Determination of Multi-Class Antibiotics Residues in Farmed Fish and Shrimp from Sri Lanka by Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry (UPLC-MS/MS)
Abstract
1. Introduction
2. Materials and Methodology
2.1. Reagents
2.2. Fish and Shrimp Samples
2.3. Solid-Liquid Extraction of Antibiotics
2.4. HPLC-MS/MS Analysis
2.5. Method Validation
2.6. Statistical Analysis
3. Results and Discussion
3.1. Optimization of Extraction of Antibiotics
3.2. Matrix Effect
3.3. Limit of Detection (LOD) and Limit of Quantification (LOQ)
3.4. Precision and Accuracy
3.5. Determination of Multi-Class Antibiotics Residues in Farmed Fish and Shrimp
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lucchetti, D.; Fabrizi, L.; Esposito, A.; Guandalini, E.; Di Pasquale, M.; Coni, E. Simple Confirmatory Method for the Determination of Erythromycin Residues in Trout: A Fast Liquid−Liquid Extraction Followed by Liquid Chromatography-Tandem Mass Spectrometry. J. Agric. Food Chem. 2005, 53, 9689–9694. [Google Scholar] [CrossRef] [PubMed]
- Samanidou, V.F.; Evaggelopoulou, E.N. Analytical strategies to determine antibiotic residues in fish. J. Sep. Sci. 2007, 30, 2549–2569. [Google Scholar] [CrossRef]
- Heuer, O.E.; Kruse, H.; Grave, K.; Collignon, P.; Karunasagar, I.; Angulo, F.J. Human health consequences of use of antimicrobial agents in aquaculture. Clin. Infect. Dis. 2009, 49, 1248–1253. [Google Scholar] [CrossRef]
- Saxena, S.K.; Rangasamy, R.; Krishnan, A.A.; Singh, D.P.; Uke, S.P.; Malekadi, P.K.; Sengar, A.S.; Mohamed, D.P.; Gupta, A. Simultaneous determination of multi-residue and multi-class antibiotics in aquaculture shrimps by UPLC-MS/MS. Food Chem. 2018, 260, 336–343. [Google Scholar] [CrossRef]
- European Commission. Commission Decision 37/2010 of 22 December 2009 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off. J. Eur. Communities 2010, 15, 1–72. [Google Scholar]
- Chen, J.; Sun, R.; Pan, C.; Sun, Y.; Mai, B.; Li, Q.X. Antibiotics and food safety in aquaculture. J. Agric. Food Chem. 2020, 68, 11908–11919. [Google Scholar] [CrossRef]
- Lulijwa, R.; Rupia, E.J.; Alfaro, A.C. Antibiotic use in aquaculture, policies and regulation, health and environmental risks: A review of the top 15 major producers. Rev. Aquacult. 2019, 12, 640–663. [Google Scholar] [CrossRef]
- Okocha, R.C.; Olatoye, I.O.; Adedeji, O.B. Food safety impacts of antimicrobial use and their residues in aquaculture. Public Health Rev. 2018, 39, 21. [Google Scholar] [CrossRef] [PubMed]
- Hishamunda, N.; Ridler, N.B.; Bueno, P.; Yap, W.G. Commercial aquaculture in Southeast Asia: Some policy lessons. Food Policy 2009, 34, 102–107. [Google Scholar] [CrossRef]
- Love, D.C.; Rodman, S.; Neff, R.A.; Nachman, K.E. Veterinary drug residues in seafood inspected by the European Union, United States, Canada, and Japan from 2000 to 2009. Environ. Sci. Technol. 2011, 45, 7232–7240. [Google Scholar] [CrossRef]
- Impens, S.; Reybroeck, W.; Vercammen, J.; Courtheyn, D.; Ooghe, S.; De Wasch, K.; Smedts, W.; De Brabander, H. Screening and confirmation of chloramphenicol in shrimp tissue using ELISA in combination with GC–MS2 and LC–MS2. Anal. Chim. Acta 2003, 483, 153–163. [Google Scholar] [CrossRef]
- Lundborg, C.S.; Tamhankar, A.J. Antibiotic residues in the environment of South East Asia. BMJ 2017, 358, 42–45. [Google Scholar] [CrossRef] [PubMed]
- European Commision. Rapid Alert System for Food and Feed (RSFF). 2022. Available online: https://food.ec.europa.eu/safety/rasff-food-and-feed-safety-alerts/reports-and-publications_en#latest-rasff-publications (accessed on 13 June 2022).
