Abstract
Ubiquitin-40S ribosomal protein S27a (RPS27A), ubiquitin-like protein Fubi, and ribosomal protein (S30FAU) are ubiquitin-related proteins that are involved in the regulation of immune-related functions such as cell cycle, protein expression, and apoptosis. This study aimed to confirm the molecular characteristics, gene expression analysis, and antibacterial activity of RPS27A and S30FAU identified from the starry flounder (15 starry flounders of 128.7 ± 18.2 g). An expression analysis using a normal fish showed that RPS27A was highly expressed in the head kidney, heart, and stomach. In contrast, S30FAU exhibited high expression in the stomach, heart, and head kidney. Upon simulating an artificial pathogen infection, RPS27A was highly expressed in the heart at 1 h and 3 days post-viral hemorrhagic septicemia (VHSV) infection, and had a high expression in the kidney, liver, and heart at 7 days post-Streptococcus parauberis (S. parauberis) infection. S30FAU was highly expressed in the spleen and gills at 1 day and 12 h post-VHSV infection, respectively, and exhibited a high expression in the kidney at 7 days post-S. parauberis infection. In an MIC analysis, RPS27A and S30FAU showed antimicrobial activity against all bacteria used in this study. In the biofilm assay, S30FAU was removed from S. parauberis in a concentration-dependent manner, and the cytotoxicity test showed no hemolytic activity in both RPS27A and S30FAU. Therefore, RPS27A and S30FAU of the starry flounder were confirmed to possess antimicrobial peptide abilities without limitations of cytotoxicity. This study provides valuable information on the antibacterial ability and molecular biology of the ubiquitin family isolated from the starry flounder.
Key Contribution:
This study focuses on the preparation of antibacterial peptides from RPS27A and S30FAU, two substances derived from ubiquitin, in a starry flounder, and aims to investigate the effectiveness of the prepared peptides in terms of fighting bacterial infections.
1. Introduction
High-density aquaculture, nowadays, to meet the increase in fish consumption, is causing problems such as nutritional imbalance and various viral and bacterial infections. Thus, interest in understanding the fish immune system is increasing []. The substance that solves these problems is antibiotics. Although antibiotics can solve problems that are related to a large number of diseases, indiscriminate use causes the emergence of drug-resistant and multi-drug-resistant bacteria [], which have emerged as a new problem and require a substance to replace them.
Antimicrobial peptides (AMPs) are factors of the innate immune system that consist of approximately 12–100 amino acids and act on cell membranes or intracellular targets as endogenous antibiotics in all organisms []. Therefore, it is unlikely to acquire resistance, inhibit protein synthesis, inhibit cell-wall formation, or inhibit bacterial growth [,,]. As an antimicrobial agent, it also acts as an antiviral and antiparasitic agent [,,]. Owing to these characteristics, antimicrobial peptides have been intensively discussed in terms of human drug design and pharmaceutical treatment in recent years [,]. To date, antibacterial peptides have been found in microorganisms, insects, vertebrates, and invertebrates [], and they have α-helix, β-sheet, or random coil structures; most of these structures are simultaneously hydrophilic and hydrophobic. It also has amphiphilic characteristics []. In particular, fish are exposed to numerous pathogens because they live in a water-soluble environment in which many pathogenic microorganisms exist; therefore, an effective and innate immune response is required to defend them. Although fish have a specific immune response system, including cell-mediated responses and antibodies, it takes a long time for their immune system to activate because they live in extreme environments of high salt, high pressure, and low temperature []. However, the non-specific immune system of fish responds quickly and expresses substances against pathogens; among them, antibacterial peptides play an important role in fish immunity, so they can be suggested as an alternative to antibiotics [,]. In the previous study, antimicrobial peptides were isolated by synthesizing peptides that specifically targeted antibacterial activity. These peptides were then incubated with bacteria to confirm their ability to inhibit bacterial growth, as determined by the minimum inhibitory concentration (MIC) or the minimum bactericidal concentration (MBC). The peptides that demonstrated effective antimicrobial activity in the experiment were selected as candidates for further study as potential antimicrobial peptides [,,].
Ubiquitin is a small protein consisting of 76 amino acids that was first discovered by Goldstein and his colleagues in 1975; it is also involved in the labeling process [,]. Ubiquitin is commonly found in various organs or in the cytoplasm of cells, and three enzymes (E1, E2, and E3) are involved in the protein-labeling process. The ubiquitin-activating enzyme (E1) shares several ubiquitin molecules with the substrate ubiquitin-conjugating enzyme (E2), which receives activated ubiquitin from E1, forms a high-energy thiol ester, and transfers it to ubiquitin–protein ligases (E3). In addition, E3 catalyzes stable isopeptide binding between the substrate protein and ubiquitin [,]. Ubiquitins play an important role in many immune responses, such as cell cycle regulation, protein expression regulation, and apoptosis, by influencing protein positional changes []. Ubiquitin-40S ribosomal protein S27a (RPS27A), ubiquitin-like protein Fubi, and ribosomal proteins (S30FAU) are proteins that are related to ubiquitin; all of these are related to ribosomes, which are organelles that synthesize proteins, form peptide bonds, and are present in all living cells. RPS27A, a member of the S27AE family of the ribosomal proteins, is a component of the 40S subunit of ribosomes [], and plays an important role in ribosome formation and post-translational modification []. In addition, RPS27A acts as a cell stress sensor that arrests the cell cycle. RPS27A is highly expressed in human solid tumors, and its expression level is upregulated in chronic myeloid leukemia and acute leukemia patients []. S30FAU is a gene related to the Finkel–Biskis–Reilly murine sarcoma virus (FBR-MuSV), which causes osteosarcoma in susceptible mice []. S30FAU is a member of the S30E family of ribosomal proteins and constitutes the 40S subunit of ribosomes []. In a previous study, S30FAU was isolated as an antimicrobial peptide from activated macrophages of mice and was reported to be a gene that was related to humoral immunity []. The starry flounder used in this experiment is a benthic fish belonging to the orders Pleuronectiformes and Pleuronectidae that grows to a height of 30–40 cm. It is a large species that is distributed in the low-temperature zone [,]. Despite the recent increase in the demand for and consumption of scallops, there are relatively few studies on the innate immunity of stilts that are damaged by fish diseases []. In this study, the molecular characteristics of the two antimicrobial peptides (RPS27A and S30FAU) identified in the starry flounder, tissue-specific expression analysis of normal fish, and expression analysis of artificially infected organisms (viral hemorrhagic septicemia, Streptococcus parauberis) were studied. To confirm the antibacterial activity of the two antimicrobial peptides, the minimum inhibitory concentration (MIC), biofilm assay, and cytotoxicity were analyzed.
