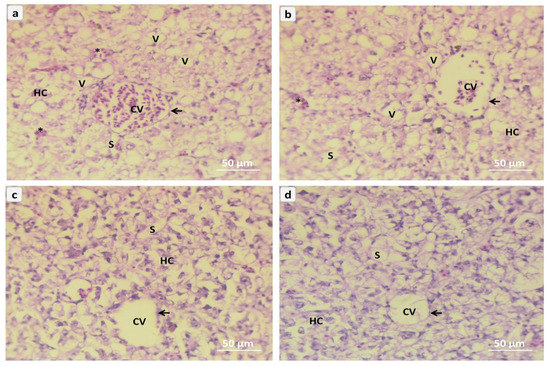
Figure 3.
Representative micrograph of liver sections of red tilapia (Oreochromis sp.) challenged with Aspergillus flavus, (a) → NC0, (b) → NC1, (c) → NC2, (d) → NC3. NC0: control diet; NC1: nanocurcumin 40 mg/kg diet; NC2: nanocurcumin 50 mg/kg diet; NC3: nano curcumin 60 mg/kg diet. CV = central hepatic vein, arrows = endothelium layer, HC = Hepatocytes, S = hepatic sinusoid, asterisk = inflammatory cells, and V = vacuoles. (H&E, Bar = 50 µm).
3.5.2. Intestinal Histomorphology
Photomicrography of intestinal tissues in red tilapia (Oreochromis sp.) (Figure 4, and Table 5); NC0 group was fish exposed to A. flavus and not fed on NC, show distorted intestinal structure in the layer of serosa, layers of mucosal fibers, the layer of the submucosa, layer of mucosa, villi which lined with simple columnar epithelial tissues with mucous gland, lamina propria, and rending the apical portion of intestinal villi that caused by A. flavus challenge (Figure 4a,b, and Table 5). The intestine tissue from the NC1 (T1) group represents a mild intact apical portion of intestinal villi (Figure 4c,d, and Table 5). The intestinal tissue from the NC2 group shows moderate amelioration of the intestinal structure, smooth the apical portion of intestinal villi, and lamina propria attached with the villi bases (Figure 4e,f, and Table 5). The intestinal tissue from the NC3 group represents a markedly significant enhancement of the intestinal architecture as in the smooth apical portion of intestinal villi and clearance of the lamina propria (Figure 4g,h, and Table 5).
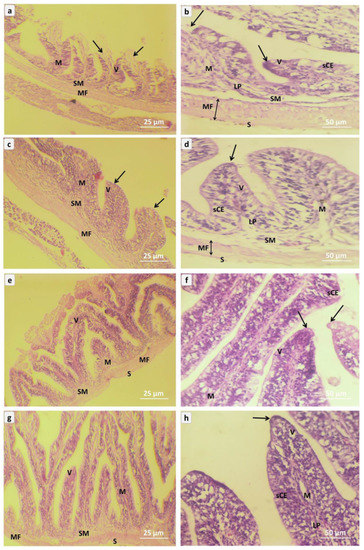
Figure 4.
Representative micrograph of intestinal sections of red tilapia (Oreochromis sp.) challenged with Aspergillus flavus, (a,b) → NC0, (c,d) → NC1, (e,f) → NC2, (g,h) → NC3. NC0: control diet; NC1: nanocurcumin 40 mg/kg diet; NC2: nanocurcumin 50 mg/kg diet; NC3: nano curcumin 60 mg/kg diet. S = layer of serosa, MF = layers of mucosal fibers, SM = layer of the submucosa, M = layer of mucosa, V = villi, sCE = simple columnar epithelial tissues, LP = lamina propria, arrows = apical portion of intestinal villi. (H&E, Bar = 25 µm and 50 µm).

Table 5.
Intestinal parameters (Mean ± SE, n = 5) for villi length, villi width, muscular layer thickness, and absorption zone of red tilapia (Oreochromis sp.) challenged with Aspergillus flavus, at the end of the experiment.
3.5.3. Splenic Tissues
Figure 5 illustrates the photomicrography of splenic tissues in red tilapia (Oreochromis sp.); the NC0 group was fish exposed to A. flavus and not fed on NC, showing distortion of the splenic capsule, and numerous of the melano inflammatory macrophages that caused by A. flavus challenge (Figure 5a). The spleen tissue from the NC1 group represents moderate inflammatory macrophages and mild distortion of splenic tissues (Figure 5b). The spleen tilapia tissues from the NC2 group show moderate amelioration of the spleen in the splenic trabecular tissues (Figure 5c). The spleen tissue from the NC3 group represents highly marked amelioration of the splenic tissues and normal melano splenic inflammatory macrophages (Figure 5d).
