Abstract
In Mexico, atrazine is one of the herbicides most widely authorized and used in different irrigation districts. Atrazine is a herbicide that contaminates aquatic systems. Previous studies have shown that atrazine causes damage to red blood cells and liver tissue in different aquatic species, including abnormalities in melanomacrophages. However, more information on amphibians is needed, since most of these studies have been done on fish. Furthermore, no study has determined the effect of atrazine on species native to Mexico. Therefore, in this study, we asked what the effects of atrazine are on the erythrocytes and melanomacrophages of the liver in the male frog (Lithobates spectabilis). In the present study, we analyzed (1) the cytotoxicity of atrazine using the micronucleus test, (2) the area of melanomacrophage centers and the presence of melanin, and (3) the characterization of liver damage using histological techniques. Our results show that atrazine is cytotoxic to erythrocytes, increases the area of and melanin presence in melanomacrophage centers, and causes liver damage in male L. spectabilis. Therefore, hepatotoxicity and cytotoxicity are indicators of environmental stress. We suggest monitoring Mexico’s aquatic systems and further analyzing atrazine effects and other pollutants on native species.
Keywords:
amphibians; Gesaprim; melanomacrophage centers; hepatotoxicity; cytotoxicity; frog; Mexico Key Contribution:
This work provides new information on the effect of the herbicide atrazine on a species of frog native to Mexico. We found a cytotoxic effect on erythrocytes and liver damage in Lithobates spectabilis. These results may contribute to monitoring environmentally relevant concentrations of atrazine in aquatic systems and analyzing its impact on other species of frogs native to Mexico.
1. Introduction
One of the main threats to amphibians is pesticide contamination []. Atrazine (2–18 chloro-4-ethylamino-6-isopropylamino-s-triazine) is widely applied worldwide, despite being one of the most toxic herbicides [,]. Analyses of aquatic systems have shown that it is more likely to find atrazine than other agrochemicals [,,]. Amphibians are excellent animal models for evaluating atrazine’s effect in the laboratory. Different investigations in the laboratory using chronic and acute exposure have demonstrated that atrazine can be genotoxic and mutagenic in amphibians [,]. However, most of these studies have considered higher doses than are environmentally relevant. Atrazine concentrations in the environment range from a maximum of 100 to a minimum of 0.01 []. In contrast, the laboratory analyses have used concentrations of up to 35 mg/L []. Atrazine has been shown to affect the morphology of the oxygen-carrying cells in the circulatory system, causing alterations to the membrane, nucleus, and cytoplasm in erythrocytes from Lithobates catesbeianus [], Dendrophryniscus minutus [], Rhinella schneideri [], Anaxyrus americanus, Xenopus laevis [], and Lithobates pipiens []. Several of these authors have also determined that an increase in micronucleus frequency is associated with liver damage [,]. The vertebrate liver is essential in the biotransformation of xenobiotics involving hepatocytes and Kupffer cells through enzymatic and non-enzymatic mechanisms []. The livers of several ectothermic animals contain melanomacrophages or pigment (melanin) cells. Pigment cells form melanomacrophage centers, whose functions include defense, melanogenesis, and sequestration of cellular degradation products or foreign substances of endogenous and exogenous origins [].
Field and laboratory studies have shown that atrazine causes an accumulation of erythrocytes, hypervascularization, and sinusoidal enlargement, along with increases in the numbers of melanomacrophages and abnormalities in erythrocytes in Rhinella schneideri []. Some studies of Xenopus laevis have found hypertrophy, vascular congestion and dilation, apoptosis and cell death of hepatocytes, numerous hematopoietic cells, increased collagen in connective tissue [], smaller livers and caspase-3-immunopositive cells []. In Sclerophrys regulari, atrazine causes hepatocyte degeneration, hemorrhage, necrosis, vasodilatation, congestion in blood vessels, and aggregation of melanomacrophage cells between hepatocytes []. However, the cytotoxic and hepatotoxic effects on L. spectabilis are unknown. Therefore, we hypothesized that atrazine causes cytotoxic damage to erythrocytes and increases the area of melanomacrophage centers, causing histological alterations in the liver of the male frog of the species L. spectabilis. This species is native to central Mexico [], which is also the location of the megalopolis of the Mexican highlands, and where the effects of atrazine on frog fauna have not been explored []. Atrazine (Gesaprim®) is the third most widely used herbicide and is applied without restriction in some irrigation districts in Mexico []. Therefore, the objective of the current study was to evaluate the subchronic effect of the atrazine herbicide on the erythrocytes’ morphology, area of melanomacrophage centers, melanin’s presence in the centers of liver melanomacrophages, and histological alterations in the liver in males of the Lithobates spectabilis.
2. Materials and Methods
2.1. Lithobates spectabilis
We selected L. spectabilis as our study species. This frog is native to and widely distributed throughout central Mexico. L. spectabilis is found primarily in oak, pine-oak, and fir forests at 1200 to 3200 m.a.s.l from eastern Michoacan to central Mexico, and north through Tlaxcala. It mainly inhabits the edges of mountain streams, and its breeding season is in July []. L. spectabilis is listed as a species of least concern due to its wide distribution and large presumed population size [].
2.2. Capture Site
Twelve adult male L. spectabilis were captured in an aquatic system using an entomological net. The aquatic system had no history of pesticide contamination and a stable population of frogs. Males were captured during their active hours (18:00–20:00 h) between August and September 2021 in the locality of Cuaxonacayo, Ixtacuixtla-Tlaxcala (19°6423.60 N 98°2153.99 W; 2919 m.a.s.l). All individuals were collected with a scientific collection permit issued by the Secretaría de Medio Ambiente y Recursos Naturales de Mexico (SEMARNAT; SGPA/DGVS/03662). Frogs were identified as L. spectabilis based on a field-identification guide for amphibians distributed in Mexico []. After capture and identification, the males were transported to the laboratory. No animals showed anatomical alterations, such as limb deformities.
2.3. Experimental Design
Animal Housing Conditions
In the laboratory, we assembled glass terrariums to simulate the environmental conditions (vegetation, humidity (75–90%), room temperature (22.3–24.9 °C), illumination) of the frogs’ natural habitat. We used 40 L fish tanks (51 × 29.5 × 26 cm). We lined the bottoms of the tanks with uncontaminated grass and added a glass container to hold water (25.5 × 25.5 × 8 cm). Temperature and humidity were monitored to track abnormal changes. In each tank, a photo-period of 12/12 h light/dark was maintained using a lamp controlled by a digital timer (lights on at 6:00 a.m.), and a sensor was used to record the ambient temperature and relative humidity using an Arduino-cloud program for 24 h. This experiment was carried out in two phases: acclimatization and atrazine exposure.
2.4. Phase 1: Acclimatization
The males were allowed to acclimate inside a tank with potable water for one hour before being placed in the terrarium. All twelve individuals were placed in terrariums for acclimatization for three weeks. During this time, the individuals were only exposed to potable water, which was replaced daily to prevent the accumulation of frog feces and urine. The potable water used in this experiment was from a non-contaminated source. The individuals were fed ad libitum with live crickets raised for pet-feeding purposes (Petmmal S.A de C.V).
2.5. Phase 2: Atrazine Exposure
The individuals were randomly allocated to two terrariums after the acclimatization phase. One terrarium was used for the control group (n = 6), and the other was used for the group exposed to the herbicide atrazine Gesaprim® (n = 6).In the treatment of subchronic exposure to atrazine, individuals were exposed to a commercial formulation of atrazine herbicide (Gesaprim® 90% purity) at a concentration of 15 g/L dissolved in potable water added to glass containers inside the terrariums for 90 days. The dose used in this study was determined based on the environmental concentration that has been previously reported in an aquatic system in Mexico [], making it an environmentally relevant and realistic dose. For the control group, the individuals were exposed to potable water only. The atrazine solution and potable water were replaced daily throughout the experiment.