2. Materials and Methods
2.1. Next-Generation Sequencing (NGS) Analysis
Next-generation sequencing (NGS) analysis was performed to determine the gene sequence. First, total RNA was isolated from the major tissues (leukocytes, red blood cells, liver, kidneys, and spleen) of the starry flounder artificially infected by Streptococcus parauberis and synthesized into cDNA using quality check (QC) for concentration and purity, and the suitability of all samples for analysis was confirmed. Samples verified by QC were sent to DNA link Co., Ltd. (Yongin, Republic of Korea) for transcriptome sequencing and primary assembly. The processed data were sent to Insilicogen Co., Ltd. (Yongsin, Republic of Korea) for secondary assembly, and the coding sequence (CDS) of RPS27A and S30FAU were confirmed.
2.2. Molecular Characteristics
The nucleotide sequences and predicted amino acid sequences of the cDNA CDS of the identified RPS27A and S30FAU were confirmed using GENETYX ver. 8.0 program (SDC Software Development, Tokyo, Japan), and National Center for Biotechnology Information (NCBI) BLASTX program (http://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 13 October 2021) was used for the analysis. Molecular weight (MW) and isoelectric point (pI) were predicted using ExPASy Proteomics Serve’s ProtParam tool (http://web.expasy.org/protparam/, accessed on 14 October 2021), and the position of a specific domain was determined using Simple Modular. It was confirmed with the Architecture Research Tool (SMART) (http://smart.embl-heidelberg.de/, accessed on 14 October 2021). Amino acid sequences (RPS27A and S30FAU) and multiple sequence alignments of mammals, humans, and other fish registered in the NCBI peptide sequence database were analyzed using ClustalW (http://www.genome.jp/tools/clustalw/, accessed on 15 October 2021). In addition, phylogenetic analysis was performed using the neighbor-joining (NJ) method of the Mega 4 program, and bootstrap sampling was repeated 1000 times.
2.3. Gene Expression Analysis
2.3.1. Experimental Fish Preparation and Tissue Extraction
Experimental fish were purchased from a private fish farm in Pohang, Gyeongsangbuk-do, and were used with an average starry flounder of 21.5 ± 1.3 cm and weight of 128.7 ± 18.2 g. VHSV and S. parauberis used for artificial infection were provided by the National Institute of Fisheries Science and acclimated to 17 ± 1 °C and 25 ± 1 °C. During the acclimatization period, seawater was maintained at dissolved oxygen at >6 mg/L, salinity at 28–30 psu, pH at 7.8–8.6, and NH3+ at <0.1 mg/L. For tissue-specific expression analysis, five healthy starry flounders were anesthetized with benzocaine (Sigma, St. Louis, MO, USA), dissected, and the head kidney, trunk kidney, spleen, liver, intestine, gills, eyes, brain, muscle, heart, skin, and stomach were removed. To confirm the expression characteristics of RPS27A and S30FAU after artificial infection with pathogens, the starry flounder was acclimated to 17 ± 1 °C and 25 ± 1 °C, respectively, and the prepared VHSV and S. parauberis were 1 × cells/fish and 1 × cells/fish, respectively. After suspension in PBS of 10 cells/fish, it was injected subcutaneously, and the control group was injected subcutaneously with the same amount of PBS. At 1 and 12 h and 1, 3, 5, and 7 days after the pathogen and PBS were injected, five fish were randomly selected from each of the experimental and control groups, and the kidney, spleen, gills, liver, brain, intestine, and heart were aseptically treated.
2.3.2. Total RNA Isolation and cDNA Synthesis
Total RNA was isolated from extracted tissues using TRIzol-based RNAiso (TaKaRa, Kyoto, Japan). First, 600 μL of RNAiso was added to each sample and ground using a homogenizer. Next, 100 μL of chloroform (Daejeon, Republic of Korea) was added, vortexed, and centrifuged at 14,000 rpm for 10 min. The separated supernatant was transferred to a new e-tube, and an equal amount of PCI (Phenol:Chloroform:Isoamyl alcohol) (Biosesang, Seongnam, Republic of Korea) was added and centrifuged at 14,000 rpm for 10 min. To remove genomic DNA, the supernatant was DNase-treated using Recombinant DNase I (TaKaRa, Kyoto, Japan) according to the manufacturer’s method, and after transferring the supernatant to a new e-tube, isopropanol (St. Louis, MO, USA) 500 μL, 5 μL of Dr.Gen (TaKaRa, Kyoto, Japan), and 50 μL of 3 M sodium acetate (TaKaRa, Kyoto, Japan) were added, followed by centrifugation at 14,000 rpm for 10 min. After removing the separated supernatant, 600 μL of 75% DEPC ethyl alcohol was added for washing and centrifuged at 14,000 rpm for 5 min. After washing and natural drying at room temperature for 10–15 min to remove the remaining supernatant, an appropriate amount of DEPC DW (Bioneer, Daejeon, Republic of Korea) was added to completely dissolve it. Before synthesizing cDNA, total RNA was measured using NanoVue (GE healthcare, Chicago, IL, USA) to measure concentration and purity, and the PrimeScript™ 1st strand cDNA Synthesis Kit (TaKaRa, Kyoto, Japan) was used to follow the manufacturer’s method. cDNA was synthesized as follows: 1 μL of DNase-treated total RNA, 1 μL of Oligo dT primer, 1 μL of dNTP mixture, and 10 μL of total amount, including 7 μL of RNase-free water, were mixed, reacted at 65 °C for 5 min, and then incubated on ice for 5 min. Then, 20 μL of a mixture of 4 μL of 5×Script Buffer, 0.5 μL of RNase Inhibitor, 1 μL of Primer Script RTase, and 4.5 μL of RNase-free water were mixed with the above reaction solution at 30 °C for 10 min, followed by 60 μL at 42 °C. min, and reacted at 95 °C for 5 min.
2.3.3. Primer Design
The specific primer sets used for quantitative real-time PCR were Primer3 ver. 0.4 (http://bioinfo.ut.ee/primer3-0.4.0/, accessed on 16 October 2021) was used to design (Table 1).

Table 1.
PCR primers used in this study.
2.3.4. Quantitative Real-Time PCR
To examine the expression levels of RPS27A and S30FAU in healthy starry flounder that was artificially infected with various pathogens, a quantitative, real-time PCR was performed using TB Green Master Mix, according to the manufacturer’s protocol. In brief, 1 μL of cDNA template, 1 μL each of forward/reverse primers, 12.5 μL of TB Green, and 9.5 μL of DDW were mixed to a total amount of 25 μL. The amplification conditions were as follows: initial denaturation at 95 °C for 10 min, followed by 20 s at 95 °C, and 1 min at 60 °C for a total of 45 cycles. The final dissociation was performed at 95 °C for 15 s, 60 °C for 30 s, and 95 °C for 15 s. Finally, the Ct (threshold cycle) values of RPS27A and S30FAU were compared with those of EF-1α mRNA and were calculated using the method [].