- Manage, P.M. Heavy use of antibiotics in aquaculture; emerging human and animal health problems—A review. Sri Lanka J. Aquat. Sci. 2018, 23, 13–27. [Google Scholar] [CrossRef]
- Khan, M.; Lively, J.A. Determination of sulfite and antimicrobial residue in imported shrimp to the USA. Aquac. Rep. 2020, 18, 100529. [Google Scholar] [CrossRef]
- Bohm, D.A.; Stachel, C.S.; Gowik, P. Validation of a multi-residue method for the determination of several antibiotic groups in honey by LC-MS/MS. Anal. Bioanal. Chem. 2012, 403, 2943–2953. [Google Scholar] [CrossRef]
- Junza, A.; Amatya, R.; Barrón, D.; Barbosa, J. Comparative study of the LC–MS/MS and UPLC–MS/MS for the multi-residue analysis of quinolones, penicillins and cephalosporins in cow milk, and validation according to the regulation 2002/657/EC. J. Chromatogr. B 2011, 879, 2601–2610. [Google Scholar] [CrossRef]
- Dasenaki, M.E.; Thomaidis, N.S. Multi-residue determination of seventeen sulfonamides and five tetracyclines in fish tissue using a multi-stage LC–ESI–MS/MS approach based on advanced mass spectrometric techniques. Anal. Chim. Acta 2010, 672, 93–102. [Google Scholar] [CrossRef]
- Johnstonm, L.; Mackaym, L.; Croft, M. Determination of quinolones and fluoroquinolones in fish tissue and seafood by high-performance liquid chromatography with electrospray ionization tandem mass spectrometric detection. J. Chromatogr. A 2002, 982, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.I.; Pettis, J.S.; Smith, I.B.; Chu, P.S. Multiclass determination and confirmation of antibiotic residues in honey using LC-MS/MS. J. Agric. Food Chem. 2008, 56, 1553–1559. [Google Scholar] [CrossRef]
- Tang, Y.Y.; Lu, H.F.; Lin, H.Y.; Shih, Y.C.; Hwang, D.F. Multiclass analysis of 23 veterinary drugs in milk by ultra-performance liquid chromatography-electrospray tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2012, 881–882, 12–19. [Google Scholar] [CrossRef]
- Fedorova, G.; Nebesky, V.; Randak, T.; Grabic, R. Simultaneous determination of 32 antibiotics in aquaculture products using LC-MS/MS. Chem. Pap. 2014, 68, 29–36. [Google Scholar] [CrossRef]
- Canada-Canada, F.; de la Pena, M.A.; Espinosa-Mansilla, A. Analysis of antibiotics in fish samples. Anal. Bioanal. Chem. 2009, 395, 987–1008. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, H.; Arnaudguilhem, C.; Jaber, F.; Lobinski, R. Multiresidue analysis of 22 sulfonamides and their metabolites in animal tissues using quick, easy, cheap, effective, rugged, and safe extraction and high resolution mass spectrometry (hybrid linear ion trap-Orbitrap). J. Chromatogr. A 2014, 1355, 61–72. [Google Scholar] [CrossRef]