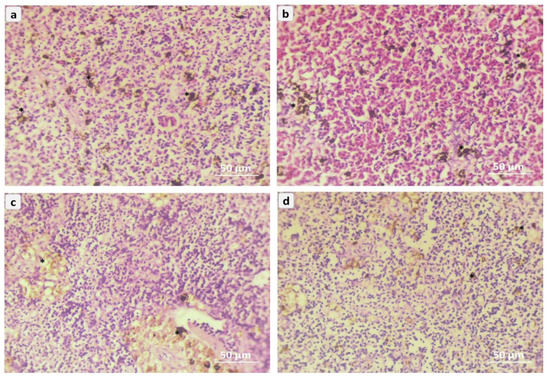
Figure 5.
Representative micrograph of splenic sections of red tilapia (Oreochromis sp.) challenged with Aspergillus flavus, (a) → NC0, (b) → NC1, (c) → NC2, (d) → NC3. NC0: control diet; NC1: nanocurcumin 40 mg/kg diet; NC2: nanocurcumin 50 mg/kg diet; NC3: nano curcumin 60 mg/kg diet. Asterisks = melano inflammatory macrophages in between splenic trabecular tissues. (H&E, Bar = 50 µm).
3.5.4. Gill Tissues
The photomicrography of gill tissues in red tilapia (Oreochromis sp.) is given in Figure 6; the NC0 group was fish exposed to A. flavus and not fed on NC, showing the warping of secondary filament, and vacuole in gill secondary filament as edema formation that affects the respiratory mechanism that caused by A. flavus challenge (Figure 6a). The gill tissue from the NC1 group represents moderate dilation of the primary lamella and deletion of some secondary filaments (Figure 6b). The gill tissue from the NC2 group shows moderate ameliorative of the gill epithelium structures for primary lamella and secondary filaments (Figure 6c). The gill tissue from the NC3 group represents highly marked amelioration of the primary epithelium of gill lamella and secondary filament (Figure 6d).
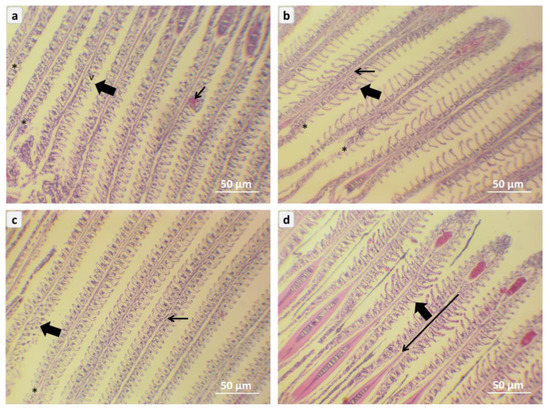
Figure 6.
Representative micrograph of gill sections of red tilapia (Oreochromis sp.) challenged with Aspergillus flavus, (a) → NC0, (b) → NC1, (c) → NC2, (d) → NC3. NC0: control diet; NC1: nanocurcumin 40 mg/kg diet; NC2: nanocurcumin 50 mg/kg diet; NC3: nano curcumin 60 mg/kg diet. Thin arrows = primary lamella, Thick arrows = secondary filaments. (H&E, Bar = 50 µm) V = vacuoles, and Asterisks = deletion of some secondary filaments.
4. Discussion
The existing research outcomes validated that the inclusion of different concentrations of NC powder in the fish diet improved the fish growth performance and health status. The trend of performance was NC3 > NC2 > NC1 > NC0, probably due to the attributes of NC, which is identified as an anti-inflammatory agent [32,33]. As illustrated in this research, the higher dose showed a significantly (p < 0.05) higher performance. This statement is advocated in various studies where a different dose of NC powder was used as a feed additive to the different fish species [2,11,33]. The current findings will serve as a new potential study on the tilapia fingerlings that NC improved the growth, feed competence, survival rate, and health status. It was more precisely understood when the treated tilapia fingerlings were challenged with the A. flavus pathogen.
