Impact of Streptococcus agalactiae Challenge on Immune Response, Antioxidant Status and Hepatorenal Indices of Nile Tilapia: The Palliative Role of Chitosan White Poplar Nanocapsule
Abstract
1. Introduction
2. Material and Methods
2.1. Preparation of the White Poplar Leave Extract
2.2. Preparation of the CWPNC
2.3. Characterization of the CWPNC
2.3.1. Characterization of CWPNC Using AFM
2.3.2. Characterization of CWPNC Using TEM
2.3.3. Characterization of CWPNC Using XRD
2.3.4. Characterization of CWPNC Using BET Surface Area and DA Pore Size
2.3.5. Characterization of CWPNC Using DLS and Zeta Potential
2.4. Isolation and Preparation of the Bacterial Isolates
2.5. In Vitro Assay
2.5.1. Antibacterial Inhibition Activity by Disc Diffusion Assay
2.5.2. Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC)
2.6. In Vivo-Assay
2.6.1. Fish and Rearing Conditions
2.6.2. Identifying CWPNC Therapeutic Dosage
2.6.3. Experimental Setup
2.6.4. Blood Sampling
2.6.5. Serum Oxidative Stress Assay
2.6.6. Hepato-Renal Related Parameters
2.6.7. Immune-Related Parameters Assay
2.7. Data Analysis
3. Results
3.1. Characterization of the CWPNC
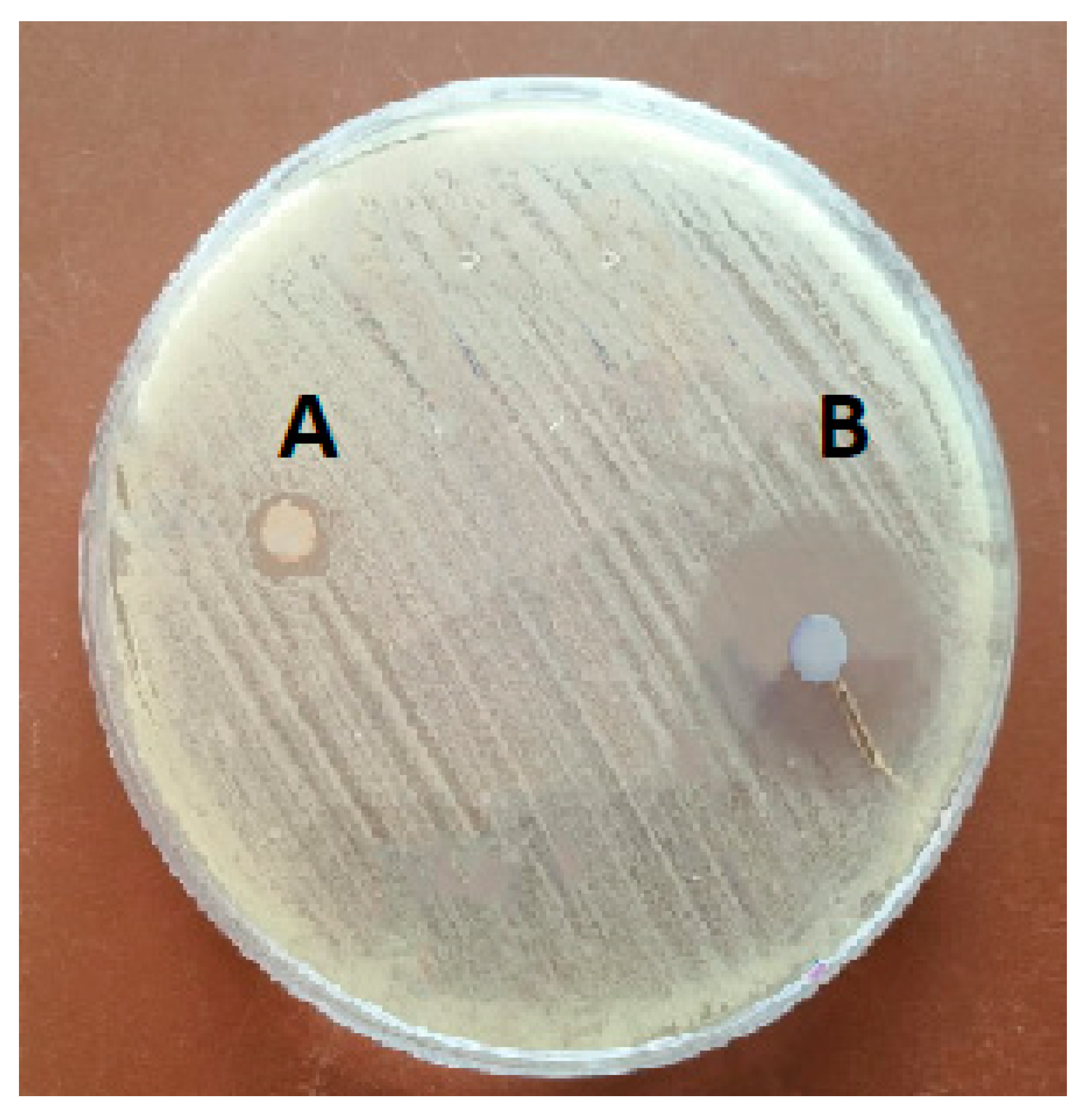
3.2. In Vitro Antibacterial Activity of CWPNC against S. agalactiae
3.3. In Vivo Assay
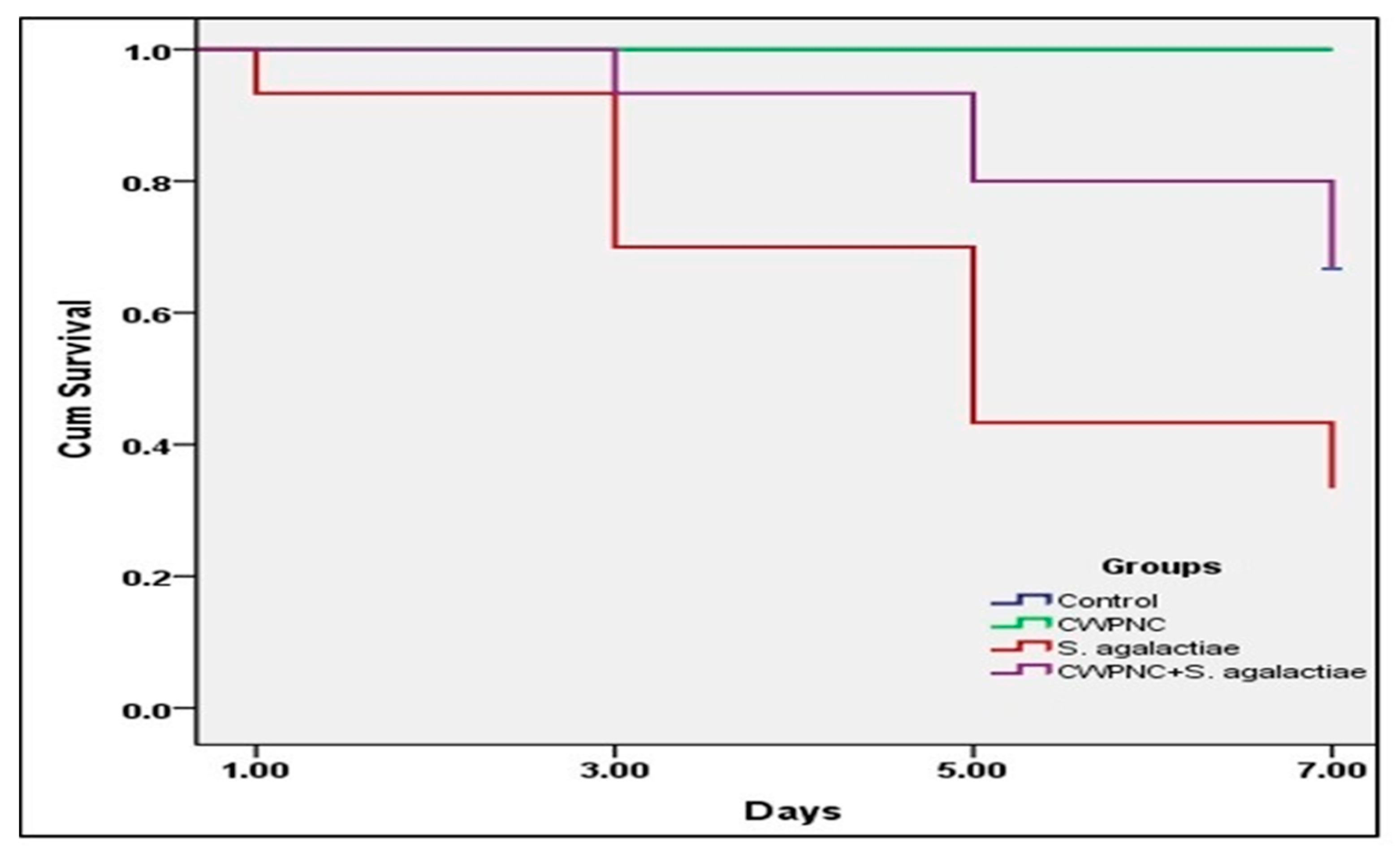
3.3.1. Clinical Observation, Behavior Response, and Survival Rate
3.3.2. The Results of the Shapiro-Wilk Test
3.3.3. Oxidative Stress-Related Parameters
3.3.4. Hepato-Renal Related Parameters
3.3.5. Immune-Related Parameters
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faggio, C.; Piccione, G.; Marafioti, S.; Arfuso, F.; Fortino, G.; Fazio, F. Metabolic response to monthly variations of Sparus aurata reared in Mediterranean on-shore tanks. Turk. J. Fish. Aquat. Sci. 2014, 14, 567–574. [Google Scholar]
- Faggio, C.; Arfuso, F.; Piccione, G.; Zumbo, A.; Fazio, F. Effect of three different anticoagulants and storage time on haematological parameters of Mugil cephalus (Linneaus, 1758). Turk. J. Fish. Aquat. Sci. 2014, 14, 615–621. [Google Scholar]
- Afewerki, S.; Asche, F.; Misund, B.; Thorvaldsen, T.; Tveteras, R. Innovation in the Norwegian aquaculture industry. Rev. Aquac. 2023, 15, 759–771. [Google Scholar] [CrossRef]
- Department, A.O. The State of World Fisheries and Aquaculture, 2000; Food & Agriculture Organization: Rome, Italy, 2000; Volume 3. [Google Scholar]
- Falk, T.M.; Abban, E.K. Genetic diversity of the Nile tilapia Oreochromis niloticus (Teleostei, Cichlidae) from the Volta System in Ghana. Biodivers. Manag. Util. West Afr Fishes. 2004, 13–15. [Google Scholar]
- Pridgeon, J.W.; Klesius, P.H. Major bacterial diseases in aquaculture and their vaccine development. CABI Rev. 2012, 11, 1–16. [Google Scholar] [CrossRef]
- Rajme-Manzur, D.; Gollas-Galvan, T.; Vargas-Albores, F.; Martínez-Porchas, M.; Hernández-Oñate, M.Á.; Hernández-López, J. Granulomatous bacterial diseases in fish: An overview of the host’s immune response. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2021, 261, 111058. [Google Scholar] [CrossRef]
- Amal, M.; Zamri-Saad, M. Streptococcosis in tilapia (Oreochromis niloticus): A review. Pertanika J. Trop. Agric. Sci. 2011, 34, 195–206. [Google Scholar]
- Ghetas, H.; Neiana, A.; Khalil, R.; AM, H.; Khallaf, M. Streptococcus agalactiae Isolation and Characterization in Nile Tilapia (Oreochromis niloticus) with Histopathological Studies. J. Curr. Vet. Res. 2021, 3, 70–79. [Google Scholar] [CrossRef]