- Jo, M.R.; Lee, H.J.; Lee, T.S.; Park, K.; Oh, E.G.; Kim, P.H.; Lee, D.S.; Horie, M. Simultaneous determination of macrolide residues in fish and shrimp by liquid chromatography-tandem mass spectrometry. Food Sci. Biotechnol. 2011, 20, 823–827. [Google Scholar] [CrossRef]
- Dufresne, G.; Fouquet, A.; Forsyth, D.; Tittlemier, S.A. Multiresidue determination of quinolone and fluoroquinolone antibiotics in fish and shrimp by liquid chromatography/tandem mass spectrometry. J. AOAC Int. 2007, 90, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.J.; Liza, A.A.; Mohsinul Reza, A.H.M.; Reza, M.S.; Absar Khan, M.N.; Kamal, M. Source identification and entry pathways of banned antibiotics nitrofuran and chloramphenicol in shrimp value chain of Bangladesh. EurAsian J. BioSciences 2014, 8, 71–83. [Google Scholar] [CrossRef]
- Villar-Pulido, M.; Gilbert-López, B.; García-Reyes, J.F.; Martos, N.R.; Molina-Díaz, A. Multiclass detection and quantitation of antibiotics and veterinary drugs in shrimps by fast liquid chromatography time-of-flight mass spectrometry. Talanta 2011, 85, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Wang, Y.; Peng, B.; Zhao, N.; Zhao, J.; Xiong, Z.; Zhao, L. Multi-class determination of steroid hormones and antibiotics in fatty hotpot ingredients by pressurized liquid extraction and liquid chromatography–tandem mass spectrometry. J. Pharm. Biomed. Anal. 2019, 171, 193–203. [Google Scholar] [CrossRef]
- Susakate, S.; Poapolathep, S.; Chokejaroenrat, C.; Tanhan, P.; Hajslova, J.; Giorgi, M.; Saimek, K.; Zhang, Z.; Poapolathep, A. Multiclass analysis of antimicrobial drugs in shrimp muscle by ultra high performance liquid chromatography-tandem mass spectrometry. J. Food Drug Anal. 2019, 27, 118–134. [Google Scholar] [CrossRef]
- Kim, J.; Suh, J.H.; Cho, H.D.; Kang, W.; Choi, Y.S.; Han, S.B. Analytical method for fast screening and confirmation of multi-class veterinary drug residues in fish and shrimp by LC-MS/MS. Food Addit. Contam. Part A 2016, 33, 420–432. [Google Scholar] [CrossRef]
- MFAR. Fisheries Statistic. 2021. Available online: http://www.fisheriesdept.gov.lk/web/index.php?lang=en (accessed on 18 June 2022).