2.6. Erythrocyte Abnormalities
The males were anesthetized by immersion with 200 mg/L MS-222 (tricaine methanesulfonate, Sigma-Aldrich) for 15 min. Heparinized syringes were used to extract 5 L of a blood sample from each individual by cardiac puncture. Blood smears were prepared on previously cleaned slides and allowed to air dry at room temperature for 24 h. The smears were fixed by applying a 3:1 (v/v) acetic acid methanol solution for 1 min. Erythrocytes were stained under the 5% Giemsa protocol, using eosin and methylene blue for 30 s, and then they were dried at room temperature. Photomicrographs of erythrocytes were taken with a camera (LEICA ICC50 E) adapted to an optical light microscope (LEICA; DM750). Slides were examined following an L-shaped path, including the smear head, body, and tail. Two observers carried out the erythrocyte counts, and each counted one thousand non-overlapping erythrocytes with intact cell and nuclear membranes per slide. The order in which the slides were analyzed was randomized, and both observers were blind with respect to the frog’s treatment. Erythrocyte alterations were divided into the following abnormalities: micronuclei, binucleated, blebbed membrane, notched nucleus, notched membrane, drop-shaped, apoptotic, and necrotic. Analyses for erythrocyte abnormalities were performed according to the protocols of Fenech and Alimba [,].
2.7. Melanomacrophages
After the individuals were euthanized with an overdose of MS-222, we removed the complete liver from each individual in the control (n = 6) and atrazine-exposed (n = 6) groups. Subsequently, we fixed the liver in a Bouin–Duboscq solution for 24 h. Livers were dehydrated with ascending alcohol concentrations (60–100%), fixed in xylol, and embedded in paraffin (Paraplast. SIGMA-ALDRICH). Then, longitudinal cuts were made with a microtome (Leica 2115) at a seven-micrometer thickness, and serial sections of tissue were stained with hematoxylin and eosin (H&E). The stain was used for qualitative (liver histology) and quantitative (area of the melanomacrophage centers and presence of melanin) histological analysis. After staining, the hepatic tissue was dehydrated with alcohols in ascending concentration (60–100%). The stained tissue samples were fixed with cytoseal TM60 and coverslipped. First, we chose a complete tissue sample that was not torn or folded. Photomicrographs were then taken under an optical light microscope (Leica; DM750) with a camera adapted to LAS EZ 3.3.0 program. Subsequently, we measured and determined the area occupied by melanomacrophage centers using the program AxionVision Rel.4.8 []. We randomly selected 100 melanomacrophage centers from the whole liver of each individual. The melanomacrophage centers were analyzed in duplicate for the control and atrazine groups. In addition, we counted the number of melanomacrophage centers with and without melanin in each group.
2.8. Histological Analysis of the Liver
Masson’s trichrome stain was used for the histological analysis of the liver in L. spectabilis. Masson’s trichrome stain is one of the most commonly used to determine liver damage []. The livers of the control group (n = 6) and atrazine (n = 6) were stained. Then, photomicrographs were taken under a light microscope (Leica; DM750) with a camera adapted to the LAS EZ 3.3.0 program.
2.9. Statistical Analysis
All analyzes were performed using RStudio (version 1.4.1717). The erythrocyte abnormalities were classified as (1) membrane bleb, (2) apoptosis, and (3) necrosis. First, 1000 erythrocytes from each individual were counted [,]. The number of anomalies observed was divided by 1000 and multiplied by 100, considering the percentage of anomalies following []. To see if the data conformed to a normal distribution, we used a Shapiro–Wilk test. Additionally, we looked at the homoscedasticity of the variances in the data. Finally, to determine if the control and atrazine-exposed groups differed in their numbers of abnormalities, the area occupied by melanomacrophage centers, or melanin-containing melanomacrophages, a student’s t-test was used for each classification ("t.test" function). The statistical significance threshold was . Data are reported as mean ± standard error unless otherwise stated.
3. Results
3.1. Erythrocyte Morphology
In this experiment, we observed erythrocytes with the normal morphology and abnormalities in both the control and atrazine-exposed groups (Figure 1). The morphologically normal erythrocytes were elliptical (Figure 1a; el), spherical (Figure 1b; sp), and biconvex (Figure 1b; bx) in all cases. The least-frequent classes of erythrocyte abnormalities had micronuclei (Figure 1c), were comma-shaped (Figure 1d; cs), had a notched membrane (Figure 1e; nm), had a notched nucleus (Figure 1f; nn), had a binucleated cell (Figure 1g; bi), and were drop-shaped (Figure 1h; d), according to some authors’ classifications [,] with the control group’s normal morphology erythrocytes (Figure 1i). The most frequently observed abnormalities in the atrazine-exposed group were: blebbed membrane (Figure 1j; bm), apoptosis (Figure 1k; ap), and necrotic erythrocytes (Figure 1l; ne). Our results indicate that atrazine induced higher frequencies of erythrocytes with blebbed membranes (t = −2.44, df = 10, p = 0.03), apoptotic erythrocytes (t = −2.96, df = 10,162, p = 0.01), and necrotic erythrocytes (t = −3.10, df = 10, p = 0.01) than the control group (Figure 2). In addition, the only individual we detected with micronuclei belonged to the control group.
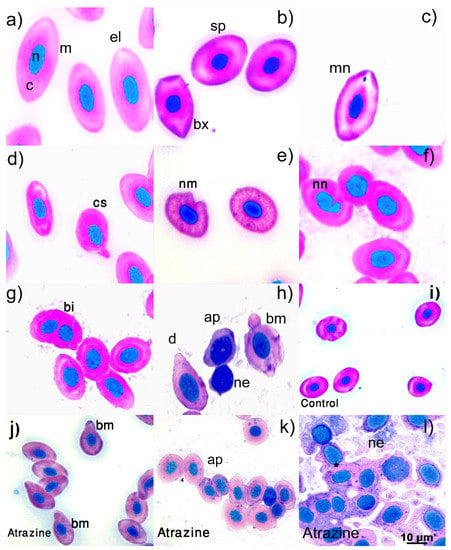
Figure 1.
Photomicrographs of erythrocyte abnormalities in male L. spectabilis. Erythrocytes with normal morphologies (a,b) and abnormalities (c–l). Erythrocytes with normal elliptical (a), spherical, or biconvex (b) shapes. Erythrocyte abnormalities: micronuclei (c), comma-shaped (d), notched membrane (e), notched nucleus (f), binucleated (g), drop-shaped (h), blebbed membrane, apoptotic, and necrotic (i). The herbicide atrazine induced erythrocytes to have a blebbed membrane (j) and undergo more apoptosis (k) and necrosis (l) compared to normal erythrocytes in the control group (i). Abbreviations: c: cytoplasm, m: membrane, n: nucleus, el; elliptical, sp: spherical, bx: biconvex, d: droplet, bm: blebbed membrane, bi: binucleated, cs: comma-shaped, nm: notched membrane, nn: notched nucleus, ap: apoptotic, ne: necrotic, *: released cytoplasm. Giemsa stain. Bar: 10 m.