As clarified in our data, the upper doses of NC powder exhibited greater growth performance and feed utilization of red tilapia. These results were advocated in the previous study of Nile Tilapia [17]. The origin of nano curcumin is turmeric, a polyphenolic molecule that acts as a growth promoter for perfect intestinal bacteria (similar to a postbiotic), potentially inhibiting the growth of fungal and microbial pathogens [8,9,34]. The feed efficiency (FI and FCR) was enhanced in fish fed with dietary NC, probably due to its ability to improve feed palatability. A fish feed with supplementation of natural curcumin and its nano form has an intrinsic outreach performance on freshwater fish, e.g., in rainbow trout [9,35], crucian carp [36], grass carp [37], common carp [38,39], Nile tilapia [11,40], and Mozambique tilapia [41]. The existing investigation notified the recital trend of growth performance, feed competence and survival rate of red tilapia were NC3 ≥ NC2 > NC1 > NC0. There was no significant alteration among NC2 and NC3 treatments, although, the treatment NC3 (60 mg nano curcumin/kg of diet) showed the highest efficacy after 56 days of feeding trial. This result was very much similar to a previous study [10]. Dietary inclusion of NC significantly increased fish body protein and ash content after eight weeks of feeding period with a simple reduction in body lipid levels. Recent different investigations found that long-term curcumin feeding significantly increased the ash and protein contents of Nile tilapia muscles [34,42]. A healthy diet improved the ash levels of the dorsal muscles and the whole body, while decreasing the lipid amounts in juvenile gibel carp as evidenced by [43]. These findings confirmed data on growth performance with NC diets.
The haemato–biochemical parameters are critical in determining aquatic animal health. All of the haemato–biochemical parameters for red tilapia used in this study were within normal ranges, according to the previous study conducted by [44]. Blood variables are significant indicators for assessing fish health, nutritional status, and capability to acclimate to their surroundings [45,46]. The consequences showed that RBC counts and Hb levels were significantly greater in fish that received NC than in the untreated group. These findings demonstrated that alimentary NC had a constructive effect on erythrogram indices in Red Tilapia without anaemia signs. This feature might be associated with the NC that can stimulate chemosynthesis and erythropoiesis, thus implying improved fish health. These verdicts are consistent with earlier investigations, which found that including curcumin in the diet improves haematological parameters in various fish species [9,40].
The levels of AST and ALT are important aminotransferases in hepatocytes that reflect hepatic health [47]. The findings imply that the fish in the current trial were not stressed. In previous research, [48] validated substantial reductions in serum ALT and AST activities detected in fish that received curcumin in their diets. Moreover, [47], informed that ALT and AST diminished in P. vannamei fed various levels of NC supplements relative to the un-supplemented group, principally at the management of a 150 mg NC/kg diet. Additionally, significant reductions in glucose levels were evidenced in Red tilapia given NC diets relative to the control group. Similar to our data, fish diets enriched with curcumin showed lower glucose than un-treated [41]. The authors proposed that curcumin might increase glucose consumption and thus enhance the glycogenesis process, finally, lowering blood glucose levels.
Blood protein fraction such as total proteins (TP), which is composed of albumin and globulin, is frequently dignified as a pointer of aquatic animal immune and health profiles [49], and its elevation indicates a more robust innate immune eminence [50]. In addition, serum immunoglobulins are an important constituent of the humoral immune scheme in vertebrates. They are important in immune processes such as opsonization, phagocytosis, and neutralizing toxins, viruses, and pathogenic bacteria in the host body [50,51]. As mentioned in our data, there are significant escalations in blood proteins such as TP, ALB, and GLO levels in fish that received NC, which is connected with the induction of humoral immunity in Red tilapia. A similar study [52] described that P. vannamei fed NC-enriched diets had greater TP and ALB levels than the control group. Accumulative reports [9,11] indicated that curcumin has been established to favorably impact the non-specific immunity of various fish classes. Previously, curcumin administration boosted fish immunity and health by stimulating neutrophils and macrophages [53]. Additionally, curcumin stimulates cytokine synthesis, which plays important role in immune response regulation [54]. The addition of NC led to an improvement in the levels of glucose. These outcomes are consistent with the reports of [55], who stated vast escalations of glucose in fish exposed to inferior environmental issues. Results show that the serum levels of MCH and MCHC were affected by the addition of NC to Red Tilapia fish, while MCV was not influenced by dietary NC inclusion.