- Liyanage, G.Y.; Manage, P.M.; de Alwis, A. Study on the Occurrence of Antibiotic Contaminations in the Aquatic Environment, Sri Lanka, Proceeding of international conference on Multidiciplinary Approach; University of Sri Jayawardanaapura: Nugegoda, Sri Lanka, 2014. [Google Scholar]
- Analysis of Veterinary Drugs in Meat with the Agilent 6495 Triple Quadrupole LC/MS. 2017. Available online: https://www.agilent.com/cs/library/applications/5991-7895EN.pdf (accessed on 31 May 2022).
- Eurachem, the fitness for purpose of Analytical Method. 2014. Available online: https://www.eurachem.org/index.php/publications/guides/mv (accessed on 2 April 2022).
- Zhou, W.; Yang, S.; Wang, P.G. Matrix effects and application of matrix effect factor. Bioanalysis 2017, 9, 1839–1844. [Google Scholar] [CrossRef]
- Lopes, R.P.; Reyes, R.C.; Romero-González, R.; Vidal, J.L.M.; Frenich, A.G. Multiresidue determination of veterinary drugs in aquaculture fish samples by ultra high performance liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. B 2012, 895, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Pashaei, R.; Dzingelevičienė, R.; Abbasi, S.; Szultka-Młyńska, M.; Buszewski, B. Determination of 15 human pharmaceutical residues in fish and shrimp tissues by high-performance liquid chromatography-tandem mass spectrometry. Environ. Monit. Assess. 2022, 194, 325. [Google Scholar] [CrossRef]
- Khataei, M.M.; Epi, S.B.H.; Lood, R.; Spégel, P.; Yamini, Y.; Turner, C. A review of green solvent extraction techniques and their use in antibiotic residue analysis. J. Pharm. Biomed. Anal. 2022, 209, 114487. [Google Scholar] [CrossRef]
- Schwaiger, B.; König, J.; Lesueur, C. Development and validation of a multi-class UHPLC-MS/MS method for determination of antibiotic residues in dairy products. Food Anal. Methods 2018, 11, 1417–1434. [Google Scholar] [CrossRef]
- Nebot, C.; Regal, P.; Miranda, J.; Cepeda, A.; Fente, C. Simultaneous Determination of Sulfonamides, Penicillins and Coccidiostats in Pork by High-Performance Liquid Chromatography–Tandem Mass Spectrometry. J. Chromatogr. Sci. 2012, 50, 414–425. [Google Scholar] [CrossRef]
- Meras, D.T.; Diaz, G.F.; Lopez, S.M.I.; Caceres, R. Comparison of different methods for the determination of several quinolonic and cinolonic antibiotics in trout muscle tissue by HPLC with fluorescence detection. Chromatographia 2000, 51, 163. [Google Scholar] [CrossRef]