2.4. Antibacterial Activity of Antimicrobial Peptides
2.4.1. Peptide Synthesis
The antibacterial peptide sequences of RPS27A and S30FAU were determined using the AntiBP Server (http://webs.iiitd.deu.in/raghava/antibp/submit.html, accessed on 24 May 2022), and the peptides were synthesized by GL Biochem (Shanghai, China), Ltd. The synthesized peptide was purified to 95% purity by high-performance liquid chromatography (HPLC) and lyophilized (Table 2).

Table 2.
Synthesis peptides used in this study.
2.4.2. Strains
To analyze the antibacterial activity of synthetic peptides RPS27A and S30FAU, S. parauberis PH0710, S. parauberis KSP28, S. iniae, Vibrio harveyi, and Vibrio campbellii were used. Each strain was plated on brain heart infusion agar (BHIA) and cultured at 28 °C or 37 °C for 24 h, and the obtained colonies were cultured in brain heart infusion broth (BHIB) at 28 °C or 37 °C for 24 h. Then, centrifugation was performed at 4000 rpm for 10 min. The concentration of each bacterial suspension was diluted with PBS to an absorbance of 0.15 at 540 nm and resuspended up to 1 × CFU/fish.
2.4.3. Minimum Inhibitory Concentration (MIC)
To confirm the minimum inhibitory concentrations (MIC) of RPS27A and S30FAU, the peptides dissolved in 2.5% acetonitrile (ACN) were diluted in a 96-well plate using PBS in a two-fold serial dilution method. All strains were diluted with Mueller–Hinton (MH) medium to 1 × CFU/fish, and 50 μL of the bacteria were mixed with 50 μL of two-fold serially diluted peptides and incubated at 28 °C or 37 °C for 12 h. After culturing, the absorbance was measured at 540 nm using a VICTOR3 1420 Multilabel Counter (PerkinElmer, Waltham, MA, USA). MIC experiments were performed in triplicates to increase accuracy.
2.4.4. Cytotoxicity
Blood was collected from the starry flounder using a heparinized syringe and added to Leibovitz’s L-15 medium. Then, a Percoll gradient method was performed with 53% Percoll solution, and erythrocytes were separated by centrifugation at 400 × g for 15 min. After washing the separated red blood cells three times with PBS, 100 μL of different concentrations (7.81–250 μg/mL) of antimicrobial peptides were mixed and incubated at room temperature for 30 min. PBS was used as the negative control, and 0.1% TritonX-100 (Sigma-Aldrich) was used as the positive control. After incubation, centrifugation was performed at 1000× g for 10 min, the supernatant was transferred to a 96-well plate, and the absorbance was measured at 540 nm.
2.4.5. Biofilm Assay
To determine whether the antimicrobial peptide affected the bacterial biofilm, the bacteria were grown at a concentration of 1 × CFU/fish in BHIA by the method described for the strains mentioned above, and M9 medium (Welgene, Precision Solution™, Gyeongsan, Republic of Korea) was added. The bacteria were cultured in a 96-well plate at 28 °C or 37 °C for 24 h, washed with PBS, and treated with peptides of different concentrations (7.81–250 μg/mL), followed by incubation at 28 °C or 37 °C for 24 h. After incubation, the supernatant was removed and 150 μL of absolute ethanol was added, followed by incubation at room temperature for 30 min. After washing with PBS, staining with 1% crystal violet (Sigma-Aldrich) for 10 min, washing with distilled water, adding 150 μL of 30% acetic acid solution, incubating for 10 min at room temperature, and measuring the absorbance at 595 nm, PBS and 2.5% ACN were used as controls. The biofilm assay was performed three times to increase accuracy.
2.5. Statistical Analysis
All experiments were performed in triplicates, and all data are expressed as mean ± standard deviation. Significant differences between tissues were confirmed by one-way analysis of variance (ANOVA) test (* p < 0.05, ** p < 0.01).
3. Results
3.1. Molecular Characteristics
3.1.1. Sequence Analysis
The CDS of the cDNAs of RPS27A and S30FAU identified in the starry flounder were 471 bp and 402 bp, respectively (Figure 1). The predicted amino acid (aa) sequence of RPS27A was 156 aa, which contained the UBQ domain (1–72 aa) and the Ribosomal_S27 domain (102–147 aa); the isoelectric point (pI) was predicted to be 9.68 with a molecular weight of 17.99 kDa. The expected amino acid of S30FAU was 133 aa, which contained the UBQ domain (1–70 aa), and its pI was predicted to be 9.86 with a molecular weight of 14.76 kDa.
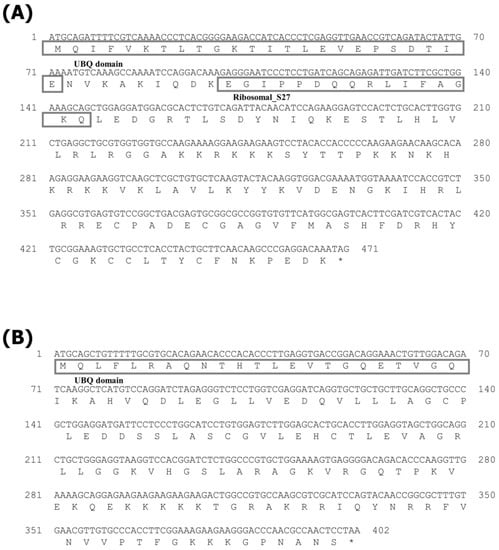
Figure 1.
cDNA and deduced amino acid sequence of the RPS27A (A) and S30FAU (B). The box indicates UBQ and Ribosomal_S27 domain in (A); box indicate UBQ domain in (B).
3.1.2. Multiple Alignment Analysis
Multiple alignment analysis of the amino acid sequences of RPS27A and S30FAU in the starry flounder and the amino acid sequences of RPS27A and S30FAU in other fish and mammals showed that the UBQ domain and ribosomal_S27 domain of RPS27A in the starry flounder were highly conserved in other fish and mammals (Figure 2). In addition, it showed 100% homology with Senegalese sole (Solea senegalensis), mud hopper (Boleophthalmus Pectinirostris), chum salmon (Oncorhynchus keta), tongue sole (Cynoglossidae), rainbow trout (Oncorhynchus mykiss), and Pacific halibut (Hippoglossus stenolepis) and relatively low homology with mammalian species at 98.08%. The UBQ domain of S30FAU of the starry flounder is also highly conserved in other fishes and mammals. In particular, it showed 100% homology with the Atlantic halibut and relatively low homology with mammalian species at 78.95%.