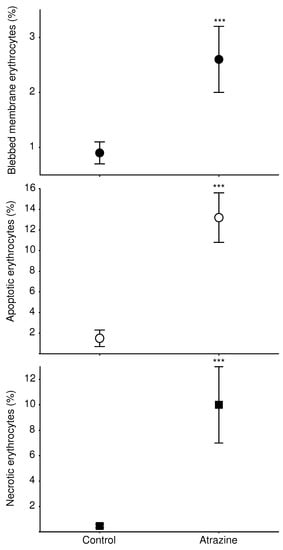
Figure 2.
Cytotoxicity of the herbicide atrazine in the erythrocytes of L. spectabilis, expressed as significant differences in the frequencies of erythrocyte abnormalities in the control group (left) versus the atrazine-exposed group (right). The group exposed to the herbicide atrazine had higher percentages of erythrocytes with membrane blebbing (upper panel), apoptotic erythrocytes (middle panel), and necrotic erythrocytes (lower panel). Standard error bars are shown. Asterisks show statistical differences.
3.2. Melanomacrophages and Melanine
We found that the area occupied by melanomacrophage centers in the livers of L. spectabilis males was larger in the atrazine-exposed group (t = −22.65, df = 10, p = 0.02) than in the control group (Figure 3a). They also had more melanin-containing melanomacrophages (t = −2.85, df = 10, p = 0.01) (Figure 3b).
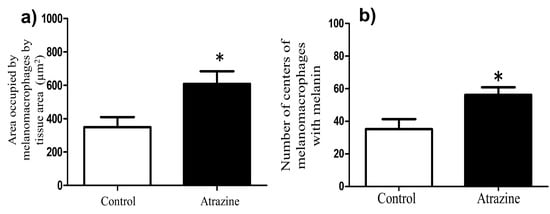
Figure 3.
The area occupied and melanin presence of melanomacrophage centers. The area occupied by melanomacrophage centers (mean ± SE) in the livers of male L. spectabilis was larger in the atrazine-exposed group compared to the control group (a). The atrazine group also had significantly higher numbers of melanomacrophage centers in livers with melanin (mean ± SE) compared to the control group (b). An asterisk indicates a statistically significant difference compared to the control group.
3.3. Liver Histopathology
The histological description of the liver of the male L. spectabilis exposed to atrazine included a larger area occupied by melanomacrophage centers (Figure 4b) compared to the control group (Figure 4a). Hepatic lesions were only observed in the atrazine-exposed group, and the most common lesion types were infiltration of immune cells (Figure 4c) and lymphocytes (Figure 4d), hemorrhagic patches (Figure 4e), blood vessel congestion (Figure 4f), and liver tissue degeneration (Figure 4g). No recognizable changes were observed in the hepatic tissues of control frogs (Figure 4h). Localized collagen accumulation was also visible in the livers of individuals from the atrazine group (Figure 4i)—specifically, collagen deposits between hepatocytes (Figure 4j). Regarding the hepatic triad (Figure 4k), we located degeneration and sloughing of the biliary duct epithelium (Figure 4l), atrophy of the hepatocytes (Figure 4m), collagen deposition in the subcapsular hematopoietic tissue and disorganized parenchyma (Figure 4n), some reticular collagen fibers distributed among the hepatic cords (Figure 4o), and erythrocyte infiltration in the livers of individuals from the atrazine group (Figure 4p).
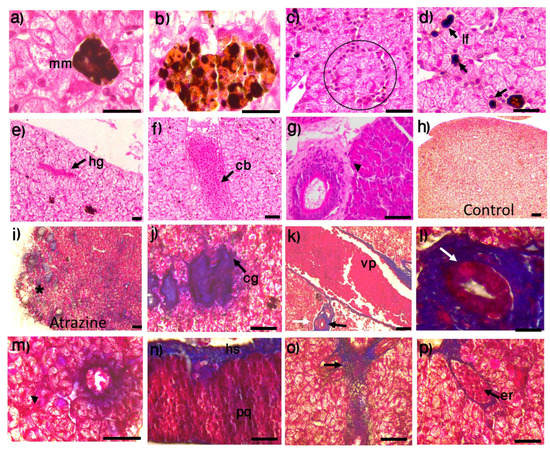
Figure 4.
Cytological preparations showing liver tissue in male L. spectabilis frogs exposed to the herbicide atrazine and controls. The melanomacrophage (mm) area was smaller in the control group (a) than the atrazine-exposed group (b). In the atrazine group, there were liver lesions, including: (c) infiltration of immune cells (circle), (d) infiltration of lymphocytes (lf) in the liver tissue, (e) patches of hemorrhage (hg), (f) congestion of blood vessels (cb), (g) degeneration of liver tissue (arrowhead), (i) liver tissue with collagen (*) deposition, and (j) collagen (cg) deposition between hepatocytes (compare to (h) liver tissue without collagen in the control group). In the hepatic triad (k), portal vein (vp), bile duct (black arrow), and hepatic artery (white arrow), there were (l) degeneration and sloughing of epithelium in the biliary duct (black arrows), (m) atrophy of hepatocytes (arrowhead), (n) deposition of collagen in the subcapsular hematopoietic tissue (hs) and disorganized parenchyma (p), (o) reticular collagen fibers distributed between the hepatic cords (arrow), and (p) erythrocyte infiltration (er). (a–g) H&E stain; (h–p) Masson’s trichrome. Scale bars represent 10 and 20 m.
4. Discussion
Our results indicate that atrazine induced a cytotoxic effect in the erythrocytes of male L. spectabilis. We found that the herbicide atrazine Gesaprim® is cytotoxic, since it induces increases in membrane blebbing, apoptosis, and necrosis of erythrocytes of L. spectabilis. The increase in the number of blebbed erythrocytes could result from the alterations in the spectrin ( and ), actin, and ankyrin proteins that maintain the structure of the membrane []. Consequently, an increase in membrane-blebbed erythrocytes may be associated with the onset of apoptosis []. In addition, our results determined that atrazine Gesaprim® caused blebbing in the erythrocytes of L. spectabilis. The apoptosis of the erythrocytes could be due to the following alterations: a decrease in ATP [], the activation of caspases [], alteration of phosphatidylserine [,], increased calcium, and the formation of reactive oxygen species (ROS) [,]. Studies on exposed fish to xenobiotics have found that caspase 3 can be considered a universal indicator of erythrocyte apoptosis [,]. Similarly, fishes exposed to fipronil have elevated expression of caspase 3 in blood tissue []. Apoptosis of erythrocytes may also result from oxidative stress that indirectly damages the deoxyribonucleic acid (DNA) and causes cell death [,]. It has been determined that atrazine increases ROS in fish []. Thus, it is possible that atrazine generates ROS, causes DNA damage, modulates the P53 gene, and provokes apoptosis in frogs []. In fact, the formation of apoptotic bodies would rapidly induce the death of erythrocytes, as occurs in Rana esculenta []. In addition, oxygen transport can be reduced in L. spectabilis because erythrocytes form vacuoles and unevenly distribute hemoglobin due to an alteration in oxidative phosphorylation []. Atrazine has determined increased DNA damage in erythrocytes and decreased hemoglobin in fishes []. In addition, apoptosis can increase ion and water influx, causing cells to swell and rupture [].
We found other abnormalities in the erythrocytes of L. spectabilis in both groups. The abnormalities could have been due to alterations in the calcium-ionophore and extracellular calcium, which induce changes in the biconvex and ellipsoid shape of the erythrocyte membrane []. The formation of binucleated cells could result from blocking cytokinesis or cell fusion []. Notched nuclei may be due to aneuploidy, as in micronuclei formation []. This work mainly found the formation of "notches in the membrane of erythrocytes of L. spectabilis". It has been determined that an alteration of the erythrocytes’ metabolism and ATP causes membrane fragmentation and the consequent formation of notches or vesicles []. Therefore, our results suggest necrosis of the erythrocytes.