- Potter, R.A.; Burns, B.G.; van de Riet, J.M.; North, D.H.; Darvesh, R. Simultaneous determination of 17 sulfonamides, the potentiators ormetoprim, trimethoprim in salmon muscle by liquid chromatography with tandem mass spectrometry detection. J. AOAC Int. 2007, 90, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Dickson, L.C. Performance characterization of a quantitative liquid chromatography–tandem mass spectrometric method for 12 macrolide and lincosamide antibiotics in salmon, shrimp and tilapia. J. Chromatogr. B 2014, 967, 203–210. [Google Scholar] [CrossRef]
- Gracia-Lor, E.; Sancho, J.V.; Hernández, F. Multi-class determination of around 50 pharmaceuticals, including 26 antibiotics, in environmental and wastewater samples by ultra-high performance liquid chromatography–tandem mass spectrometry. J. Chromatogr. A 2011, 1218, 2264–2275. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Li, P.; Wang, Y.; Zhu, K. Analysis of veterinary antibiotic residues in swine wastewater and environmental water samples using optimized SPE-LC/MS/MS. Chemosphere 2009, 74, 1090–1097. [Google Scholar] [CrossRef]
- Liu, X.; Steele, J.C.; Meng, X.Z. Usage, residue, and human health risk of antibiotics in Chinese aquaculture: A review. Environ. Pollut. 2017, 223, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Lulijwa, R.; Kajobe, R. Aquaculture production and its contribution to development in the Rwenzori region Uganda Use of abattoir wastes for livestock feeding View project Artemia Study View project. Afr. J. Trop Hydrobiol. Fish 2018, 16, 56–62. [Google Scholar]
- Chen, B.; Lin, L.; Fang, L.; Yang, Y.; Chen, E.; Yuan, K.; Zou, S.; Wang, X.; Luan, T. Complex pollution of antibiotic resistance genes due to beta-lactam and aminoglycoside use in aquaculture farming. Water Res. 2018, 134, 200–208. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Deng, M.; Wang, Q.; Yang, Y.; Yang, Y.; Nie, X. Residues and health risk assessment of quinolones and sulfonamides in cultured fish from Pearl River Delta, China. Aquaculture 2016, 458, 38–46. [Google Scholar] [CrossRef]
- Dinh, Q.T.; Munoz, G.; Duy, S.V.; Do, D.T.; Bayen, S.; Sauvé, S. Analysis of sulfonamides, fluoroquinolones, tetracyclines, triphenylmethane dyes and other veterinary drug residues in cultured and wild seafood sold in Montreal, Canada. J. Food Compos. Anal. 2020, 94, 103630. [Google Scholar] [CrossRef]