- Cabello, F.C. Heavy use of prophylactic antibiotics in aquaculture: A growing problem for human and animal health and for the environment. Environ. Microbiol. 2006, 8, 1137–1144. [Google Scholar] [CrossRef]
- Maron, D.; Smith, T.; Nachman, K. Restrictions on antimicrobial use in food animal production: An international regulatory and economic survey. Glob. Health 2013, 9, 48–58. [Google Scholar] [CrossRef]
- Binh, V.N.; Dang, N.; Anh, N.T.K.; Thai, P.K. Antibiotics in the aquatic environment of Vietnam: Sources, concentrations, risk and control strategy. Chemosphere 2018, 197, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Forouzi, A.; Ghasemnezhad, A.; Nasrabad, R.G. Phytochemical response of Stevia plant to growth promoting microorganisms under salinity stress. S. Afr. J. Bot. 2020, 134, 109–118. [Google Scholar] [CrossRef]
- Karrenberg, S.; Edwards, P.J.; Kollmann, J. The life history of Salicaceae living in the active zone of floodplains. Freshw. Biol. 2002, 47, 733–748. [Google Scholar] [CrossRef]
- Nassima, B.; Nassima, B.; Riadh, K. Antimicrobial and antibiofilm activities of phenolic compounds extracted from Populus nigra and Populus alba buds (Algeria). Brazilian J. Pharm. Sci. 2019, 55, e18114. [Google Scholar] [CrossRef]
- Emerich, D.F.; Thanos, C.G. Targeted nanoparticle-based drug delivery and diagnosis. J. Drug Target. 2007, 15, 163–183. [Google Scholar] [CrossRef]
- Shaffer, C. Nanomedicine transforms drug delivery. Drug Discov. Today 2005, 10, 1581–1582. [Google Scholar] [CrossRef]
- Yoshikawa, T.; Tsutsumi, Y.; Nakagawa, S. Development of nanomedicine using intracellular DDS. Nihon. Rinsho. 2006, 64, 247–252. [Google Scholar]
- Moghimi, S.M.; Hunter, A.C.; Murray, J.C. Nanomedicine: Current status and future prospects. FASEB J. 2005, 19, 311–330. [Google Scholar] [CrossRef]
- Emerich, D.F. Nanomedicine–prospective therapeutic and diagnostic applications. Expert Opin. Biol. Ther. 2005, 5, 1–5. [Google Scholar] [CrossRef]
- Sahoo, S.; Parveen, S.; Panda, J. The present and future of nanotechnology in human health care. Nanomedicine 2007, 3, 20–31. [Google Scholar] [CrossRef]
- Pillai, C.K.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Ibrahim, R.E.; Elshopakey, G.E.; Abd El-Rahman, G.I.; Ahmed, A.I.; Altohamy, D.E.; Zaglool, A.W.; Younis, E.M.; Abdelwarith, A.A.; Davies, S.J.; Al-Harthi, H.F. Palliative role of colloidal silver nanoparticles synthetized by moringa against Saprolegnia spp. infection in Nile Tilapia: Biochemical, immuno-antioxidant response, gene expression, and histopathological investigation. Aquac. Rep. 2022, 26, 101318. [Google Scholar] [CrossRef]
- El-Houseiny, W.; Mansour, M.F.; Mohamed, W.A.; Al-Gabri, N.A.; El-Sayed, A.A.; Altohamy, D.E.; Ibrahim, R.E. Silver nanoparticles mitigate Aeromonas hydrophila-induced immune suppression, oxidative stress, and apoptotic and genotoxic effects in Oreochromis niloticus. Aquaculture 2021, 535, 736430. [Google Scholar] [CrossRef]
- Ma, Z.; Garrido-Maestu, A.; Jeong, K.C. Application, mode of action, and in vivo activity of chitosan and its micro-and nanoparticles as antimicrobial agents: A review. Carbohydr. Polym. 2017, 176, 257–265. [Google Scholar] [CrossRef]
- Liu, C.; Wang, L.; Xu, H.; Wang, S.; Gao, S.; Ji, X.; Xu, Q.; Lan, W. “One pot” green synthesis and the antibacterial activity of g-C3N4/Ag nanocomposites. Mater. Lett. 2016, 164, 567–570. [Google Scholar] [CrossRef]