Several reports have found that NC significantly improved blood erythrogram indices in various fish classes [5,8,17]. Clinical marks observed in red tilapia after infection with A. flavus were following the previous study [14]. The investigational injection of Red Tilapia with A. flavus revealed that it is greatly pathogenic, with 80% increasing mortality in the challenged control group within 15 days. The challenge test with Aspergillus flavus of Oreochromis sp. revealed a decreasing trend in mortality rates (i.e., 80%, 55%, 45%, and 40% in control, NC1, NC2, and NC3 groups, respectively). The present results are consistent with the previous study on rainbow trout (Oncorhynchus mykiss) conducted by [56]. By boosting the amount and activity of host glutathione peroxidase, the fish given diets enriched with NC decreased the cytopathic impact, mortality rate, and cellular mortality of SHK-1 and intracellular bacterial capacity [57]. The survivalist percentages of infected fish given NC demonstrated the compound’s shielding effects alongside A. flavus infection [48,49,54]. This histopathological study noticed the distortion according to A. flavus exposure and the important role of the different levels of nano-curcumin in the increased immune resistance of red tilapia (Oreochromis sp.) challenged with A. flavus infection. The NC3 group showed more significantly enhanced hepatopancreatic tissues, intestinal layers, spleen structure, and gill tissues.
5. Conclusions
Including dietary NCs in different concentrations in the Red tilapia diet improved its growth performance, feed utilization, survival rate, and disease resistance against A. flavus infection. These results indicate that nano curcumin can be considered a beneficial dietary supplement for Red tilapia at optimal inclusion levels of 50–60 mg/kg diet.
Author Contributions
Conceptualization, E.-S.H.E., M.B.M., S.S. and Y.M.A.E.-A.; methodology, B.A.A., M.B.M. and S.b.I.; software, S.D.J. and M.E.S.; validation, E.-S.H.E., S.D.J., M.E.S., M.E.H.E. and B.A.A.; formal analysis, N.N.B.A.E.-H.; investigation, B.A.A., S.b.I., W.K.B. and S.D.J.; histological preparations and examinations, Y.M.A.E.-A.; resources, M.P., F.S.A.; data curation, M.E.A.E.-H., M.E.H.E. and W.K.B.; writing—original draft preparation, N.N.B.A.E.-H. and M.E.A.E.-H.; writing—review and editing, M.B.M., S.b.I., E.-S.H.E. and M.E.A.E.-H.; visualization, N.N.B.A.E.-H. and M.E.A.E.-H.; funding acquisition, M.P. and F.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
The current trial received no external funding.
Institutional Review Board Statement
This experiment was implemented in a private fish farm in Ismailia Governorate, Egypt, upon receiving animal ethical approval from the Suez Canal University (Ref. 68/2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data of this research are available upon request.
Acknowledgments
Authors thank their respective universities and institutes for their support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Eissa, E.-S.H.; Baghdady, E.S.; Gaafar, A.Y.; El-Badawi, A.A.; Bazina, W.K.; Al-Kareem, O.M.A.; El-Hamed, N.N.B.A. Assessing the Influence of Dietary Pediococcus acidilactici Probiotic Supplementation in the Feed of European Sea Bass (Dicentrarchus labrax L.) (Linnaeus, 1758) on Farm Water Quality, Growth, Feed Utilization, Survival Rate, Body Composition, Blood Biochemical Parameters, and Intestinal Histology. Aquac. Nutr. 2022, 2022, 5841220. [Google Scholar] [CrossRef]
- Alagawany, M.; Farag, M.R.; Abdelnour, S.A.; Dawood, M.A.; Elnesr, S.S.; Dhama, K. Curcumin and its different forms: A review on fish nutrition. Aquaculture 2021, 532, 736030. [Google Scholar] [CrossRef]
- Zheng, B.; Peng, S.; Zhang, X.; McClements, D.J. Impact of delivery system type on curcumin bioaccessibility: Comparison of curcumin-loaded nanoemulsions with commercial curcumin supplements. J. Agric. Food Chem. 2018, 66, 10816–10826. [Google Scholar] [CrossRef]