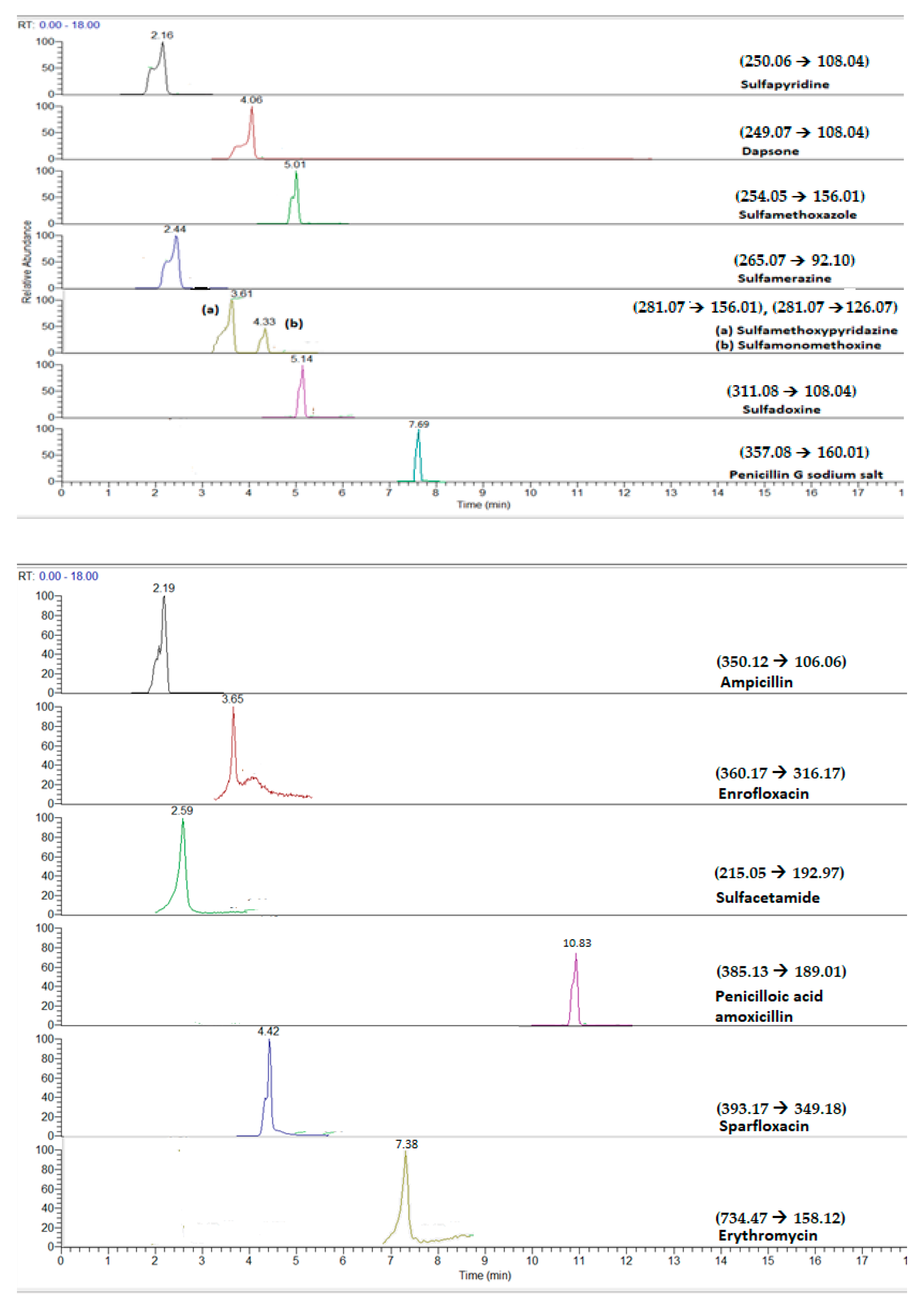
| Compound | Precursor Ion | Product Ions | Collision Energy (v) |
|---|---|---|---|
| Sulfamethoxypyridazine | 281.07 | 156.01 | 25 |
| Sulfapyridine | 250.06 | 108.04 | 20 |
| Sulfamethoxazole | 254.05 | 156.01 | 25 |
| Sulfadoxine | 311.08 | 108.04 | 25 |
| Sulfamerazine | 265.07 | 92.10 | 28 |
| Sulfamonomethoxine | 281.07 | 126.07 | 30 |
| Sulfacetamide | 215.05 | 192.97 | 45 |
| Enrofloxacin | 360.17 | 316.17 | 35 |
| Sparfloxacin | 393.17 | 349.18 | 20 |
| Ampicillin | 350.12 | 106.06 | 30 |
| Dapsone | 249.07 | 108.04 | 25 |
| Erythromycin | 734.47 | 158.12 | 32 |
| Penicilloic acid of amoxicillin | 385.13 | 189.01 | 30 |
| Penicillin G sodium salt | 357.08 | 160.01 | 35 |
| Antibiotic | Fish | Shrimp | ||||
|---|---|---|---|---|---|---|
| ME (%) | LOD (µg kg−1) | LOQ (µg kg−1) | ME (%) | LOD (µg kg−1) | LOQ (µg kg−1) | |
| Sulfamethoxypyridazine | 45 | 0.42 | 1.32 | 58 | 0.48 | 1.62 |
| Sulfapyridine | 26 | 0.31 | 1.03 | 33 | 0.22 | 0.72 |
| Sulfamethoxazole | 21 | 0.36 | 1.19 | 28 | 0.37 | 1.26 |
| Sulfadoxine | 56 | 0.10 | 0.35 | 74 | 0.14 | 0.44 |
| Sulfamerazine | 29 | 0.19 | 0.63 | 41 | 0.25 | 0.82 |
| Sulfamonomethoxine | 73 | 0.17 | 0.56 | 81 | 0.21 | 0.68 |
| Sulfacetamide | 29 | 0.30 | 1.10 | 39 | 0.38 | 1.26 |
| Enrofloxacin | 22 | 0.33 | 1.11 | 35 | 0.36 | 1.20 |
| Sparfloxacin | 37 | 0.07 | 0.24 | 42 | 0.13 | 0.42 |
| Ampicillin | 80 | 0.35 | 1.15 | 71 | 0.45 | 1.50 |
| Dapsone | 45 | 0.26 | 0.85 | 53 | 0.19 | 0.64 |
| Erythromycin | 97 | 0.25 | 0.82 | 82 | 0.30 | 1.00 |
| Penicilloic acid of Amoxicillin | 35 | 0.32 | 1.05 | 40 | 0.33 | 1.10 |
| Penicillin G | 18 | 0.18 | 0.59 | 30 | 0.25 | 0.80 |
| Tilapia Fish | Shrimp | |||||||
|---|---|---|---|---|---|---|---|---|
| Compound | Intra Day | Inter-Day | Intra Day | Inter-Day | ||||
| AR % | RSD | AR % | RSD | AR % | RSD | AR % | RSD | |
| Sulfamethoxypyridazine | ||||||||
| 1 | 102 ± 1 | 10 | 105 ± 1 | 17 | 101 ± 1 | 11 | 96 ± 8 | 9 |
| 5 | -a | -a | 102 ± 1 | 11 | -a | -a | 91 ± 7 | 16 |
| 10 | 89 ± 1 | 8 | 82 ± 1 | 12 | 88 ± 1 | 8 | 81 ± 2 | 14 |
| 50 | -a | -a | 96 ± 5 | 11 | -a | -a | 96 ± 6 | 13 |
| 100 | 87 ± 6 | 7 | 83 ± 7 | 10 | 84 ± 3 | 4 | 80 ± 8 | 10 |
| Sulfapyridine | ||||||||
| 1 | 94 ± 1 | 12 | 93 ± 1 | 16 | 87 ± 3 | 4 | 93 ± 1 | 18 |
| 5 | -a | -a | 92 ± 1 | 13 | -a | -a | 92 ± 7 | 15 |