- Aliasghari, A.; Rabbani Khorasgani, M.; Vaezifar, S.; Rahimi, F.; Younesi, H.; Khoroushi, M. Evaluation of antibacterial efficiency of chitosan and chitosan nanoparticles on cariogenic streptococci: An in vitro study. Iran. J. Microbiol. 2016, 8, 93–100. [Google Scholar]
- Ibrahim, R.E.; Amer, S.A.; Farroh, K.Y.; Al-Gabri, N.A.; Ahmed, A.I.; El-Araby, D.A.; Ahmed, S.A. The effects of chitosan-vitamin C nanocomposite supplementation on the growth performance, antioxidant status, immune response, and disease resistance of Nile tilapia (Oreochromis niloticus) fingerlings. Aquaculture 2021, 534, 736269. [Google Scholar] [CrossRef]
- Zetterberg, C.; Öfverholm, T. Carpal tunnel syndrome and other wrist/hand symptoms and signs in male and female car assembly workers. Int. J. Ind. Ergon. 1999, 23, 193–204. [Google Scholar] [CrossRef]
- Jenkins, J.A.; Bart, H., Jr.; Bowker, J.D.; Bowser, P.; MacMillan, J.; Nickum, J.; Rose, J.; Sorensen, P.; Whitledge, G.; Rachlin, J.W.J.B.; et al. Guidelines for the Use of Fishes in Research; American Fisheries Society: Bethesda, MD, USA, 2014; p. 104. [Google Scholar]
- Nishikimi, M.; Rao, N.A.; Yagi, K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef]
- Aebi, H. [13] Catalase in vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analyt. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Murray, R. Aspartate aminotransferase. In Clinical Chemistry; Kaplan, L.A., Pesce, A.J., Eds.; The CV Mosby Co.: Toronto, ON, Canada, 1984; pp. 418, 437, 1257–1260. [Google Scholar]
- Burtis, C.A.; Ashwood, E.R. Tietz Textbook of Clinical Chemistry; Amer Assn for Clinical Chemistry: Washington, DC, USA, 1994. [Google Scholar]
- Bartles, H.; Bohmer, M.; Heirli, C. Colorimetric kinetic method for creatinine determination in serum and urine. Clin. Chem. Acta 1972, 37, 193–195. [Google Scholar]
- Caruso, D.; Schlumberger, O.; Dahm, C.; Proteau, J.-P. Plasma lysozyme levels in sheatfish Silurus glanis (L.) subjected to stress and experimental infection with Edwardsiella tarda. Aquac. Res. 2002, 33, 999–1008. [Google Scholar] [CrossRef]
- Pérez-Sánchez, T.; Mora-Sánchez, B.; Balcázar, J.L. Biological Approaches for Disease Control in Aquaculture: Advantages, Limitations and Challenges. Trends Microbiol. 2018, 26, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Defoirdt, T.; Sorgeloos, P.; Bossier, P. Alternatives to antibiotics for the control of bacterial disease in aquaculture. Curr. Opin. Microbiol. 2011, 14, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Seil, J.T.; Webster, T.J. Antimicrobial applications of nanotechnology: Methods and literature. Int. J. Nanomed. 2012, 7, 2767–2781. [Google Scholar]
- Banna, A.H.E.; Youssef, F.S.; Elzorba, H.Y.; Soliman, A.M.; Mohamed, G.G.; Ismail, S.H.; Mousa, M.R.; Elbanna, H.A.; Osman, A.S. Evaluation of the wound healing effect of neomycin-silver nano-composite gel in rats. Int. J. Immunopathol. Pharmacol. 2022, 36, 03946320221113486. [Google Scholar] [PubMed]
- Rajendran, R.; Radhai, R.; Balakumar, C.; Ahamed, H.A.M.; Vigneswaran, C.; Vaideki, K. Synthesis and characterization of neem chitosan nanocomposites for development of antimicrobial cotton textiles. J. Eng. Fiber. Fabr. 2012, 7, 155892501200700116. [Google Scholar] [CrossRef]
- Amina, C.H.; Souraya, E.G.; Abdellatif, H.; Suzanna, D.; Hakima, S.; Saad, I.; Mohammed, I. Antimycobacterial activity of Populus alba leaf extracts. J. Med. Plant. Res. 2013, 7, 1015–1021. [Google Scholar]