- Zhou, C.; Huang, Z.; Zhou, S.; Hu, J.; Yang, R.; Wang, J.; Wang, Y.; Yu, W.; Lin, H.; Ma, Z. The Impacts of Dietary Curcumin on Innate Immune Responses and Antioxidant Status in Greater Amberjack (Seriola dumerili) under Ammonia Stress. J. Mar. Sci. Eng. 2023, 11, 300. [Google Scholar] [CrossRef]
- Abdel-Ghany, H.M.; El-Sisy, D.M.; Salem, M.E.-S. A comparative study of effects of curcumin and its nanoparticles on the growth, immunity and heat stress resistance of Nile tilapia (Oreochromis niloticus). Sci. Rep. 2023, 13, 2523. [Google Scholar] [CrossRef]
- Chen, M.N.; Yue, D.Y.; Liu, H.; Yang, Y.; Yu, H. Effects of Dietary Nano-Curcumin Supplementation on Growth Performance, Glucose Metabolism, and Endoplasmic Reticulum Stress in Juvenile Largemouth Bass. New Prog. Eff. Funct. Feed Addit. Mar. Aquat. Anim. 2022, 9, 924569. [Google Scholar] [CrossRef]
- More, S.; Pawar, A. Brain Targeted Curcumin Loaded Turmeric Oil Microemulsion Protects Against Trimethyltin Induced Neurodegeneration in Adult Zebrafish: A Pharmacokinetic and Pharmacodynamic Insight. Pharm. Res. 2023, 40, 675–687. [Google Scholar] [CrossRef]
- El Basuini, M.F.; Zaki, M.A.; El-Hais, A.M.; Elhanafy, M.G.; El-Bilawy, E.H.; Zaineldin, A.I.; Abdel-Aziz, M.F.; Abouelsaad, I.A.; El-Ratel, I.T.; Mzengereza, K. Microbial, immune and antioxidant responses of Nile tilapia with dietary nano-curcumin supplements under chronic low temperatures. Aquac. Fish. 2022. [Google Scholar] [CrossRef]
- Yonar, M.E.; Yonar, S.M.; İspir, Ü.; Ural, M.Ş. Effects of curcumin on haematological values, immunity, antioxidant status and resistance of rainbow trout (Oncorhynchus mykiss) against Aeromonas salmonicida subsp. achromogenes. Fish Shellfish Immunol. 2019, 89, 83–90. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Eissa, E.-S.H.; Tawfik, W.A.; Abd Elnabi, H.E.; Saadony, S.; Bazina, W.K.; Ahmed, R.A. Dietary curcumin nanoparticles promoted the performance, antioxidant activity, and humoral immunity, and modulated the hepatic and intestinal histology of Nile tilapia fingerlings. Fish Physiol. Biochem. 2022, 48, 585–601. [Google Scholar] [CrossRef]
- Mahmoud, H.K.; Al-Sagheer, A.A.; Reda, F.M.; Mahgoub, S.A.; Ayyat, M.S. Dietary curcumin supplement influence on growth, immunity, antioxidant status, and resistance to Aeromonas hydrophila in Oreochromis niloticus. Aquaculture 2017, 475, 16–23. [Google Scholar] [CrossRef]
- Nair, M.; Jayant, R.D.; Kaushik, A.; Sagar, V. Getting into the brain: Potential of nanotechnology in the management of NeuroAIDS. Adv. Drug Deliv. Rev. 2016, 103, 202–217. [Google Scholar] [CrossRef] [PubMed]
- Klahan, R.; Yuangsoi, B.; Whangchai, N.; Ramaraj, R.; Unpaprom, Y.; Khoo, K.S.; Deepanraj, B.; Pimpimol, T. Biorefining and biotechnology prospects of low-cost fish feed on Red tilapia production with different feeding regime. Chemosphere 2023, 311, 137098. [Google Scholar] [CrossRef] [PubMed]
- A Abd El Ghany, N.; S. Elias, N. A Risk Assessment of Fungal Infection with Aspergillus flavus in Oreochromis niloticus through a Laboratory-Acquired Infection. Zagazig Vet. J. 2014, 42, 91–103. [Google Scholar] [CrossRef]
- El-Deen, A.; Osman, H.; Zaki, M. Mass mortality in cultured Nile tilapia Oreochromis niloticus in Kafr El-Sheikh Province, Egypt due to saprolegniosis with emphasis on treatment trials. J. Biol. Sci. 2018, 18, 39–45. [Google Scholar] [CrossRef]
- El-Tawab, A.; El-Hofy, F.; Moustafa, E.; Halawa, M. Insight into isolation, identification and antimicrobial sensitivity of some moulds isolated from fresh water fishes. Adv. Anim. Vet. Sci 2020, 8, 174–182. [Google Scholar]