Finally, an alteration in erythrocytes’ morphology can affect individuals’ health. For example, an increase in apoptotic and necrotic erythrocytes can lead to degenerative diseases, inflammatory processes, hypoxemia, or hypercapnia []. In addition, the cytotoxic stress caused by exposure to atrazine can alter blood tissue due to severe erythrocyte damage [,,]. However, we consider it essential to carry out more trials that analyze the effects of atrazine on the immune system and oxidative stress. Similarly, it is also necessary to investigate herbicides’ effects on the proteins involved in cell-cycles phases, such as caspases or P53. Studies performed on Xenopus laevis tadpoles determined significantly more active caspase-3 and apoptosis in the kidneys []. Atrazine induces more active caspase-3 immunopositive cells in liver tissue, suggesting higher apoptosis rates []. Therefore, atrazine could be involved in signaling pathways and induction of apoptosis in cells through the modulation of caspase 3. Unfortunately, information regarding the cell-cycle phases and proteins altered by the atrazine in frogs is unknown. Therefore, early detection of erythrocytes with membrane blisters can be essential to preventing apoptotic or necrotic states in amphibian cells [].
The Gesaprim® formulation, which contains atrazine, has been determined to be genotoxic, but atrazine is not []. In this investigation, the individuals were not exposed to atrazine alone but to a commercial formulation (including other substances). Therefore, we do not rule out the possibility that the additive substances in the commercial formulation may also be responsible for some effects. Unlike most authors [,,], in the present study, we did not report an increase in the micronuclear formation due to herbicide atrazine Gesaprim® [,]. Previous toxicity studies in amphibians have focused on the lethal effects of the herbicide atrazine in the short term in tadpoles. In Dendropsophus minutus exposed to a concentration of 18 mg/L of the atrazine herbicide Atanor 50 SC® (50% atrazine) for 96 h, it induces a higher frequency of micronuclei []. In Rhinella Schneider exposed to SIPTRAN 500 SC® (6 and 19 mg/L), it was determined that the atrazine-based agrochemical increased the frequencies of micronuclei and other nuclear abnormalities in erythrocytes, in addition to liver damage after a period of 48 or 96 h of exposure []. In Dendropsophus minutus exposed to up to 40 mg/L of the herbicide Atanor 50 SC®, the frequency of micronuclei is increased, determining that atrazine is mutagenic and genotoxic []. However, in the present analysis, we did not find any genotoxicity of Gesaprim® atrazine in L. spectabilis. Unlike these works, we used a different commercial formulation and a much lower concentration. Therefore, the genotoxicity found in these works could have been due to the higher concentrations of some comercial formulations containing atrazine. The only micronuclei we detected were in the erythrocytes of an individual not exposed to atrazine Gesaprim®. In that case, the formation of micronuclei could have been due to malfunctioning of the mitotic spindle and the presence of acentric chromosome fragments during cell division or mitosis []. However, the organisms respond differently to xenobiotics [], the active ingredients of the formulations [,], doses [,], and bioaccumulation []. This alteration may explain why some species of frogs are highly susceptible to atrazine, yet others are not.
On the other hand, the erythrocyte cytotoxicity and liver tissue degeneration found in L. spectabilis may also be related to a larger area of melanomacrophages centers, as has also been determined in previous studies [,]. It has been shown that cytotoxicity in erythrocytes increases the recruitment of macrophages, as these cells function as metabolic dumping grounds []. These results are consistent with previous studies that found erythrocyte abnormalities and liver damage in Rana castebianus [] and Leptodactylus latinasus [] exposed to other herbicides.
The presence of melanomacrophages indicates detoxification of exogenous and endogenous substances []. Furthermore, the melanomacrophages synthesize and degrade melanin [,]. Our results demonstrated an increase in the number of melanin-bearing melanomacrophage centers in the livers of L. spectabilis. Various works have determined that some pollutants increase melanin synthesis in frogs [], toads [], and fish [] exposed to pesticides. The results of these works show that atrazine suppresses nitric oxide [], and melanomacrophages release nitric oxide []. In this sense, an increase in the number of melanomacrophage centers with melanin could neutralize free radicals and toxic agents in the liver []. In addition, the increase in melanin-containing melanomacrophages could be a protective mechanism for liver pigment cells in frogs exposed to atrazine, as occurs with other herbicides [,]. In Leptodactylus latinasus exposed to glyphosate, an increase in the melanin area in melanomacrophages, modification of melanomacrophage pigmentation, and erythrocyte nuclear abnormalities were determined. It is suggested that this may interfere with hepatic metabolism []. In another study, atrazine was found to have an immunomodulatory effect by altering the melanomacrophage area of the spleen in Oreochromis niloticus []. Therefore, melanomacrophages can be used as biomarkers for toxicological evaluation. Then, the changes observed in melanomacrophages centers can be considered biomarkers of environmental stress [].
We also found a larger area occupied by melanomacrophages upon atrazine Gesaprim® exposure, which could have been due to the activation of a defense mechanism to eliminate atrazine, as has already been reported in fish [,]. Organisms may undergo hematological changes to cope with pollutant stress [,] after an inflammatory process []. In this sense, liver damage caused by atrazine could increase susceptibility to diseases, as occurs in fish living in polluted environments [], because melanomacrophages act as a defensive system and trap antigens for lymphoid cells in fish and frogs []. Consistently with this idea, we observed infiltration of lymphocytes in the liver parenchyma in the present study. These results coincide with the analyses reported on fish [] and frogs [] exposed to insecticides, and they may be due to atrazine’s ability to induce an immune response. In addition, lymphocyte infiltration is related to inflammation, edema, fibrosis, and liver degeneration [].
Necrosis is related to the loss of cytoplasm and nuclei []. Hepatocyte and parenchymal necrosis caused by atrazine Gesaprim® could have been due to the alteration of liver enzymes, such as alanine aminotransferase, as observed in fish exposed to atrazine []. It has been determined that the alteration of this enzyme causes hepatocyte necrosis []. Therefore, the damage in the liver parenchyma is indicative of the deterioration of cellular activity due to the alterations in enzymes due to atrazine toxicity []. Other studies determine that herbicides alter some of the proteins in the cytoplasm of hepatocytes and cause cell destruction or necrosis [,]. Therefore, atrazine Gesaprim® could also modify the expression of some proteins, causing damage to liver cells []. Furthermore, atrazine could increase ROS [] and cause necrosis, as occurs in frogs and fishes [,]. However, comparing the agents that induce ROS formation is relevant to determining if oxidative stress is the leading cause of damage. An alteration in calcium homeostasis and the formation of ROS can cause the death of hepatocytes exposed to Cu []. Therefore, atrazine Gesaprim® could stimulate higher production of ROS, since oxygen radicals are the main toxic effect that alters cell viability []. Hepatocytes can also eliminate or excrete toxic metabolites to avoid possible irreversible damage caused by atrazine []. Regarding hepatocyte hypertrophy, inflammation and enlargement of hepatocytes have been found to cause cell atrophy []. However, hepatocyte degeneration could cause an alteration in lipid metabolism; this is supported by findings that fish exposed to aldicarb have been found to have massive hepatocyte degeneration, excess fat, and hypertrophied hepatocytes []. Thus, hypertrophy can also be attributed to a cellular response to stress caused by atrazine. We propose, as future work, to analyze effect biomarkers to evaluate the chemical exposure of amphibians. The analysis of liver biomarkers related to biochemical and histopathological alterations—for example, of aspartate transaminase, alanine transaminase, -glutamyl transferase, alkaline phosphatase, catalase, glutathione reductase, glutathione peroxidase, total protein, serum albumin, total bilirubin, and creatinine [].