- Devlieghere, F.; Vermeulen, A.; Debevere, J. Chitosan: Antimicrobial activity, interactions with food components and applicability as a coating on fruit and vegetables. Food Microbiol. 2004, 21, 703–714. [Google Scholar] [CrossRef]
- Yildirim-Aksoy, M.; Beck, B. Antimicrobial activity of chitosan and a chitosan oligomer against bacterial pathogens of warmwater fish. J. Appl. Microbiol. 2017, 122, 1570–1578. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.-C.; Su, Y.P.; Chen, C.-C.; Jia, G.; Wang, H.L.; Wu, J.G.; Lin, J.G. Relationship between antibacterial activity of chitosan and surface characteristics of cell wall. Acta Pharmacol. Sin. 2004, 25, 932–936. [Google Scholar]
- Ulanowska, K.; Tkaczyk, A.; Konopa, G.; Węgrzyn, G. Differential antibacterial activity of genistein arising from global inhibition of DNA, RNA and protein synthesis in some bacterial strains. Arch. Microbiol. 2006, 184, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Bouarab-Chibane, L.; Forquet, V.; Lantéri, P.; Clément, Y.; Léonard-Akkari, L.; Oulahal, N.; Degraeve, P.; Bordes, C. Antibacterial Properties of Polyphenols: Characterization and QSAR (Quantitative Structure-Activity Relationship) Models. Front. Microbiol. 2019, 10, 829. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xie, S.; Ahmed, S.; Wang, F.; Gu, Y.; Zhang, C.; Chai, X.; Wu, Y.; Cai, J.; Cheng, G. Antimicrobial activity and resistance: Influencing factors. Front. Pharm. 2017, 8, 364. [Google Scholar] [CrossRef] [PubMed]
- Chusnie, T.; Lamb, A.J. Antimicrobial activity of flavonoid. Int. J. Antimicrob. Agent 2005, 26, 343–356. [Google Scholar]
- Goy, R.C.; Britto, D.d.; Assis, O.B. A review of the antimicrobial activity of chitosan. Polímeros 2009, 19, 241–247. [Google Scholar] [CrossRef]
- Ganan, M.; Carrascosa, A.; Martinez-Rodriguez, A. Antimicrobial activity of chitosan against Campylobacter spp. and other microorganisms and its mechanism of action. J. Food Prot. 2009, 72, 1735–1738. [Google Scholar] [CrossRef]
- Raafat, D.; Von Bargen, K.; Haas, A.; Sahl, H.-G. Insights into the mode of action of chitosan as an antibacterial compound. Appl. Environ. Microbiol. 2008, 74, 3764–3773. [Google Scholar] [CrossRef]
- Figueiredo, H.; Carneiro, D.; Faria, F.; Costa, G. Streptococcus agalactiae associado à meningoencefalite e infecção sistêmica em tilápia-do-Nilo (Oreochromis niloticus) no Brasil. Arq. Bras. Med. Vet. Zootec. 2006, 58, 678–680. [Google Scholar] [CrossRef]
- Pretto-Giordano, L.G.; Müller, E.E.; Freitas, J.C.d.; Silva, V.G.d. Evaluation on the Pathogenesis of Streptococcus agalactiae in Nile Tilapia (Oreochromis niloticus). Brazilian Arch. Biol. Technol. 2010, 53, 87–92. [Google Scholar] [CrossRef]
- Salvador, R.; Muller, E.E.; Freitas, J.C.d.; Leonhadt, J.H.; Pretto-Giordano, L.G.; Dias, J.A. Isolation and characterization of Streptococcus spp. group B in Nile tilapias (Oreochromis niloticus) reared in hapas nets and earth nurseries in the northern region of Parana State, Brazil. Ciência Rural 2005, 35, 1374–1378. [Google Scholar] [CrossRef]
- Lin, F.P.-Y.; Lan, R.; Sintchenko, V.; Gilbert, G.L.; Kong, F.; Coiera, E. Computational bacterial genome-wide analysis of phylogenetic profiles reveals potential virulence genes of Streptococcus agalactiae. PLoS ONE 2011, 6, e17964. [Google Scholar] [CrossRef] [PubMed]