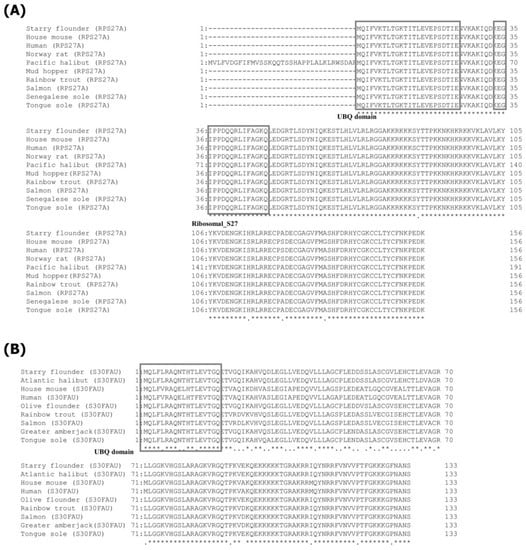
Figure 2.
Multiple sequence alignment analysis of starry flounder RPS27A (A) and S30FAU (B) sequence with other species amino acid sequences. An asterisk denotes the presence of homology in all amino acid sequences, while a dot signifies the absence of homology in all or part of the sequence. NCBI accession numbers of dicentracin are as follows: house mouse (Mus musculus), NP_001029037.1; human (Homo sapiens), AAH53371.1; Norway rat (Rattus norvegicus), NP_001292372.1; Pacific halibut (Hippoglossus stenolepis), XP_035028116.1; mud hopper (Boleophthalmus Pectinirostris), XP_033835162.1; rainbow trout (Oncorhynchus mykiss), XP_021468602.1; chum salmon, XP_036510427.1; Senegalese sole (Solea senegalensis), BAF45917.1; tongue sole (Cynoglossidae), XP_008319624.1; Atlantic halibut (Hippoglossus hippoglossus), XP_034436125.1; house mouse, NP_001153711.1; human, NP_001988.1; olive flounder (Paralichthys oilvaceus) XP_019947565.1; rainbow trout, XP_021468126.1; chum salmon, XP_035649649.1; greater amberjack (Seriola dumerili), and XP_022626116.1; tongue sole, XP_008311372.1.
3.1.3. Phylogenetic Analysis
The phylogenetic tree was analyzed using the Mega 4 program to confirm the phylogenetic position of RPS27A and S30FAU in the starry flounder (Figure 3). RPS27A in the starry flounder was included in the fish cluster and was classified in a different cluster from mammals. S30FAU was also included in the fish cluster and was classified in a cluster different from that of mammals. In particular, S30FAU of the starry flounder confirmed the closest relationship with the Atlantic halibut.
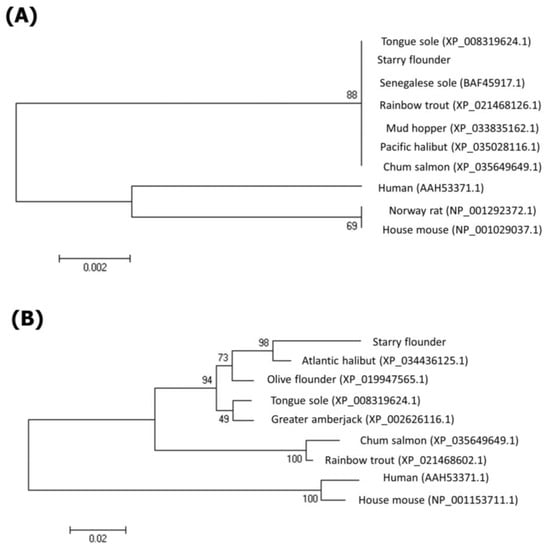
Figure 3.
Phylogenetic analysis of deduced RPS27A (A) and S30FAU (B) amino acid sequences in fish. The phylogenetic tree was constructed using the neighbor-joining method within MEGA 4 software. Bootstrap sampling was performed with 1000 replicates.
3.2. Gene expression Analysis
3.2.1. Expression Analysis by Normal Fish Tissue
Tissue-specific expression analysis in healthy starry flounders showed that RPS27A mRNA was highly expressed in the order of the trunk kidney, heart, and stomach based on the lowest expression in the intestine, and S30FAU mRNA showed high expression in the order of the stomach, heart, and trunk kidney based on the lowest expression in the intestine (Figure 4).
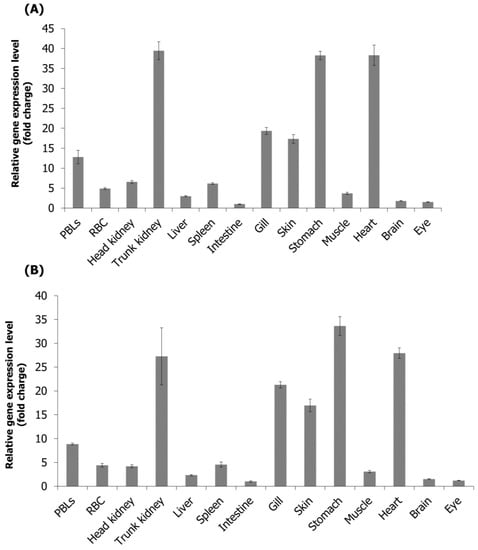
Figure 4.
Detection of RPS27A (A) and S30FAU (B) genes in different tissues of healthy starry flounder by real-time PCR. EF-1α was used for normalizing the real-time PCR results. Data are presented as the mean ± SD from three independent cDNA samples with three replicates from each sample.
3.2.2. Analysis of Expression by Time after Artificial Infection with Pathogens
After artificial infection with VHSV and S. paruberis in a healthy starry flounder, mRNA expression in the kidney, spleen, gills, liver, brain, intestine, and heart was confirmed using RT-qPCR (Figure 5). After the infection with VHSV, RPS27A showed the highest expression in the heart at 1 h and 3 days after the infection, and S30FAU showed significantly higher expression at 1 day and 12 h after the infection in the spleen and gills. After the infection with S. parauberis, RPS27A showed the highest expression in the kidney, liver, and heart 7 days after the infection, and S30FAU showed the highest expression in the kidney 7 days after the infection.
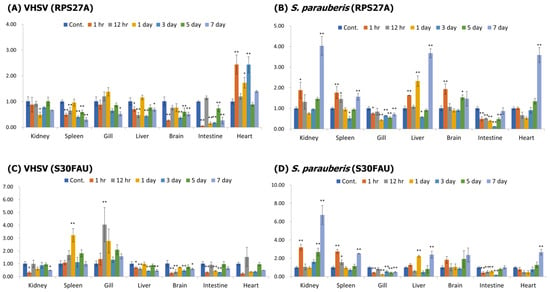
Figure 5.