We hypothesize that atrazine could affect the obstruction and reduction of blood flow in L. spectabilis, as occurs in fish exposed to 4-nonylphenol []. In other words, atrazine-induced hepatotoxicity causes lesions that could be related to blood flow []. In fact, biliary epithelial hyperplasia may be associated with fibrosis, inflammation, and proliferation or may be the result of atrazine. In fish, bile-duct-epithelium hyperplasia is caused by exposure to environmental contaminants [,]. Accordingly, this damage can obstruct circulation and block blood flow []. In the present study, we observed congestion of the blood vessels, which corresponds to findings in other aquatic organisms []. Liver analysis determines that the congestion of blood vessels could also cause hepatocyte degeneration and necrosis [,]. In addition, we found the formation of hemorrhagic patches, degeneration, and necrosis in the liver. Our results coincide with the reports of frogs exposed to environmental contaminants [].
In particular, we found collagen deposits in the liver parenchyma, so we also assume that this could cause fibrosis in L. spectabilis exposed to atrazine Gesaprim®. Atrazine is suggested to interfere with liver function, causing various lesions []. For example, fibrosis has been determined around the bile ducts up to the parenchyma. It is assumed that this fibrosis could affect the pumping of blood that has to pass through the sinusoids toward the central vein in fish exposed to contaminants []. In the same way, collagen-filled deposits have been identified between hepatocytes and fibers with slight perisinusoidal fibrosis in frogs exposed to insecticides []. Other results determine that the reticular fibers are responsible for supporting the parenchyma and are juxtaposed with hepatocytes []. Therefore, the alteration of the reticular fibers found could affect the organization of the parenchyma and the connection of the hepatocytes in L. spectabilis, since extensions to hepatocytes not separated by collagen is essential for emergency regeneration. An increase in collagen could also negatively affect the regeneration of liver tissue [].
The changes observed in the histology of the liver can lead to other pathologies or even the death of individuals due to metabolic and hematological alterations in amphibians []. Such histological changes in the liver and erythrocyte cytotoxicity in individuals exposed to atrazine Gesaprim® may contribute to amphibian population declines. As such, it is essential to consider the physicochemical characteristics of herbicides to know their effects on wild species, especially under natural conditions []. The use of atrazine must be regulated in Mexico. It is essential to carry out monitoring in water bodies since most of the studies have been carried out in the soil. The mean degradation time of atrazine in Andosol soil varies from 10 to 17 days, whereas in Vertisol, it is up to 35 days []. It has been determined that the concentrations of atrazine and its metabolite (desethylatrazine) in water samples exceed the limit for human water consumption of 2 []. In surface waters of agricultural areas, concentrations of up to 15.01 have been determined []. L. spectabilis can be considered a suitable species for analyzing herbicides such as atrazine because there are no studies in Mexico regarding the effects of atrazine on amphibians. In this sense, L. spectabilis can be used as a reference for the environmental contamination of Mexico’s aquatic systems. We have determined that atrazine causes histological damage to the liver. However, more research is needed to determine its effects on other organs and how it interferes with liver or blood-function markers. We suggest conducting more toxicological, genotoxic, and hepatotoxic studies in species that are naturally exposed to atrazine. When herbicides are used intensively in the habitat, they can affect liver functions and blood tissue in frogs, making them more vulnerable to attack by diseases and predators, which can contribute to their decline [].
5. Conclusions
It is essential to quantify the risks of contamination by atrazine in amphibians from an ecological point of view. Unfortunately, few ecotoxicological studies have been carried out on species native to Mexico. Atrazine-exposed individuals had larger areas and higher melanin presence in melanomacrophage centers and alterations in the histological features of the liver. In addition, we determined an increase in the number of blebbed membrane, apoptotic, and necrotic erythrocytes. Our results may contribute to carrying out more hematological and hepatotoxic studies that facilitate understanding the contamination of aquatic systems and its effects on amphibians that inhabit central Mexico. Investigations carried out in the laboratory on the effect of atrazine using natural environmental concentrations and mimicking aquatic systems may help to understand the causes of amphibian decline, mainly because the atrazine concentration in aquatic systems and its effect on amphibians are still entirely unknown in Mexico. Finally, the increased area of melanomacrophage centers, the increase in the number of melanin-containing melanomacrophage centers, damage to liver histology, and cytotoxic damage to erythrocytes can be considered bioindicators of environmental stress caused by atrazine Gesaprim®. We suggest analyzing proteins involved in the cell cycle to determine the mechanisms of apoptosis and necrosis in erythrocytes. In addition, we propose evaluating liver biomarkers that allow the analysis of liver damage.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/fishes8040207/s1.
Author Contributions
Conceptualization, M.M.-T. and K.H.-P.; methodology, K.H.-P.; data curation, K.H.-P.; formal analysis, M.M.-T.; writing original draft preparation, M.M.-T.; writing review and editing, M.M.-T., C.M.-C., L.J.-S., S.R.C.-L., E.G.-N. and A.A.-H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All individuals were collected with a scientific collection permit issued by the Secretaría de Medio Ambiente y Recursos Naturales de Mexico (SEMARNAT; SGPA/DGVS/03662).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the Supplemental Materials.