- Pietrocola, G.; Visai, L.; Valtulina, V.; Vignati, E.; Rindi, S.; Arciola, C.R.; Piazza, R.; Speziale, P. Multiple interactions of FbsA, a surface protein from Streptococcus agalactiae, with fibrinogen: Affinity, stoichiometry, and structural characterization. Biochemistry 2006, 45, 12840–12852. [Google Scholar] [CrossRef] [PubMed]
- Buscetta, M.; Papasergi, S.; Firon, A.; Pietrocola, G.; Biondo, C.; Mancuso, G.; Midiri, A.; Romeo, L.; Teti, G.; Speziale, P. FbsC, a novel fibrinogen-binding protein, promotes Streptococcus agalactiae-host cell interactions. J. Biol. Chem. 2014, 289, 21003–21015. [Google Scholar] [CrossRef]
- Landwehr-Kenzel, S.; Henneke, P. Interaction of Streptococcus agalactiae and cellular innate immunity in colonization and disease. Front. Immunol. 2014, 5, 519. [Google Scholar] [CrossRef]
- Kannika, K.; Pisuttharachai, D.; Srisapoome, P.; Wongtavatchai, J.; Kondo, H.; Hirono, I.; Unajak, S.; Areechon, N. Molecular serotyping, virulence gene profiling and pathogenicity of Streptococcus agalactiae isolated from tilapia farms in Thailand by multiplex PCR. J. Appl. Microbiol. 2017, 122, 1497–1507. [Google Scholar] [CrossRef]
- Ibrahim, R.E.; Amer, S.A.; Shahin, S.A.; Darwish, M.I.; Albogami, S.; Abdelwarith, A.A.; Younis, E.M.; Abduljabbar, M.H.; Davies, S.J.; Attia, G.A. Effect of fish meal substitution with dried bovine hemoglobin on the growth, blood hematology, antioxidant activity and related genes expression, and tissue histoarchitecture of Nile tilapia (Oreochromis niloticus). Aquac. Rep. 2022, 26, 101276. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Ibrahim, R.E.; Elshopakey, G.E.; Khamis, T.; Abdel-Ghany, H.M.; Abdelwarith, A.A.; Younis, E.M.; Davies, S.J.; Elabd, H.; Elhady, M. Immune-antioxidant trait, growth, splenic cytokines expression, apoptosis, and histopathological alterations of Oreochromis niloticus exposed to sub-lethal copper toxicity and fed thyme and/or basil essential oils enriched diets. Fish Shellfish Immunol. 2022, 131, 1006–1018. [Google Scholar] [CrossRef]
- Amer, S.A.; Farahat, M.; Khamis, T.; Abdo, S.A.; Younis, E.M.; Abdel-Warith, A.-W.A.; Reda, R.; Ali, S.A.; Davies, S.J.; Ibrahim, R.E. Evaluation of Spray-Dried Bovine Hemoglobin Powder as a Dietary Animal Protein Source in Nile Tilapia, Oreochromis niloticus. Animals 2022, 12, 3206. [Google Scholar] [CrossRef]
- Fontagné-Dicharry, S.; Lataillade, E.; Surget, A.; Larroquet, L.; Cluzeaud, M.; Kaushik, S. Antioxidant defense system is altered by dietary oxidized lipid in first-feeding rainbow trout (Oncorhynchus mykiss). Aquaculture 2014, 424, 220–227. [Google Scholar] [CrossRef]
- Abdel Rahman, A.N.; Van Doan, H.; Elsheshtawy, H.M.; Dawood, A.; Salem, S.M.; Sheraiba, N.I.; Masoud, S.R.; Abdelnaeim, N.S.; Khamis, T.; Alkafafy, M. Dietary Salvia officinalis leaves enhances antioxidant-immune-capacity, resistance to Aeromonas sobria challenge, and growth of Cyprinus carpio. Fish Shellfish Immunol. 2022, 127, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Abdel Rahman, A.N.; Shakweer, M.S.; Algharib, S.A.; Abdelaty, A.I.; Kamel, S.; Ismail, T.A.; Daoush, W.M.; Ismail, S.H.; Mahboub, H.H. Silica nanoparticles acute toxicity alters ethology, neuro-stress indices, and physiological status of African catfish (Clarias gariepinus). Aquac. Rep. 2022, 23, 101034. [Google Scholar] [CrossRef]
- Garcia, D.; Lima, D.; da Silva, D.G.H.; de Almeida, E.A. Decreased malondialdehyde levels in fish (Astyanax altiparanae) exposed to diesel: Evidence of metabolism by aldehyde dehydrogenase in the liver and excretion in water. Ecotoxicol. Environ. Saf. 2020, 190, 110107. [Google Scholar] [CrossRef]