- Eissa, E.S.H.; Ezzo, O.H.; Khalil, H.S.; Tawfik, W.A.; El-Badawi, A.A.; Abd Elghany, N.A.; Mossa, M.I.; Hassan, M.M.; Hassan, M.M.; Eissa, M.E. The effect of dietary nanocurcumin on the growth performance, body composition, haemato-biochemical parameters and histopathological scores of the Nile tilapia (Oreochromis niloticus) challenged with Aspergillus flavus. Aquac. Res. 2022, 53, 6098–6111. [Google Scholar] [CrossRef]
- Ferreira, F.D.; Mossini, S.A.G.; Ferreira, F.M.D.; Arrotéia, C.C.; da Costa, C.L.; Nakamura, C.V.; Machinski Junior, M. The inhibitory effects of Curcuma longa L. essential oil and curcumin on Aspergillus flavus link growth and morphology. Sci. World J. 2013, 2013, 343804. [Google Scholar] [CrossRef]
- Kakran, M.; Sahoo, N.G.; Tan, I.-L.; Li, L. Preparation of nanoparticles of poorly water-soluble antioxidant curcumin by antisolvent precipitation methods. J. Nanopart. Res. 2012, 14, 1–11. [Google Scholar] [CrossRef]
- Carvalho, D.d.M.; Takeuchi, K.P.; Geraldine, R.M.; Moura, C.J.d.; Torres, M.C.L. Production, solubility and antioxidant activity of curcumin nanosuspension. Food Sci. Technol. 2015, 35, 115–119. [Google Scholar] [CrossRef]
- Munir, M.B.; Hashim, R.; Manaf, M.S.A.; Nor, S.A.M. Dietary prebiotics and probiotics influence the growth performance, feed utilisation, and body indices of snakehead (Channa striata) fingerlings. Trop. Life Sci. Res. 2016, 27, 111. [Google Scholar] [CrossRef] [PubMed]
- Fath El-Bab, A.F.; Majrashi, K.A.; Sheikh, H.M.; Shafi, M.E.; El-Ratel, I.T.; Neamat-Allah, A.N.; El-Raghi, A.A.; Elazem, A.Y.A.; Abd-Elghany, M.F.; Abdelnour, S.A. Dietary supplementation of Nile tilapia (Oreochromis niloticus) with β-glucan and/or Bacillus coagulans: Synergistic impacts on performance, immune responses, redox status and expression of some related genes. Front. Vet. Sci. 2022, 23, 1011715. [Google Scholar] [CrossRef]
- Horwitz, W. Official Methods of Analysis of AOAC International. Volume I, Agricultural Chemicals, Contaminants, Drugs; Horwitz, W., Ed.; AOAC International: Gaithersburg, MD, USA, 2010. [Google Scholar]
- Feldman, B.V.; Zinkl, J.G.; Jain, N.C.; Schalm, O.W. Schalm’s Veterinary Hematology; Feldman, B.F., Zinkl, J.G., Jain, N.C., Gasper, P.E., Giger, U., De Gopegui, R.R., Grindem, C.B., Kristensen, A.t., Latimer, K.S., Rogers, K., et al., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2000. [Google Scholar]
- Blaxhall, P.; Daisley, K. Routine haematological methods for use with fish blood. J. Fish Biol. 1973, 5, 771–781. [Google Scholar] [CrossRef]
- Trinder, P. Determination of glucose in blood using glucose oxidase with an alternative oxygen acceptor. Ann. Clin. Biochem. 1969, 6, 24–27. [Google Scholar] [CrossRef]
- Reitman, S.; Frankel, S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Bussy, U.; Chung-Davidson, Y.-W.; Buchinger, T.; Li, K.; Smith, S.A.; Daniel Jones, A.; Li, W. Metabolism of a sea lamprey pesticide by fish liver enzymes part B: Method development and application in quantification of TFM metabolites formed in vivo. Anal. Bioanal. Chem. 2018, 410, 1763–1774. [Google Scholar] [CrossRef]
- Adjovi, Y.; Tiko, G.; Gnonlonfin, B.; Sanni, A. Morphologic and molecular characterization of Aspergillus flavus isolated from smoked, fermented and dried fishes sold in main markets of Cotonou (Benin). J. Food Ind. Microbiol. 2019, 5, 2. [Google Scholar]
- Abdel-Tawwab, M.; Shukry, M.; Farrag, F.A.; El-Shafai, N.M.; Dawood, M.A.O.; Abdel-Latif, H.M.R. Dietary sodium butyrate nanoparticles enhanced growth, digestive enzyme activities, intestinal histomorphometry, and transcription of growth-related genes in Nile tilapia juveniles. Aquaculture 2021, 536, 736467. [Google Scholar] [CrossRef]
- Yossa, R.; Verdegem, M. Misuse of multiple comparison tests and underuse of contrast procedures in aquaculture publications. Aquaculture 2015, 437, 344–350. [Google Scholar] [CrossRef]