Acknowledgments
The second author acknowledges Facultad de Ciencias de la Salud de la Universidad Autónoma de Tlaxcala for an academic stay during the fall of 2022. The first author wishes to thank the student Anahí Pérez-Garzón for her field and laboratory support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Egea-Serrano, A.; Relyea, R.A.; Tejedo, M.; Torralva, M. Understanding of the impact of chemicals on amphibians: A meta-analytic review. Ecol. Evol. 2012, 2, 1382–1397. [Google Scholar] [CrossRef]
- de Oliveira, J.S.P.; Gonçalves Vieira, L.; Fernandes Carvalho, W.; Benvindo de Souza, M.; de Lima Rodrigues, A.S.; Simões, K.; de Melo De Silva, D.; dos Santos Mendonça, J.; Hirano, L.Q.L.; Quagliatto Santos, A.L.; et al. Mutagenic, genotoxic and morphotoxic potential of different pesticides in the erythrocytes of Podocnemis expansa neonates. Sci. Total Environ. 2020, 737, 140304. [Google Scholar] [CrossRef]
- Cavas, T. In vivo genotoxicity evaluation of atrazine and atrazine–based herbicide on fish Carassius auratus using the micronucleus test and the comet assay. Food Chem. Toxicol. 2011, 49, 1431–1435. [Google Scholar] [CrossRef]
- Battaglin, W.A.; Rice, K.C.; Focazio, M.J.; Salmons, S.; Barry, R.X. The occurrence of glyphosate, atrazine, and other pesticides in vernal pools and adjacent streams in Washington, DC, Maryland, Iowa, and Wyoming, 2005–2006. Environ. Monit. Assess. 2009, 155, 281–307. [Google Scholar] [CrossRef]
- Černoch, I.; Fránek, M.; Diblíková, I.; Hilscherová, K.; Randák, T.; Ocelka, T.; Bláha, L. Determination of atrazine in surface waters by combination of POCIS passive sampling and ELISA detection. J. Environ. Monit. 2011, 13, 2582–2587. [Google Scholar] [CrossRef]
- Snyder, M.N.; Henderson, W.M.; Glinski, D.A.; Purucker, S.T. Biomarker analysis of American toad (Anaxyrus americanus) and grey tree frog (Hyla versicolor) tadpoles following exposure to atrazine. Aquat. Toxicol. 2017, 182, 184–193. [Google Scholar] [CrossRef]
- Sparling, D.W.; Fellers, G.M. Toxicity of two insecticides to california, USA, anurans and its relevance to declining amphibian populations. Environ. Toxicol. Chem. 2009, 28, 1696–1703. [Google Scholar] [CrossRef]
- Graymore, M.; Stagnitti, F.; Allinson, G. Impacts of atrazine in aquatic ecosystems. Environ. Int. 2001, 26, 483–495. [Google Scholar] [CrossRef]
- Lenkowski, J.R.; Reed, J.M.; Deininger, L.; McLaughlin, K.A. Perturbation of Organogenesis by the Herbicide Atrazine in the Amphibian Xenopus laevis. Environ. Health Perspect. 2008, 116, 223–230. [Google Scholar] [CrossRef]
- Clements, C.; Ralph, S.; Petras, M. Genotoxicity of select herbicides in Rana catesbeiana tadpoles using the alkaline single-cell gel DNA electrophoresis (comet) assay. Environ. Mol. Mutagen. 1997, 29, 277–288. [Google Scholar] [CrossRef]
- Gonçalves, M.W.; Marins de Campos, C.B.; Guerra Batista, V.; da Cruz, A.D.; de Marco Junior, P.; Pereira Bastos, R.; de Melo e Silva, D. Genotoxic and mutagenic effects of Atrazine Atanor 50 SC on Dendropsophus minutus Peters, 1872 (Anura: Hylidae) developmental larval stages. Chemosphere 2017, 182, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Iglesias, J.M.; Franco-Belussi, L.; Natale, G.S.; de Oliveira, C. Biomarkers at different levels of organisation after atrazine formulation (SIPTRAN 500SC®) exposure in Rhinella schineideri (Anura: Bufonidae) Neotropical tadpoles. Environ. Pollut. 2019, 244, 733–746. [Google Scholar] [CrossRef] [PubMed]
- Freeman, J.; Rayburn, A. In vivo genotoxicity of atrazine to anuran larvae. Mutat. Res. Toxicol. Environ. Mutagen. 2004, 560, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Howe, G.E.; Gillis, R.; Mowbray, R.C. Effect of chemical synergy and larval stage on the toxicity of atrazine and alachlor to amphibian larvae. Environ. Toxicol. Chem. 1998, 17, 519–525. [Google Scholar] [CrossRef]
- Sena, L.; Asouzu Johnson, J.; Nkomozepi, P.; Mbajiorgu, E.F. Atrazine-Induced Hepato-Renal Toxicity in Adult Male Xenopus laevis Frogs. Appl. Sci. 2021, 11, 1776. [Google Scholar] [CrossRef]
- Dornelles, M.F.; Oliveira, G.T. Effect of Atrazine, Glyphosate and Quinclorac on Biochemical Parameters, Lipid Peroxidation and Survival in Bullfrog Tadpoles (Lithobates catesbeianus). Arch. Environ. Contam. Toxicol. 2014, 66, 415–429. [Google Scholar] [CrossRef]
- Fenoglio, C.; Boncompagni, E.; Fasola, M.; Gandini, C.; Comizzoli, S.; Milanesi, G.; Barni, S. Effects of environmental pollution on the liver parenchymal cells and Kupffer-melanomacrophagic cells of the frog Rana esculenta. Ecotoxicol. Environ. Saf. 2005, 60, 259–268. [Google Scholar] [CrossRef]
- Steinel, N.C.; Bolnick, D.I. Melanomacrophage Centers As a Histological Indicator of Immune Function in Fish and Other Poikilotherms. Front. Immunol. 2017, 8, 827. [Google Scholar] [CrossRef]
- Zaya, R.M.; Amini, Z.; Whitaker, A.S.; Kohler, S.L.; Ide, C.F. Atrazine exposure affects growth, body condition and liver health in Xenopus laevis tadpoles. Aquat. Toxicol. 2011, 104, 243–253. [Google Scholar] [CrossRef]
- Mahmoud, F.A.b.R.; Gadel-Rab, A.G.; Said, R.E.S.; Saber, S.A.L.; ElSalkh, B.A.A.; Said, A.S.; Atia, M.M. Impact of atrazine and nitrate on liver and kidney of egyptian toad Sclerophrys regularis: Bioindicator alarming on ecosystem. Acta Sci. Biol. Sci. 2022, 44, e56386. [Google Scholar] [CrossRef]
- IUCN SSC Amphibian Specialist Group. Lithobates Spectabilis. The IUCN Red List of Threatened Species 2020: e.T58722A53971736. 2020. Available online: https://www.iucnredlist.org/species/58722/53971736 (accessed on 19 September 2022).
- Hernández-Antonio, A.; Hansen, A.M. Uso de plaguicidas en dos zonas agrícolas de México y evaluación de la contaminación de agua y sedimentos. Rev. Int. Contam. Ambient 2011, 27, 115–127. [Google Scholar]
- Villada Canela, M. Estimation of Contaminating Risk Groundwater Tables by Infiltration of Atrazine Herbicide in Irrigation Districts in Mexico. Master’s Thesis, Universidad Nacional Autónoma de México, Mexico City, Mexico, 2006. (In Spanish). [Google Scholar]
- Hillis, D.M.; Frost, J.S. Three new species of leopard frogs (Rana pipiens complex) from the Mexican Plateau. In Occasional Papers of the Museum of Natural History; University of Kansas: Lawrence, KS, USA, 1985; Volume 117, pp. 1–14. [Google Scholar]
- Fenech, M. The in vitro micronucleus technique. Mutat. Res. Mol. Mech. Mutagen. 2000, 455, 81–95. [Google Scholar] [CrossRef]