- Ozmen, I.; Bayir, A.; Cengiz, M.; Sirkecioglu, A.; Atamanalp, M. Effects of water reuse system on antioxidant enzymes of rainbow trout (Oncorhynchus mykiss W., 1792). Vet. Med. 2004, 49, 373. [Google Scholar] [CrossRef]
- Zelko, I.N.; Mariani, T.J.; Folz, R.J. Superoxide dismutase multigene family: A comparison of the CuZn-SOD (SOD1), Mn-SOD (SOD2), and EC-SOD (SOD3) gene structures, evolution, and expression. Free Radic. Biol. Med. 2002, 33, 337–349. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Souza, C.F.; da Silva, A.S.; Velho, M.C.; Ourique, A.F.; Baldisserotto, B. Benefits of nanotechnology: Dietary supplementation with nerolidol-loaded nanospheres increases survival rates, reduces bacterial loads and prevents oxidative damage in brains of Nile tilapia experimentally infected by Streptococcus agalactiae. Microb. Pathog. 2020, 141, 103989. [Google Scholar] [CrossRef] [PubMed]
- Danise, T.; Innangi, M.; Curcio, E.; Piccolella, S.; Fioretto, A.; Pacifico, S. White poplar (Populus alba L.) leaf waste recovery and intercropping outcome on its polyphenols. Ind. Crops Prod. 2021, 171, 113866. [Google Scholar] [CrossRef]
- Mohamed, W.A.; El-Houseiny, W.; Ibrahim, R.E.; Abd-Elhakim, Y.M. Palliative effects of zinc sulfate against the immunosuppressive, hepato-and nephrotoxic impacts of nonylphenol in Nile tilapia (Oreochromis niloticus). Aquaculture 2019, 504, 227–238. [Google Scholar] [CrossRef]
- Abdel Rahman, A.N.; Mohamed, A.A.-R.; Dahran, N.; Farag, M.F.; Alqahtani, L.S.; Nassan, M.A.; AlThobaiti, S.A.; El-Naseery, N.I. Appraisal of sub-chronic exposure to lambada-cyhalothrin and/or methomyl on the behavior and hepato-renal functioning in Oreochromis niloticus: Supportive role of taurine-supplemented feed. Aquat. Toxicol. 2022, 250, 106257. [Google Scholar] [CrossRef]
- Abdel Rahman, A.N.; El-Bouhy, Z.; Wahbah, M.; Ahmed, S. Fisheries. Effects of dietary turmeric and clove powder on growth and immune response of the Nile tilapia. Egypt. J. Aquat. Biol. Fish. 2020, 24, 589–608. [Google Scholar] [CrossRef]
- Zamri-Saad, M.; Amal, M.N.A.; Siti-Zahrah, A. Pathological Changes in Red Tilapias (Oreochromis spp.) Naturally Infected by Streptococcus agalactiae. J. Comp. Pathol. 2010, 143, 227–229. [Google Scholar] [CrossRef] [PubMed]
- Uribe, C.; Folch, H.; Enríquez, R.; Moran, G. Innate and adaptive immunity in teleost fish: A review. Vet. Med. 2011, 56, 486. [Google Scholar] [CrossRef]
- Reda, R.M.; Maricchiolo, G.; Quero, G.M.; Basili, M.; Aarestrup, F.M.; Pansera, L.; Mirto, S.; Abd El-Fattah, A.H.; Alagawany, M.; Abdel Rahman, A.N. Rice protein concentrate as a fish meal substitute in Oreochromis niloticus: Effects on immune response, intestinal cytokines, Aeromonas veronii resistance, and gut microbiota composition. Fish Shellfish Immunol. 2022, 126, 237–250. [Google Scholar] [CrossRef]
- Jones, E.M.; Oliver, L.P.; Ma, J.; Leeuwis, R.H.J.; Myrsell, V.; Arkoosh, M.R.; Dietrich, J.P.; Schuster, C.M.; Hawkyard, M.; Gamperl, A.K.; et al. Production of a monoclonal antibody specific to sablefish (Anoplopoma fimbria) IgM and its application in ELISA, western blotting, and immunofluorescent staining. Fish Shellfish Immunol. 2022, 130, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Kania, P.W.; Buchmann, K. Complement Activation in Fish with Emphasis on MBL/MASP. In Principles of Fish Immunology; Springer: Berlin/Heidelberg, Germany, 2022; pp. 279–300. [Google Scholar]