Gene expression of RPS27A (A,B) and S30FAU (C,D) in the kidney, spleen, gill, liver, brain, intestine, and heart after infection with VHSV and S. parauberis. Levels of RPS27A and S30FAU transcripts were quantified relative to that of EF-1α levels. Data are presented as the mean ± SD from three independent cDNA samples with three replicates for each sample. Asterisks represent significant differences compared to the control (PBS) group by ANOVA (* p < 0.05 and ** p < 0.01).
3.3. Antibacterial Activity of Antimicrobial Peptides
3.3.1. Minimum Inhibitory Concentration (MIC)
As a result of the MIC experiments to confirm the antibacterial activities of RPS27A and S30FAU, S. parauberis PH0710, S. parauberis KSP28, S. iniae, V. harveyi, and V. campbellii all showed antibacterial activity (Table 3).

Table 3.
Antimicrobial activity of RPS27A and S30FAU.
3.3.2. Cytotoxicity
As a result of confirming the hemolytic activity of the starry flounder on red blood cells, RPS27A and S30FAU did not show a significant difference from the negative control group, PBS, but showed a significant difference from the positive control group, 0.1% Triton X-100 (Figure 6). However, no hemolysis was observed in any of the concentration ranges (7.81–250 μg/mL) used.
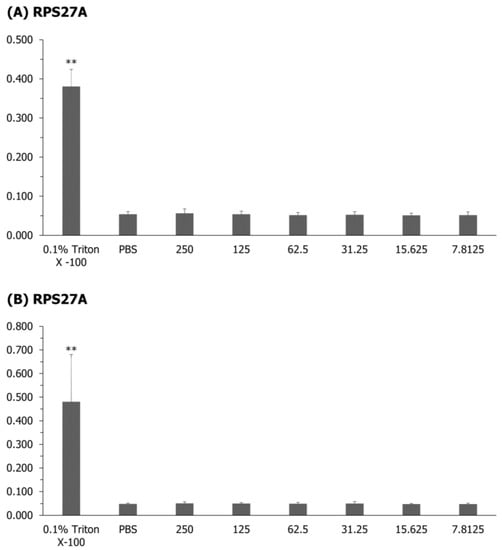
Figure 6.
The hemolytic and cytotoxicity of RPS27A (A) and S30FAU (B) were analyzed using starry flounder red blood cells. The positive control was measured by adding 0.1% Triton X-100 solution. Values are expressed as the mean ± SD, and asterisks indicate a significant difference from the negative control (PBS buffer) (** p < 0.01).
3.3.3. Biofilm Assay
Confirming the biofilm destruction effect of RPS27A and S30FAU on Gram-negative and Gram-positive bacteria, RPS27A showed no significant difference according to the concentration in all strains used in the experiment, but S30FAU showed a concentration-dependent effect on S. parauberis PH0710 and S. parauberis KSP28. This confirmed that the biofilm had been destroyed. In addition, the ACN 2.5% control group was used to determine whether the solvent was toxic and not significantly different from the PBS control group.
4. Discussion
Antimicrobial peptides were first discovered in the epidermal secretions of frogs in 1962 [], and piscidins, gaduscidins, moronecidins, grammistins, and pleurocidin were found in fish []. Ubiquitin was first isolated from bovine thymus as a stimulatory polypeptide of adenylate cyclase and it is well known as a proteolytic marker [,]. Ubiquitin has multiple functions, such as functioning as a hormone or autocrine growth factor and activating B-cell differentiation and adenylation cyclase in many tissues [,]. Additionally, research has shown that ubiquitin plays an essential role in the antimicrobial response of living organisms [,,]. To focus on the antimicrobial response function of ubiquitin, this study obtained two antibacterial peptide sequences (RSP27A and S30FAU) from immune cells, including mast cells, rodlet cells phagocytic granulocytes, and eosinophilic granular cells, which are known to highly express piscidins (a representative) []. These immune cells were sourced from the kidney, spleen, and liver, which are immune organs of fish, as well as from leukocytes and red blood cells. The study also investigated the molecular characteristics, expression analysis for each tissue, and antibacterial activity of the obtained peptides. Ubiquitin proteins are highly conserved in a variety of organisms and they promote the replication of several types of ubiquitin-like proteins that can form conjugates with other proteins. In particular, ubiquitin-like proteins are known to consist only of UBQ domains [,], and, in this study, S30FAU was found to have only UBQ domains, confirming that it is a ubiquitin-like protein. However, the function of the ubiquitin-like protein of S30FAU, called “Fubi”, has not yet been clearly identified []. In this study, the RPS27A of a starry flounder showed a high homology to rainbow trout, Senegalese sole, mud hopper, chum salmon, tongue sole, and Pacific halibut, and both the UBQ = and Ribosomal_S27 domains were well conserved. S30FAU showed a high homology to the Atlantic halibut, and all the UBQ domains were well conserved. Therefore, RPS27A and S30FAU are considered as highly conserved genes within the fish lineage. In the tissue-specific expression analysis of normal fish, RPS27A and S30FAU showed higher expressions in the body, gills, skin, stomach, and heart than in other tissues. The result of showing RPS27A in Senegalese sole, which was in a previous study, showed the highest expression in the heart and the lowest expression in the brain as a result of a tissue-specific expression analysis []. In addition, RPS27A detected in the gills and skin of mud hoppers was studied as an antibacterial peptide []. In this study, expression of RPS27A was confirmed in the gills and skin of the starry flounder. In addition, the RPS27A of a mud hopper and RPS27A of a starry flounder showed a 100% homology; hence, RPS27A in starry flounder is also considered to have antibacterial ability. In the results of artificial infection with pathogens, the expression of RPS27A was the highest in the heart during VHSV infection and high in the heart, kidney, and liver during S. parauberis infection. The kidney, gill, and spleen are hematopoietic organs and immune cells of fish, so they seem to be upregulated for immune regulation. High expression in the liver is caused of antimicrobial peptides or immunological proteins, as liver activation is thought to be upregulated []. In this study, especially after artificial infection with the pathogen, RPS27A was highly expressed in the heart, and further experiments are needed to investigate the correlation between RPS27A and heart tissue. S30FAU was highly expressed in the gills and kidneys following artificial infection with VHSV and in the kidneys following infection with S. parauberis. In a previous study, it was also reported that S30FAU