- Alimba, C.G.; Aladeyelu, A.M.; Nwabisi, I.A.; Bakare, A.A. Micronucleus cytome assay in the differential assessment of cytotoxicity and genotoxicity of cadmium and lead in Amietophrynus regularis. EXCLI J. 2018, 17, 89–101. [Google Scholar] [CrossRef]
- Manrique, W.G.; da Silva Claudiano, G.; Petrillo, T.R.; de Castro, M.P.; Pereira Figueiredo, M.A.; de Andrade Belo, M.A.; de Moraes, J.R.E.; de Moraes, F.R. Response of splenic melanomacrophage centers of Oreochromis niloticus (Linnaeus, 1758) to inflammatory stimuli by BCG and foreign bodies. J. Appl. Ichthyol. 2014, 30, 1001–1006. [Google Scholar] [CrossRef]
- Lefkowitch, J.H. Special stains in diagnostic liver pathology. Semin. Diagn. Pathol. 2006, 23, 190–198. [Google Scholar] [CrossRef]
- Botelho, R.G.; Monteiro, S.H.; Christofoletti, C.A.; Moura-Andrade, G.C.R.; Tornisielo, V.L. Environmentally Relevant Concentrations of Atrazine and Ametrine Induce Micronuclei Formation and Nuclear Abnormalities in Erythrocytes of Fish. Arch. Environ. Contam. Toxicol. 2015, 69, 577–585. [Google Scholar] [CrossRef]
- Bruno-Franco, M.; Mazzei, C. The red blood cell membrane: Structure and functions. Blood Transf. 2004, 2, 160–180. [Google Scholar]
- de Campos Ventura, B.; de Angelis, D.d.F.; Marin-Morales, M.A. Mutagenic and genotoxic effects of the Atrazine herbicide in Oreochromis niloticus (Perciformes, Cichlidae) detected by the micronuclei test and the comet assay. Pestic. Biochem. Physiol. 2008, 90, 42–51. [Google Scholar] [CrossRef]
- Betz, T.; Lenz, M.; Joanny, J.F.; Sykes, C. ATP-dependent mechanics of red blood cells. Proc. Natl. Acad. Sci. USA 2009, 106, 15320–15325. [Google Scholar] [CrossRef]
- Uçar, A.; Parlak, V.; Yeltekin, A.Ç.; Özgeriş, F.B.; Çağlar, O.; Türkez, H.; Alak, G.; Atamanalp, M. Assesment of hematotoxic, oxidative and genotoxic damage potentials of fipronil in rainbow trout Oncorhynchus mykiss, Walbaum. Toxicol. Mech. Methods 2021, 31, 73–80. [Google Scholar] [CrossRef]
- Weed, R.I.; Reed, C.F. Membrane alterations leading to red cell destruction. Am. J. Med. 1966, 41, 681–698. [Google Scholar] [CrossRef] [PubMed]
- Bratosin, D.; Estaquier, J.; Slomianny, C.; Tissier, J.P.; Quatannens, B.; Bulai, T.; Mitrofan, L.; Marinescu, A.; Trandaburu, I.; Ameisen, J.C.; et al. On the evolution of erythrocyte programmed cell death: Apoptosis of Rana esculenta nucleated red blood cells involves cysteine proteinase activation and mitochondrion permeabilization. Biochimie 2004, 86, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Martínez, M.A.; Wu, Q.; Ares, I.; Martínez-Larrañaga, M.R.; Anadón, A.; Yuan, Z. Fipronil insecticide toxicology: Oxidative stress and metabolism. Crit. Rev. Toxicol. 2016, 46, 876–899. [Google Scholar] [CrossRef] [PubMed]
- Officioso, A.; Manna, C.; Alzoubi, K.; Lang, F. Bromfenvinphos induced suicidal death of human erythrocytes. Pestic. Biochem. Physiol. 2016, 126, 58–63. [Google Scholar] [CrossRef]
- Mohanty, J.; Nagababu, E.; Rifkind, J. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front. Physiol. 2014, 5, 84. [Google Scholar] [CrossRef]
- Islas-Flores, L.; Novoa-Luna, K.A.; Islas-Flores, H.; San Juan-Reyes, N.; Gómez-Oliván, L.M. Evaluation of the Toxicity of Municipal Effluents from a Locality in the State of Mexico Using Hyalella azteca as a Bioindicator. In Pollution of Water Bodies in Latin America: Impact of Contaminants on Species of Ecological Interest; Gómez-Oliván, L.M., Ed.; Springer: Cham, Switzerland, 2019; pp. 97–111. [Google Scholar] [CrossRef]
- Bayero, U. Toxicity of Atrazine (Herbicide) to Juveniles of the African Catfish, Clarias gariepinus (Bürchell, 1822). Master’s Thesis, Ahmadu Bello University, Zaria, Nigeria, 2017. [Google Scholar]
- Ratn, A.; Awasthi, Y.; Kumar, M.; Singh, S.K.; Tripathi, R.; Trivedi, S.P. Phorate induced oxidative stress, DNA damage and differential expression of p53, apaf-1 and cat genes in fish, Channa punctatus (Bloch, 1793). Chemosphere 2017, 182, 382–391. [Google Scholar] [CrossRef]
- Farag, M.R.; Alagawany, M. Erythrocytes as a biological model for screening of xenobiotics toxicity. Chem.-Biol. Interact. 2018, 279, 73–83. [Google Scholar] [CrossRef]
- Akhtar, N.; Fiaz Khan, M.; Tabassum, S.; Zahran, E. Adverse effects of atrazine on blood parameters, biochemical profile and genotoxicity of snow trout (Schizothorax plagiostomus). Saudi J. Biol. Sci. 2021, 28, 1999–2003. [Google Scholar] [CrossRef]
- Rodilla, V. Origin and evolution of binucleated cells and binucleated cells with micronuclei in cisplatin-treated CHO cultures. Mutat. Res. Toxicol. 1993, 300, 281–291. [Google Scholar] [CrossRef]
- Jindal, R.; Batoye, S. SEM Studies on Erythrocyte Alterations in Ctenopharyngodon idellus (Cuvier and Valenciennes) induced by Fenvalerate. Res. J. Anim. Vet. Fish. Sci. 2015, 3, 1–5. [Google Scholar]
- do Nascimento Monteiro, J.A.; Araújo da Cunha, L.; Helen, P.M.; Souza dos Reis, H.; da Silva Aguiar, A.C.; Lobato de Oliveira-Bahia, V.R.; Rodríguez Burbano, R.M.; Machado da Rocha, C.A. Mutagenic and histopathological effects of hexavalent chromium in tadpoles of Lithobates catesbeianus (Shaw, 1802) (Anura, Ranidae). Ecotoxicol. Environ. Saf. 2018, 163, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Zeljezic, D.; Garaj-Vrhovac, V.; Perkovic, P. Evaluation of DNA damage induced by atrazine and atrazine-based herbicide in human lymphocytes in vitro using a comet and DNA diffusion assay. Toxicol. Vitr. 2006, 20, 923–935. [Google Scholar] [CrossRef] [PubMed]
- Edginton, A.N.; Rouleau, C. Toxicokinetics of 14C-Atrazine and Its Metabolites in Stage-66 Xenopus laevis. Environ. Sci. Technol. 2005, 39, 8083–8089. [Google Scholar] [CrossRef] [PubMed]
- Solomon, K.R.; Carr, J.A.; Du Preez, L.H.; Giesy, J.P.; Kendall, R.J.; Smith, E.E.; Van Der Kraak, G.J. Effects of Atrazine on Fish, Amphibians, and Aquatic Reptiles: A Critical Review. Crit. Rev. Toxicol. 2008, 38, 721–772. [Google Scholar] [CrossRef]
- Agius, C. On the failure to detect haemosiderin in the melano-macrophages of dogfish Scyliorhinus canicula (L.) after prolonged starvation. Experientia 1983, 39, 64–66. [Google Scholar] [CrossRef]
- Brown, C.L.; George, C. Age-dependent accumulation of macrophage aggregates in the yellow perch, Perca flavescens (Mitchill). J. Fish Dis. 1985, 8, 135–138. [Google Scholar] [CrossRef]
- Santos, A.T.; Valverde, B.S.L.; De Oliveira, C.; Franco-Belussi, L. Genotoxic and melanic alterations in Lithobates catesbeianus (anura) tadpoles exposed to fipronil insecticide. Environ. Sci. Pollut. Res. 2021, 28, 20072–20081. [Google Scholar] [CrossRef]