- Mahboub, H.H.; Shahin, K.; Mahmoud, S.M.; Altohamy, D.E.; Husseiny, W.A.; Mansour, D.A.; Shalaby, S.I.; Gaballa, M.M.; Shaalan, M.; Alkafafy, M.; et al. Silica nanoparticles are novel aqueous additive mitigating heavy metals toxicity and improving the health of African catfish, Clarias gariepinus. Aquat. Toxicol. 2022, 249, 106238. [Google Scholar] [PubMed]
- Wang, J.; Lu, D.-Q.; Jiang, B.; Luo, H.-L.; Lu, G.-L.; Li, A.-X. The effect of intermittent hypoxia under different temperature on the immunomodulation in Streptococcus agalactiae vaccinated Nile tilapia (Oreochromis niloticus). Fish Shellfish Immunol. 2018, 79, 181–192. [Google Scholar] [CrossRef]
- Wu, Y.-R.; Gong, Q.-F.; Fang, H.; Liang, W.-W.; Chen, M.; He, R.-J. Effect of Sophora flavescens on non-specific immune response of tilapia (GIFT Oreochromis niloticus) and disease resistance against Streptococcus agalactiae. Fish Shellfish Immunol. 2013, 34, 220–227. [Google Scholar] [CrossRef]
- Lin, Y.-S.; Saputra, F.; Chen, Y.-C.; Hu, S.-Y. Dietary administration of Bacillus amyloliquefaciens R8 reduces hepatic oxidative stress and enhances nutrient metabolism and immunity against Aeromonas hydrophila and Streptococcus agalactiae in zebrafish (Danio rerio). Fish Shellfish Immunol. 2019, 86, 410–419. [Google Scholar] [CrossRef]

| Conc. (mg/L) | Clinical Observations | |||
|---|---|---|---|---|
| Mortality (N = 10) | Loss of Escape Reflex | Abnormal Swimming | External Skin Lesion | |
| 0.0 | 0/10 | - | - | - |
| 1 | 0/10 | - | - | - |
| 2 | 0/10 | - | - | - |
| 3 | 0/10 | - | - | - |
| 4 | 1/10 | - | - | - |
| 5 | 1/10 | + | + | - |
| 6 | 2/10 | + | + | + |
| 7 | 2/10 | ++ | ++ | ++ |
| 8 | 3/10 | +++ | +++ | +++ |
| Test | CWPNC | Chitosan |
|---|---|---|
| inhibition zone (mm) | 22 ± 1.23 | 2.5 ± 0.20 |
| MIC (µg/mL) | 10 | 80 |
| MBC (µg/mL) | 20 | 100 |
| Behavior | Control | CWPNC | S. agalactiae | CWPNC + S. agalactiae |
|---|---|---|---|---|
| Surfacing | - | - | +++ | + |
| Abnormal swimming | - | - | +++ | + |
| Loss of escape reflex | - | - | +++ | ++ |
| Groups | MDA | CAT | SOD | AST | ALT | Creatinine | IgM | LYZ | C3 |
|---|---|---|---|---|---|---|---|---|---|
| Control | 0.19 | 1.00 | 0.24 | 1.00 | 0.32 | 0.17 | 0.87 | 0.95 | 0.67 |
| CWPNC | 0.35 | 0.79 | 0.92 | 0.99 | 1.00 | 1.00 | 0.98 | 0.88 | 1.00 |
| S. agalactiae | 1.00 | 0.89 | 1.00 | 1.00 | 1.00 | 1.00 | 0.98 | 1.00 | 0.90 |
| CWPNC + S. agalactiae | 0.99 | 1.00 | 1.00 | 1.00 | 1.00 | 0.17 | 1.00 | 1.00 | 0.78 |
| S. agalactiae Infection | CWPNC (mg/L) | MDA (nmol/mL) | CAT (ng/mL) | SOD (ng/mL) |
|---|---|---|---|---|
| Effect of S. agalactiae infection | ||||
| Non-Infected | 1.42 ± 1.96 b | 154.22 ± 9.94 a | 162.46 ± 6.52 a | |
| Infected | 12.85 ± 2.98 a | 30.80 ± 3.52 b | 33.10 ± 1.66 b | |
| Effect of CWPNC as a water additive | ||||
| 0 | 11.01 ± 1.96 a | 86.29 ± 9.02 | 72.46 ± 4.23 b | |
| 3 | 3.26 ± 2.03 b | 98.73 ± 3.52 | 123.09 ± 1.20 a | |
| Interaction | ||||
| Non-infected | 0 | 1.14 ± 0.04 c | 152.15 ± 5.40 a | 133.90 ± 3.04 b |
| 3 | 1.71 ± 0.01 c | 156.30 ± 6.60 a | 191.02 ± 0.96 a | |
| Infected | 0 | 20.89 ± 0.32 a | 20.43 ± 0.44 c | 11.03 ± 0.49 d |
| 3 | 4.82 ± 0.17 b | 41.17 ± 1.05 b | 55.16 ± 0.93 c | |
| Two-way Anova p-value | ||||
| S. agalactiae infection | 0.01 | 0.03 | 0.02 | |
| CWPNC water additive | 0.02 | 0.39 | 0.01 | |
| Interaction | 0.01 | 0.001 | 0.002 | |
| S. agalactiae Infection | CWPNC (mg/L) | AST (U/L) | ALT (U/L) | Creatinine (mg/dL) |