modulates the T-cell receptor (TCR) α-like protein and Bcl g in murine cells to regulate immunity and apoptosis []. Bcl g is a protein that regulates the endogenous pathway of apoptosis, and the endogenous pathway occurs when the growth factors are lost []. Therefore, it is thought that S30FAU was highly expressed for immunomodulation in these tissues after artificial infection. As a result of investigating the tissue-specific expression of RPS27A and S30FAU in a starry flounder, it was confirmed that the ubiquitin protein is distributed in various tissues. Despite various studies conducted on ubiquitin in humans and mammals, research on its role in fish is insufficient. In particular, no previous study has investigated the RPS27A and S30FAU genes in the starry flounder. The tissue-specific gene expression results obtained in this study confirm the differences in normal biological processes and disease processes, as well as the immune response associated with the two genes in the starry flounder. These data are considered valuable as basic information in related academic fields for future studies. The MIC analysis confirmed the antibacterial activity of all bacteria. RPS27A and S30FAU showed antibacterial activity without any difference between the Gram-positive and Gram-negative bacteria used in the experiment. This non-specific antibacterial activity is thought to be related to the broad range of antibacterial activities of antimicrobial peptides against pathogens []. Most of the antimicrobial peptides produced in nature are known to have a wide range of action against pathogenic bacteria and fungi []. Biofilms are three-dimensional structures that form in a multimeric matrix self-secreted by microorganisms that can form on almost all types of solid surfaces. Tissues of living organisms can also form biofilms on medical devices [,]. Bacteria within the biofilm are 10–1000 times more resistant to antibiotics than bacteria in a suspended state due to reduced drug shear force and reduced growth rate. Therefore, to remove the biofilm, there is no choice but to administer high concentrations of antibiotics, which not only causes serious damage to the surrounding tissues, but also increases the risk of resistant bacteria []. Antimicrobial peptides that have the potential to solve these problems show strong antibacterial activity against biofilm-forming pathogen and antibiotic-resistant bacteria. Antimicrobial peptides are known for their mechanism of action: membrane-active, which acts on cell membranes, and non-membrane-active, which does not directly act on cell membranes []. This experiment confirmed that S30FAU inhibited the formation of biofilms by S. parauberis PH0710 and S. parauberis KSP28 in a concentration-dependent manner. Therefore, it is believed that RPS27A and S30FAU destroy some bacteria (S. parauberis PH0710, S. parauberis KSP28) by forming an electrostatic bond with negatively charged molecules on the bacterial cell membrane and they pass through the cell membrane in a concentration-dependent manner []. In this study, it was found that both RPS27A and S30FAU exhibited antibacterial activity against S. parauberis PH0710, S. parauberis KSP28, V. harveyi, V. campbellii, and S. iniae. However, S30FAU only showed a limited antibacterial activity against S. parauberis PH0710 and S. parauberis KSP28 in the biofilm. The substrate components of biofilm are known to vary depending on the type of pathogen, which suggests that the method of resistance to biofilm is different for each microorganism []. Therefore, it is believed that the peptides used in this experiment may not have been effective in removing the biofilm of all pathogenic bacteria. The application of antimicrobial peptides as natural antibiotics is limited because of their cytotoxicity []. Cytotoxicity was not observed for either RPS27A or S30FAU in the present study. These results may alleviate the limitations of cytotoxicity in the future commercialization of antimicrobial peptides. In this study, specific domains were identified through the molecular characteristics of RPS27A and S30FAU identified in the starry flounder, expression analysis in normal fish and artificially infected fish, and MIC, biofilm assays, and cytotoxicity experiments. It was confirmed that both RPS27A and S30FAU shared the UBQ domain and showed antibacterial activity against both Gram-positive and Gram-negative bacteria without any difference. Research on ubiquitin has been conducted extensively, but there is still insufficient research on ubiquitin in the starry flounder, and even the limited research available has not focused on ubiquitin’s function. This study aimed to investigate the antibacterial ability, one of the various functions of ubiquitin, and to provide evidence for the antibacterial ability of ubiquitin-related genes in the starry flounder. These findings can serve as crucial research material for future studies.
5. Conclusions
In conclusion, this study isolated and prepared ubiquitin as an antibacterial peptide from the starry flounder, which has been extensively studied in humans and mammals.
The study confirmed the expression of the isolated genetic material in tissues, determined the range of antibacterial activity, and evaluated the cytotoxicity, suggesting that the antibacterial peptide can be used practically.
These results provide important basic data for research concerning antimicrobial peptides as a substitute for antibiotics in fish.
Author Contributions
Conceptualization, H.-J.S. and C.-I.P.; methodology, H.-J.S.; formal analysis, H.-J.S., G.K., W.-S.W., K.-H.K., M.-Y.S., J.-W.P. and D.L.; investigation, H.-J.S.; resources, C.-I.P.; software, W.-S.W.; writing—original draft preparation, H.-J.S.; writing—review and editing, W.-S.W. and C.-I.P.; supervision, C.-I.P.; project administration, C.-I.P.; funding acquisition, C.-I.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a grant from the National Institute of Fisheries Science (NIFS), Republic of Korea (R2023031).