- Shehzad, Q.; Rehman, A.; Jafari, S.M.; Zuo, M.; Khan, M.A.; Ali, A.; Khan, S.; Karim, A.; Usman, M.; Hussain, A. Improving the oxidative stability of fish oil nanoemulsions by co-encapsulation with curcumin and resveratrol. Colloids Surf. B Biointerfaces 2021, 199, 111481. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Min, T. Curcumin, curcumin nanoparticles and curcumin nanospheres: A review on their pharmacodynamics based on monogastric farm animal, poultry and fish nutrition. Pharmaceutics 2020, 12, 447. [Google Scholar] [CrossRef] [PubMed]
- Amer, S.A.; El-Araby, D.A.; Tartor, H.; Farahat, M.; Goda, N.I.; Farag, M.F.; Fahmy, E.M.; Hassan, A.M.; Abo El-Maati, M.F.; Osman, A. Long-Term Feeding with Curcumin Affects the Growth, Antioxidant Capacity, Immune Status, Tissue Histoarchitecture, Immune Expression of Proinflammatory Cytokines, and Apoptosis Indicators in Nile Tilapia, Oreochromis niloticus. Antioxidants 2022, 11, 937. [Google Scholar] [CrossRef]
- Aqmasjed, B.; Sajjadi, M.M.; Falahatkar, B.; Safari, R. Effect of curcumin and ginger extract on growth and biochemical indices in rainbow trout (Oncorhynchus mykiss). J. Aquac. Dev. 2022, 16, 35–48. [Google Scholar]
- Jiang, J.; Wu, X.-Y.; Zhou, X.-Q.; Feng, L.; Liu, Y.; Jiang, W.-D.; Wu, P.; Zhao, Y. Effects of dietary curcumin supplementation on growth performance, intestinal digestive enzyme activities and antioxidant capacity of crucian carp Carassius auratus. Aquaculture 2016, 463, 174–180. [Google Scholar] [CrossRef]
- Ming, J.; Ye, J.; Zhang, Y.; Xu, Q.; Yang, X.; Shao, X.; Qiang, J.; Xu, P. Optimal dietary curcumin improved growth performance, and modulated innate immunity, antioxidant capacity and related genes expression of NF-κB and Nrf2 signaling pathways in grass carp (Ctenopharyngodon idella) after infection with Aeromonas hydrophila. Fish Shellfish Immunol. 2020, 97, 540–553. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Tawwab, M.; Abbass, F.E. Turmeric powder, Curcuma longa L., in common carp, Cyprinus carpio L., diets: Growth performance, innate immunity, and challenge against pathogenic Aeromonas hydrophila infection. J. World Aquac. Soc. 2017, 48, 303–312. [Google Scholar] [CrossRef]
- Rohmah, M.K.; Salahdin, O.D.; Gupta, R.; Muzammil, K.; Qasim, M.T.; Al-Qaim, Z.H.; Abbas, N.F.; Jawad, M.A.; Yasin, G.; Mustafa, Y.F. Modulatory role of dietary curcumin and resveratrol on growth performance, serum immunity responses, mucus enzymes activity, antioxidant capacity and serum and mucus biochemicals in the common carp, Cyprinus carpio exposed to abamectin. Fish Shellfish Immunol. 2022, 129, 221–230. [Google Scholar] [CrossRef]
- Mohamed, A.A.-R.; El-Houseiny, W.; Abd Elhakeem, E.-M.; Ebraheim, L.L.; Ahmed, A.I.; Abd El-Hakim, Y.M. Effect of hexavalent chromium exposure on the liver and kidney tissues related to the expression of CYP450 and GST genes of Oreochromis niloticus fish: Role of curcumin supplemented diet. Ecotoxicol. Environ. Saf. 2020, 188, 109890. [Google Scholar] [CrossRef]
- Sruthi, M.; Nair, A.B.; Arun, D.; Thushara, V.; Sheeja, C.; Vijayasree, A.S.; Oommen, O.V.; Divya, L. Dietary curcumin influences leptin, growth hormone and hepatic growth factors in Tilapia (Oreochromis mossambicus). Aquaculture 2018, 496, 105–111. [Google Scholar] [CrossRef]
- Abd El-Hakim, Y.M.; El-Houseiny, W.; Abd Elhakeem, E.-M.; Ebraheim, L.L.; Moustafa, A.A.; Mohamed, A.A.R. Melamine and curcumin enriched diets modulate the haemato-immune response, growth performance, oxidative stress, disease resistance, and cytokine production in Oreochromis niloticus. Aquat. Toxicol. 2020, 220, 105406. [Google Scholar] [CrossRef]
- Cao, S.P.; Zou, T.; Zhang, P.Y.; Han, D.; Jin, J.Y.; Liu, H.K.; Yang, Y.X.; Zhu, X.M.; Xie, S.Q. Effects of dietary fishmeal replacement with Spirulina platensis on the growth, feed utilization, digestion and physiological parameters in juvenile gibel carp (Carassis auratus gibelio var. CAS III). Aquac. Res. 2018, 49, 1320–1328. [Google Scholar] [CrossRef]