|---|---|---|---|---|
| Effect of S. agalactiae infection | ||||
| Non-Infected | 84.87 ± 2.01 b | 16.65 ± 3.15 b | 0.25 ± 0.04 | |
| Infected | 113.72 ± 5.82 a | 36.68 ± 4.32 a | 0.36 ± 0.06 | |
| Effect of CWPNC as a water additive | ||||
| 0 | 104.07 ± 2.01 a | 32.40 ± 3.12 a | 0.31 ± 0.03 | |
| 3 | 94.52 ± 4.52 b | 20.93 ± 4.02 b | 0.30 ± 0.02 | |
| Interaction | ||||
| Non-infected | 0 | 85.74 ± 0.98 c | 15.81 ± 1.12 c | 0.23 ± 0.01 c |
| 3 | 84.00 ± 2.00 c | 17.50 ± 1.00 c | 0.27 ± 0.02 c | |
| Infected | 0 | 122.40 ± 2.00 a | 49.00 ± 2.00 a | 0.40 ± 0.20 a |
| 3 | 105.05 ± 3.05 b | 24.37 ± 1.81 b | 0.33 ± 0.11 b | |
| Two-way Anova p-value | ||||
| S. agalactiae infection | 0.01 | 0.001 | 0.11 | |
| CWPNC water additive | 0.001 | 0.02 | 0.82 | |
| Interaction | 0.01 | 0.03 | 0.001 | |
| S. agalactiae Infection | CWPNC (mg/L) | IgM (ng/mL) | LYZ (ng/mL) | C3 (mg/dL) |
|---|---|---|---|---|
| Effect of S. agalactiae infection | ||||
| Non-Infected | 406.11 ± 6.43 a | 5.88 ± 0.58 a | 11.67 ± 3.42 a | |
| Infected | 177.92 ± 4.63 b | 1.04 ± 0.11 b | 3.34 ± 0.45 b | |
| Effect of CWPNC as a water additive | ||||
| 0 | 269.41 ± 6.22 b | 2.41 ± 0.49 b | 5.51 ± 3.22 b | |
| 3 | 314.62 ± 4.12 a | 4.51 ± 0.19 a | 9.49 ± 0.44 a | |
| Interaction | ||||
| Non-infected | 0 | 371.23 ± 10.13 b | 4.60 ± 0.20 b | 9.23 ± 0.49 b |
| 3 | 441.00 ± 10.00 a | 7.16 ± 0.10 a | 14.11 ± 2.00 a | |
| Infected | 0 | 167.60 ± 2.74 d | 0.23 ± 0.02 d | 1.80 ± 0.10 d |
| 3 | 188.25 ± 2.01 c | 1.86 ± 0.02 c | 4.88 ± 0.02 c | |
| Two-way Anova p-value | ||||
| S. agalactiae infection | 0.001 | 0.01 | 0.01 | |
| CWPNC water additive | 0.01 | 0.001 | 0.001 | |
| Interaction | 0.01 | 0.001 | 0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdel Rahman, A.N.; Ismail, S.H.; Fouda, M.M.S.; Abdelwarith, A.A.; Younis, E.M.; Khalil, S.S.; El-Saber, M.M.; Abdelhamid, A.E.; Davies, S.J.; Ibrahim, R.E. Impact of Streptococcus agalactiae Challenge on Immune Response, Antioxidant Status and Hepatorenal Indices of Nile Tilapia: The Palliative Role of Chitosan White Poplar Nanocapsule. Fishes 2023, 8, 199. https://doi.org/10.3390/fishes8040199
Abdel Rahman AN, Ismail SH, Fouda MMS, Abdelwarith AA, Younis EM, Khalil SS, El-Saber MM, Abdelhamid AE, Davies SJ, Ibrahim RE. Impact of Streptococcus agalactiae Challenge on Immune Response, Antioxidant Status and Hepatorenal Indices of Nile Tilapia: The Palliative Role of Chitosan White Poplar Nanocapsule. Fishes. 2023; 8(4):199. https://doi.org/10.3390/fishes8040199
Chicago/Turabian StyleAbdel Rahman, Afaf N., Sameh H. Ismail, Moustafa M. S. Fouda, Abdelwahab A. Abdelwarith, Elsayed M. Younis, Samah S. Khalil, Mahmoud M. El-Saber, Ahmed E. Abdelhamid, Simon J. Davies, and Rowida E. Ibrahim. 2023. "Impact of Streptococcus agalactiae Challenge on Immune Response, Antioxidant Status and Hepatorenal Indices of Nile Tilapia: The Palliative Role of Chitosan White Poplar Nanocapsule" Fishes 8, no. 4: 199. https://doi.org/10.3390/fishes8040199
APA StyleAbdel Rahman, A. N., Ismail, S. H., Fouda, M. M. S., Abdelwarith, A. A., Younis, E. M., Khalil, S. S., El-Saber, M. M., Abdelhamid, A. E., Davies, S. J., & Ibrahim, R. E. (2023). Impact of Streptococcus agalactiae Challenge on Immune Response, Antioxidant Status and Hepatorenal Indices of Nile Tilapia: The Palliative Role of Chitosan White Poplar Nanocapsule. Fishes, 8(4), 199. https://doi.org/10.3390/fishes8040199