Institutional Review Board Statement
The study was approved by the Gyeongsang National University Animal Testing Ethics Committee. Approval Code: GNU-150907-M0050; Approval date: 7 September 2015.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- He, S.W.; Wang, G.H.; Yue, B.; Zhou, S.; Zhang, M. TO17: A teleost antimicrobial peptide that induces degradation of bacterial nucleic acids and inhibits bacterial infection in red drum, Sciaenops ocellatus. J. Fish. Shellfish Immunol. 2018, 72, 639–645. [Google Scholar] [CrossRef]
- Su, Y.L.; Chen, G.; Chen, L.S.; Li, J.Z.; Wang, G.; He, J.Y.; Zhan, T.Y.; Li, Y.W.; Yan, M.T.; Huang, Y.H.; et al. Effects of antimicrobial peptides on serum biochemical parameters, antioxidant activity and non-specific immune responses in Epinephelus coioides. J. Fish. Shellfish Immunol. 2019, 86, 1081–1087. [Google Scholar] [CrossRef]
- Park, S.C.; Park, Y.K.; Hahm, K.S. The Role of Antimicrobial Peptides in Preventing Multidrug-Resistant Bacterial Infections and Biofilm Formation. Int. J. Mol. Sci. 2011, 12, 5971–5992. [Google Scholar] [CrossRef]
- Sun, E.; Belanger, C.R.; Haney, E.F.; Hancook, R.E.W. Host Defense (Antimicrobial) Peptides. Pept. Appl. Biomed. Biotechnol. Bioeng. 2018, 253–285. [Google Scholar] [CrossRef]
- Li, T.; Liu, Q.; Chen, H.; Li, J. Antibacterial activity and mechanism of the cell-penetrating peptide CF-14 on the gram-negative bacteria, Escherichia coli. J. Fish. Shellfish Immunol. 2020, 100, 489–495. [Google Scholar] [CrossRef]
- Takiguchi, T.; Morizane, S.; Yamamoto, T.; Kajita, A.; Iwatsuki, K. Cathelicidin antimicrobial peptide LL-37 augments interferon-β expression and antiviral activity induced by double-stranded RNA in keratinocytes. Br. J. Dermatol. 2015, 171, 492–498. [Google Scholar] [CrossRef]
- Lacerda, A.F.; Pelegrini, P.B.; de Oliveira, D.M.; Vasconcelos, É.A.; Grossi-de-Sá, M.F. Anti-parasitic peptides from arthropods and their application in drug therapy. J. Front. Microbiol. 2016, 7, 91. [Google Scholar] [CrossRef]
- Delattin, N.; De Brucker, K.; De Cremer, K.; Cammue, B.P.A.; Thevissen, K. Antimicrobial peptides as a strategy to combat fungal biofilms. J. Curr. Top. Med. Chem. 2017, 17, 604–612. [Google Scholar] [CrossRef]
- Conlon, J.M.; Mechkarska, M.; Abdel-Wahab, Y.H.; Flatt, P.R. Peptides from frog skin with potential for development into agents for type 2 diabetes therapy. J. Pept. 2018, 100, 275–281. [Google Scholar] [CrossRef]
- Kim, T.Y.; Go, H.J.; Park, N.G. Purification of an Antibacterial Peptide from the Gills of the Pufferfish Takifugu pardalis. J. Life Sci. 2017, 27, 50–56. [Google Scholar] [CrossRef]
- Oh, R.; Lee, M.J.; Kim, Y.O.; Nam, B.H.; Kong, H.J.; Kim, J.W.; An, C.M.; Kim, D.G. Isolation and Purification of Antimicrobial Peptide from Hard-shelled Mussel, Mytilus coruscus. J. Life Sci. 2016, 26, 1259–1268. [Google Scholar] [CrossRef]
- Acosta, J.; Montero, V.; Carpio, Y.; Velázquez, J.; Garay, H.E.; Reyes, O.; Cabrales, A.; Masforrol, Y.; Morales, A.; Estrada, M.P. Cloning and functional characterization of three novel antimicrobial peptides from tilapia (Oreochromis niloticus). J. Aquac. 2013, 372–375, 9–18. [Google Scholar] [CrossRef]
- Pasupuleti, M.; Schmidtchen, A.; Malmsten, M. Antimicrobial peptides: Key components of the innate immune system. J. Crit. Rev. Biotechnol. 2012, 32, 143–171. [Google Scholar] [CrossRef] [PubMed]
- Imjongjirak, C.; Amparyup, P.; Tassanakajon, A.; Sittipraneed, S. Antilipopolysaccharide factor (ALF) of mud crab Scylla paramamosain: Molecular cloning, genomic organization and the antimicrobial activity of its synthetic LPS binding domain. J. Mol. Immunol. 2007, 44, 3195–3203. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Cao, Z.; Diao, Q.; Zhou, Y.; Ao, J.; Liu, C.; Sun, Y. Antimicrobial activity and mechanisms of a derived antimicrobial peptide TroNKL-27 from golden pompano (Trachinotus ovatus) NK-lysin. J. Fish. Shellfish Immunol. 2022, 126, 357–369. [Google Scholar] [CrossRef]
- Phuket, T.R.N.; Charoensapsri, W.; Amparyup, P.; Imjongjirak, C. Antibacterial activity and immunomodulatory role of a proline-rich antimicrobial peptide SpPR-AMP1 against Vibrio campbellii infection in shrimp Litopenaeus vannamei. J. Fish. Shellfish Immunol. 2023, 132, 108479. [Google Scholar] [CrossRef]
- Hershko, A.; Ciechanover, A.; Heller, H.; Haas, A.L.; Rose, I.A. Proposed role of ATP in protein breakdown: Conjugation of protein with multiple chains of the polypeptide of ATP-dependent proteolysis. Proc. Natl. Acad. Sci. USA 1980, 77, 1783–1786. [Google Scholar] [CrossRef]
- Ciechanover, A.; Heller, H.; Elias, S.; Hass, A.L.; Hershko, A. ATP-dependent conjugatron of retrculocyte proteins with the polypeptide requrred for protein degradation. J. Natl. Acad. Sci. USA 1980, 77, 1362–1368. [Google Scholar]
- Glickman, M.H.; Ciechanover, A. The Ubiquitin-Proteasome Proteolytic Pathway: Destruction for the Sake of Construction. J. Physiol. Rev. 2002, 82, 373–428. [Google Scholar] [CrossRef]
- Pickard, M. FAU (Finkel-Biskis-Reilly murine sarcoma virus (FBR-MuSV) ubiquitously expressed). Atlas Genet. Cytogenet. Oncol. Haematol. 2012, 16, 12–17. [Google Scholar] [CrossRef]
- Jung, J.H.; Lee, J.Y.; Lee, S.M.; Choe, T.B.; An, S.K. Regulation of cellular functions of p53 by ubiquitination. J. KSBB 2009, 24, 217–226. [Google Scholar]
- Almamy, A.; Schwerk, C.; Schroten, H.; Ishikawa, H.; Asif, A.R.; Reuss, B. Interactions of antisera to different Chlamydia and Chlamydophila species with the ribosomal protein RPS27a correlate with impaired protein synthesis in a human choroid plexus papilloma cell line. J. Immunol. Res. 2017, 65, 1110–1123. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.W.; Kim, S.M.; Jin, D.H.; Kim, Y.S.; Hur, D.Y. RPS27a enhances EBV-encoded LMP1-mediated proliferation and invasion by stabilizing of LMP1. Biochem. Biophys. Res. Commun. 2017, 419, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Yu, J.; Zhang, L.; Xiong, Y.; Chen, S.; Xing, H.; Tian, Z.; Tang, K.; Wei, H.; Rao, Q.; et al. RPS27a promotes proliferation, regulates cell cycle progression and inhibits apoptosis of leukemia cells. J. Biochem. Biophys. Res. Commun. 2014, 446, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Michiels, L.; Van der Rauwelaert, E.; Van Hasselt, F.; Kas, K.; Merregaert, J. fau cDNA encodes a ubiquitin-like-S30 fusion protein and is expressed as an antisense sequence in the Finkel-Biskis-Reilly murine sarcoma virus. J. Oncogene 1993, 8, 2537–2546. [Google Scholar]