- Pérez-Iglesias, J.M.; Fanali, L.Z.; Franco-Belussi, L.; Natale, G.S.; De oliveira, C.; Brodeur, J.C.; Larramendy, M.L. Multiple Level Effects of Imazethapyr on Leptodactylus latinasus (Anura) Adult Frogs. Arch. Environ. Contam. Toxicol. 2021, 81, 492–506. [Google Scholar] [CrossRef]
- Mela, M.; Guiloski, I.; Doria, H.; Randi, M.; de Oliveira Ribeiro, C.; Pereira, L.; Maraschi, A.; Prodocimo, V.; Freire, C.; Silva de Assis, H. Effects of the herbicide atrazine in neotropical catfish (Rhamdia quelen). Ecotoxicol. Environ. Saf. 2013, 93, 13–21. [Google Scholar] [CrossRef]
- Barni, S.; Vaccarone, R.; Bertone, V.; Fraschini, A.; Bernini, F.; Fenoglio, C. Mechanisms of changes to the liver pigmentary component during the annual cycle (activity and hibernation) of Rana esculenta L. J. Anat. 2002, 200, 185–194. [Google Scholar] [CrossRef]
- Różanowska, M.; Sarna, T.; Land, E.J.; Truscott, T. Free radical scavenging properties of melanin: Interaction of eu- and pheo-melanin models with reducing and oxidising radicals. Free. Radic. Biol. Med. 1999, 26, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Saad, E.M.; Elassy, N.M.; Salah-Eldein, A.M. Effect of induced sublethal intoxication with neonicotinoid insecticides on Egyptian toads (Sclerophrys regularis). Environ. Sci. Pollut. Res. 2022, 29, 5762–5770. [Google Scholar] [CrossRef] [PubMed]
- Agius, C.; Roberts, R.J. Melano-macrophage centres and their role in fish pathology. J. Fish Dis. 2003, 26, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.E.; Melo Costa, P.; Batista Nascimento, S.; Castro, W.V.; de Azambuja Ribeiro, R.I.M.; Batista Santos, H.; Thomé, R.G. Atrazine promotes immunomodulation by melanomacrophage centre alterations in spleen and vascular disorders in gills from Oreochromis niloticus. Aquat. Toxicol. 2018, 202, 57–64. [Google Scholar] [CrossRef]
- Chen, J.Y.; Song, Y.; Zhang, L.S. Immunotoxicity of atrazine in Balb/c mice. J. Environ. Sci. Heal. Part B 2013, 48, 637–645. [Google Scholar] [CrossRef]
- Pérez-Iglesias, J.M.; Franco-Belussi, L.; Moreno, L.; Tripole, S.; de Oliveira, C.; Natale, G.S. Effects of glyphosate on hepatic tissue evaluating melanomacrophages and erythrocytes responses in neotropical anuran Leptodactylus latinasus. Environ. Sci. Pollut. Res. 2016, 23, 9852–9861. [Google Scholar] [CrossRef]
- Ribeiro, H.J.; Procópio, M.S.; Matsumura Gomes, J.M.; Oliveira Vieira, F.; Castro Russo, R.; Balzuweit, K.; Chiarini-Garcia, H.; Santana Castro, A.C.; Rizzo, E.; Dias Corrêa, J. Functional dissimilarity of melanomacrophage centres in the liver and spleen from females of the teleost fish Prochilodus argenteus. Cell Tissue Res. 2011, 346, 417–425. [Google Scholar] [CrossRef]
- Santos, R.M.B.; Monteiro, S.M.V.; Cortes, R.M.V.; Pacheco, F.A.L.; Fernandes, L.F.S. Seasonal Differences in Water Pollution and Liver Histopathology of Iberian Barbel (Luciobarbus bocagei) and Douro Nase (Pseudochondrostoma duriense) in an Agricultural Watershed. Water 2022, 14, 444. [Google Scholar] [CrossRef]
- Kelly-Reay, K.; Weeks-Perkins, B.A. Determination of the macrophage chemiluminescent response in Fundulus heteroclitus as a function of pollution stress. Fish Shellfish Immunol. 1994, 4, 95–105. [Google Scholar] [CrossRef]
- Opute, P.A.; Oboh, I.P. Hepatotoxic Effects of Atrazine on Clarias gariepinus (Burchell, 1822): Biochemical and Histopathological Studies. Arch. Environ. Contam. Toxicol. 2021, 80, 414–425. [Google Scholar] [CrossRef]
- MacFarlane, M.; Merrison, W.; Dinsdale, D.; Cohen, G.M. Active Caspases and Cleaved Cytokeratins Are Sequestered into Cytoplasmic Inclusions in Trail-Induced Apoptosis. J. Cell Biol. 2000, 148, 1239–1254. [Google Scholar] [CrossRef] [PubMed]
- Akulenko, N. Changes in Liver Parenchyma of Green Frogs (Pelophylax Esculentus Complex) Under Conditions of Anthropogenic Pollution and Their Use in Monitoring of Water Bodies. Vestn. Zool. 2015, 49, 453–458. [Google Scholar] [CrossRef]
- Krumschnabel, G.; Manzl, C.; Berger, C.; Hofer, B. Oxidative stress, mitochondrial permeability transition, and cell death in Cu-exposed trout hepatocytes. Toxicol. Appl. Pharmacol. 2005, 209, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Ziegler, U.; Groscurth, P. Morphological Features of Cell Death. Physiology 2004, 19, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Jayawardena, U.A.; Angunawela, P.; Wickramasinghe, D.D.; Ratnasooriya, W.D.; Udagama, P.V. Heavy metal–induced toxicity in the Indian green frog: Biochemical and histopathological alterations. Environ. Toxicol. Chem. 2017, 36, 2855–2867. [Google Scholar] [CrossRef]
- Mekkawy, I.A.; Mahmoud, U.M.; Sayed, A.E.D.H. Effects of 4-nonylphenol on blood cells of the African catfish Clarias gariepinus (Burchell, 1822). Tissue Cell 2011, 43, 223–229. [Google Scholar] [CrossRef]
- Ahmadivand, S.; Farahmand, H.; Mirvaghefi, A.; Eagderi, S.; Shokrpoor, S.; Rahmati-Holasoo, H. Histopathological and haematological response of male rainbow trout (Oncorhynchus mykiss) subjected to butachlor. Vet. Med. 2014, 59, 433–439. [Google Scholar] [CrossRef]
- Mohamed, F.A. Histopathological Studies on Tilapia zillii and Solea vulgaris from Lake Qarun, Egypt. World J. Fish Mar. Sci. 2009, 1, 29–39. [Google Scholar]
- Loumbourdis, N.S. Liver Histopathologic Alterations in the Frog Rana ridibunda from a Small River of Northern Greece. Arch. Environ. Contam. Toxicol. 2007, 53, 418–425. [Google Scholar] [CrossRef]
- Păunesco, A.; Ponepal, C.M.; Grigorean, V.T.; Popescu, M. Histopathological changes in the liver and kidney tissues of marsh frog (Pelophylax ridibundus) induced by the action of talstar 10EC insecticide. Analele Univ. Din Oradea -Fasc. Biol. 2012, 19, 5–10. [Google Scholar]
- Fanali, L.Z.; Freitas, J.S.; Franco-Belussi, L.; Taboga, S.R.; de Oliveira, C. Liver description in three neotropical anuran species: From anatomy to ultrastructure. Acta Zool. 2022, 103, 316–325. [Google Scholar] [CrossRef]
- Raymundo Raymundo, E.; Nikolskii Gavrilovç, I.; Duwig, C.; Prado Pano, B.L.; Moreno, C.I.; Gavi Reyes, F.; Figueroa Sandoval, B. Atrazine transport in an Andosol and a Vertisol of Mexico (In Spanish). Interciencia 2009, 34, 330–337. [Google Scholar]
- Hernández Vargas, J. Atrazine leaching in a vertisol from the Bajío de Guanajuato, Mexico. Master’s Thesis, Colegio de Posgraduados, Montecillo, Mexico, 2008. (In Spanish). [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).