- Rathore, S.; Murthy, H.; Girisha, S.; Nithin, M.; Nasren, S.; Mamun, M.; Puneeth, T.; Rakesh, K.; Kumar, B.; Pai, M. Supplementation of nano-selenium in fish diet: Impact on selenium assimilation and immune-regulated selenoproteome expression in monosex Nile tilapia (Oreochromis niloticus). Comp. Biochem. Physiol. Part C: Toxicol. Pharmacol. 2021, 240, 108907. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Tawwab, M. Effect of feed availability on susceptibility of Nile tilapia, Oreochromis niloticus (L.) to environmental zinc toxicity: Growth performance, biochemical response, and zinc bioaccumulation. Aquaculture 2016, 464, 309–315. [Google Scholar] [CrossRef]
- Adeshina, I.; Jenyo-Oni, A.; Emikpe, B.O.; Ajani, E.K.; Abdel-Tawwab, M. Stimulatory effect of dietary clove, Eugenia caryophyllata, bud extract on growth performance, nutrient utilization, antioxidant capacity, and tolerance of African catfish, Clarias gariepinus (B.), to Aeromonas hydrophila infection. J. World Aquac. Soc. 2019, 50, 390–405. [Google Scholar] [CrossRef]
- Wolf, J.C.; Wheeler, J.R. A critical review of histopathological findings associated with endocrine and non-endocrine hepatic toxicity in fish models. Aquat. Toxicol. 2018, 197, 60–78. [Google Scholar] [CrossRef]
- Abdelkhalek, N.; El-Adl, M.; El-Ashram, A.; Othman, M.; Gadallah, H.; El-Diasty, M.; Dawood, M.A.; Almeer, R.; Abdel Daim, M. Immunological and antioxidant role of curcumin in ameliorating fipronil toxicity in Nile tilapia (Oreochromis niloticus). Aquac. Res. 2021, 52, 2791–2801. [Google Scholar] [CrossRef]
- Moghadam, H.; Sourinejad, I.; Johari, S.A. Growth performance, haemato-immunological responses and antioxidant status of Pacific white shrimp Penaeus vannamei fed with turmeric powder, curcumin and curcumin nanomicelles. Aquac. Nutr. 2021, 27, 2294–2306. [Google Scholar] [CrossRef]
- Magnadottir, B. Immunological control of fish diseases. Mar. Biotechnol. 2010, 12, 361–379. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, J.; Ma, Y.; Li, J.; Chen, X. The effective components of herbal medicines used for prevention and control of fish diseases. Fish Shellfish Immunol. 2022, 126, 73–83. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Ghazanfar, S.; Abdel-Hamid, M.; Abdel-Latif, H.M.; Zhang, Z.; Naiel, M.A. Therapeutic uses and applications of bovine lactoferrin in aquatic animal medicine: An overview. Vet. Res. Commun. 2023, 1, 1–15. [Google Scholar] [CrossRef]
- Jagetia, G.C.; Aggarwal, B.B. “Spicing up” of the immune system by curcumin. J. Clin. Immunol. 2007, 27, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Haftcheshmeh, S.M.; Mirhafez, S.R.; Abedi, M.; Heydarlou, H.; Shakeri, A.; Mohammadi, A.; Sahebkar, A. Therapeutic potency of curcumin for allergic diseases: A focus on immunomodulatory actions. Biomed. Pharmacother. 2022, 154, 113646. [Google Scholar] [CrossRef] [PubMed]
- Rakotoarisoa, M.; Angelov, B.; Espinoza, S.; Khakurel, K.; Bizien, T.; Drechsler, M.; Angelova, A. Composition-switchable liquid crystalline nanostructures as green formulations of curcumin and fish oil. ACS Sustain. Chem. Eng. 2021, 9, 14821–14835. [Google Scholar] [CrossRef]
- Pérez-Valenzuela, J.; Mejías, M.; Ortiz, D.; Salgado, P.; Montt, L.; Chávez-Báez, I.; Vera-Tamargo, F.; Mandakovic, D.; Wacyk, J.; Pulgar, R. Increased dietary availability of selenium in rainbow trout (Oncorhynchus mykiss) improves its plasma antioxidant capacity and resistance to infection with Piscirickettsia salmonis. Vet. Res. 2021, 52, 1–14. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, S.; Han, C.; Wang, L.; Zheng, Q.; Wang, S.; Huang, Y.; Wei, S.; Qin, Q. Curcumin inhibits Singapore grouper iridovirus infection through multiple antiviral mechanisms. Aquaculture 2023, 562, 738870. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).