- Hiemstra, P.S.; Van den Barselaar, M.T.; Roest, M.; Nibbering, P.H.; Van Furth, R. Ubiquicidin, a novel murine microbicidal protein present in the cytosolic fraction of macrophages. J. Leukoc. Biol. 1999, 66, 423–428. [Google Scholar] [CrossRef]
- Cho, M.Y.; Lee, J.I.; Kim, M.S.; Choi, H.J.; Lee, D.C.; Kim, J.W. Isolation of Streptococcus parauberis from starry flounder, Platichthys stellatus Pallas. J. Fish. Pathol. 2008, 21, 209–217. [Google Scholar]
- Jung, H.S.; Kim, Y.K.; Kim, H.C.; Noh, J.K.; Lee, J.H.; Kim, D.S. Cytogenetic Analysis of Starry Flounder Platichthys stellatus from Korea. J. Fish. Aquat. 2014, 47, 431–434. [Google Scholar]
- Liu, Z.M.; Chen, J.; Lv, Y.P.; Hu, Z.H.; Dai, Q.M.; Fan, X.L. Molecular characterization of a hepcidin homologue in starry flounder (Platichthys stellatus) and its synergistic interaction with antibiotics. J. Fish. Shellfish Immunol. 2018, 83, 45–51. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. J. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Cha, Y.K.; Kim, Y.S.; Choi, Y.S. Antimicrobial Peptides as Natural Antibiotic Materials. J. KSBB 2012, 27, 9–15. [Google Scholar] [CrossRef]
- Valero, Y.; Saraiva-Fraga, M.; Costas, B.; Guardiola, F.A. Antimicrobial peptides from fish: Beyond the fight against pathogens. J. Aquac. 2020, 12, 224–253. [Google Scholar] [CrossRef]
- Schlesinger, D.G.; Goldstein, G. Molecular conservation of 74 amino acid sequence of ubiquitin between cattle and man. J. Nat. 1975, 255, 423–424. [Google Scholar] [CrossRef] [PubMed]
- Hegde, A.N. Ubiquitin-proteasome-mediated local protein degradation and synaptic plasticity. J. Prog. Neurobiol. 2004, 73, 311–357. [Google Scholar] [CrossRef] [PubMed]
- Ecker, D.J.; Butt, T.R.; Marsh, J.; Sternberg, E.J.; Margolis, N.; Monia, B.P.; Jonnalagadda, S.; Khan, M.I.; Weber, P.L.; Mueller, L.; et al. Gene synthesis, expression, structures, and functional activities of site-specific mutants of ubiquitin. J. Biol. Chem. 1987, 262, 14213–14221. [Google Scholar] [CrossRef]
- Goldstein, G.; Scheid, M.S.; Hammerling, V.; Boyse, E.A.; Schlesinger, D.H.; Niall, H.D. Isolation of a polypeptide that has lymphocyte-differentiating properties and is probably represented universally in living cells. Proc. Natl. Acad. Sci. USA 1975, 11–15. [Google Scholar] [CrossRef]
- Kieffer, A.E.; Goumon, Y.; Ruh, O.; Chasserot-Golaz, S.; Nullans, G.; Gasnier, C.; Aunis, D.; Metz-Boutigue, M.H. The N- and C-terminal fragments of ubiquitin are important for the antimicrobial activities. J. FASEB 2003, 17, 776–778. [Google Scholar] [CrossRef]
- Howell, S.J.; Wilk, D.; Yadav, S.P.; Bevins, C.L. Antimicrobial polypeptides of the human colonic epithelium. J. Pept. 2003, 24, 1763–1770. [Google Scholar] [CrossRef]
- Svensson, I.; Calles, K.; Lindskong, E.; Henriksson, H.; Eriksson, U.; Haggstrom, L. Antimicrobial activity of conditioned medium fractions from Spodoptera frugiperda Sf9 and Trichoplusia in Hi5 insect cells. J. Appl. Microbiol. Biotechnol. 2005, 69, 92–98. [Google Scholar] [CrossRef]
- Ganz, T.; Lehrer, R.I. Antimicrobial peptides of leukocytes. J. Curr. Opin. Hematol. 1997, 4, 53–58. [Google Scholar] [CrossRef]
- Matunis, M.J.; Wu, J.; Blobel, G. SUMO-1 Modification and Its Role in Targeting the Ran GTPase-Activating Protein, RanGAP1, to the Nuclear Pore Complex. J. Cell Biol. 1998, 140, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Conklin, D.; Holderman, S.; Whitmore, T.E.; Maurer, M.; Feldhaus, A.L. Molecular cloning, chromosome mapping and characterization of UBQLN3 a testis-specific gene that contains an ubiquitin-like domain. J. Gene 2000, 249, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Manchado, M.; Infante, C.; Asensio, E.; Cañavate, J.P.; Douglas, S.E. Comparative sequence analysis of the complete set of 40S ribosomal proteins in the Senegalese sole (Solea senegalensis Kaup) and Atlantic halibut (Hippoglossus hippoglossus L.) (Teleostei: Pleuronectiformes): Phylogeny and tissue- and development-specific expression. J. BMC Evol. Biol. 2007, 107, 1471–2148. [Google Scholar]
- Yi, Y.; You, X.; Bian, C.; Chen, S.; Lv, Z.; Qiu, L.; Shi, Q. High-Throughput Identification of Antimicrobial Peptides from Amphibious Mudskippers. J. Mar. Drugs 2017, 15, 364. [Google Scholar] [CrossRef]
- Soung, Y.H.; Lee, J.W.; Kim, S.Y.; Nam, S.W.; Park, W.S.; Lee, J.Y.; Yoo, N.J.; Lee, S.H. Mutational Analysis of Proapoptotic bcl-2 Family genes in Colon Carcinomas. Korean. J. Pathol. 2005, 39, 168–171. [Google Scholar]
- Hwang, B.; Lee, J.; Lee, D.G. Antimicrobial Peptides Derived from the Marine Organism(s) and Its Mode of Action. J. Korea Microbiol. Biotechnol. 2010, 38, 19–23. [Google Scholar]
- Fux, C.A.; Costerton, J.W.; Stewart, P.S.; Stoodley, P. Survival strategies of infectious biofilms. J. Trends Microbiol. 2005, 13, 34–40. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, J.H. Biofilm dispersion in Pseudomonas aeruginosa. J. Microbiol. 2016, 54, 71–85. [Google Scholar] [CrossRef]
- Hughes, D.; Andersson, D.I. Environmental and genetic modulation of the phenotypic expression of antibiotic resistance. FEMS Microbiol. Rev. 2017, 41, 374–391. [Google Scholar] [CrossRef]
- Park, S.C.; Nah, J.W. Antimicrobial Peptide as a Novel Antibiotic for Multi-Drug Resistance “Super-bacteria”. Appl. Chem. Eng. 2012, 23, 429–432. [Google Scholar]
- Yechiel, S. Mode of Action of Membrane Active Antimicrobial Peptides. J. Pept. Sci. 2002, 66, 236–248. [Google Scholar]
- Izano, E.A.; Amarante, M.A.; Kher, W.B.; Kaplan, J.B. Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Appl. Environ. Microbiol. 2008, 74, 470–476. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).