| 10 | 89 ± 3 | 11 | 79 ± 3 | 5 | 87 ± 1 | 11 | 79 ± 3 | 4 |
| 50 | -a | -a | 93 ± 6 | 13 | -a | -a | 90 ± 5 | 12 |
| 100 | 88 ± 7 | 8 | 84 ± 9 | 10 | 86 ± 7 | 8 | 83 ± 7 | 12 |
| Sulfamethoxazole | ||||||||
| 1 | 84 ± 1 | 13 | 83 ± 1 | 14 | 82 ± 1 | 13 | 82 ± 2 | 13 |
| 5 | -a | -a | 90 ± 1 | 18 | -a | -a | 91 ± 5 | 18 |
| 10 | 88 ± 1 | 11 | 87 ± 1 | 14 | 88 ± 2 | 13 | 90 ± 2 | 14 |
| 50 | -a | -a | 99 ± 8 | 17 | -a | -a | 76 ± 3 | 8 |
| 100 | 91 ± 10 | 12 | 85 ± 5 | 5 | 86 ± 7 | 8 | 84 ± 4 | 5 |
| Sulfadoxine | ||||||||
| 1 | 81 ± 1 | 10 | 87 ± 1 | 9 | 79 ± 4 | 12 | 86 ± 3 | 9 |
| 5 | -a | -a | 92 ± 1 | 17 | -a | -a | 86 ± 3 | 9 |
| 10 | 85 ± 1 | 7 | 83 ± 1 | 7 | 85 ± 6 | 8 | 84 ± 5 | 7 |
| 50 | -a | -a | 91 ± 4 | 9 | -a | -a | 92 ± 5 | 11 |
| 100 | 85 ± 6 | 7 | 80 ± 6 | 8 | 87 ± 6 | 6 | 80 ± 6 | 9 |
| Sulfamerazine | ||||||||
| 1 | 89 ± 1 | 7 | 95 ± 1 | 10 | 89 ± 6 | 7 | 81 ± 4 | 6 |
| 5 | -a | -a | 102 ± 1 | 6 | -a | -a | 92 ± 4 | 5 |
| 10 | 92 ± 1 | 4 | 101 ± 1 | 7 | 91 ± 4 | 5 | 95 ± 4 | 4 |
| 50 | -a | -a | 100 ± 5 | 10 | -a | -a | 80 ± 5 | 12 |
| 100 | 91 ± 5 | 5 | 94 ± 6 | 6 | 89 ± 4 | 6 | 87 ± 7 | 8 |
| Sulfamonomethoxine | ||||||||
| 1 | 89 ± 1 | 1 | 89 ± 1 | 1 | 84 ± 1 | 2 | 85 ± 1 | 2 |
| 5 | -a | -a | 93 ± 1 | 2 | -a | -a | 94 ± 3 | 2 |
| 10 | 81 ± 1 | 8 | 79 ± 1 | 2 | 79 ± 2 | 7 | 79 ± 2 | 5 |
| 50 | -a | -a | 77 ± 1 | 4 | -a | -a | 78 ± 1 | 12 |
| 100 | 97 ± 10 | 11 | 79 ± 4 | 3 | 91 ± 5 | 13 | 86 ± 8 | 9 |
| Sulfacetamide | ||||||||
| 1 | 97 ± 2 | 10 | 101 ± 1 | 7 | 89 ± 8 | 8 | 101 ± 8 | 7 |
| 5 | -a | -a | 92 ± 3 | 7 | -a | -a | 92 ± 3 | 7 |
| 10 | 94 ± 2 | 15 | 92 ± 1 | 12 | 94 ± 2 | 15 | 90 ± 6 | 11 |
| 50 | -a | -a | 88 ± 4 | 8 | -a | -a | 84 ± 3 | 5 |
| 100 | 82 ± 5 | 6 | 82 ± 7 | 10 | 82 ± 5 | 7 | 82 ± 6 | 11 |
| Enrofloxacin | ||||||||
| 1 | 89 ± 2 | 19 | 92 ± 1 | 14 | 85 ± 3 | 19 | 88 ± 1 | 12 |
| 5 | -a | -a | 100 ± 1 | 12 | -a | -a | 94 ± 3 | 14 |
| 10 | 87 ± 1 | 16 | 90 ± 2 | 18 | 82 ± 2 | 13 | 97 ± 2 | 13 |
| 50 | -a | -a | 91 ± 4 | 9 | -a | -a | 87 ± 2 | 3 |
| 100 | 94 ± 7 | 10 | 97 ± 7 | 7 | 96 ± 6 | 6 | 96 ± 7 | 8 |
| Sparfloxacin | ||||||||
| 1 | 87 ± 1 | 4 | 96 ± 1 | 3 | 90 ± 3 | 1 | 86 ± 3 | 3 |
| 5 | -a | -a | 87 ± 1 | 4 | -a | -a | 89 ± 2 | 5 |
| 10 | 86 ± 1 | 10 | 84 ± 1 | 12 | 85 ± 1 | 6 | 86 ± 5 | 13 |
| 50 | -a | -a | 87 ± 3 | 6 | -a | -a | 88 ± 2 | 3 |
| 100 | 91 ± 8 | 11 | 88 ± 2 | 2 | 91 ± 2 | 1 | 88 ± 4 | 2 |
| Ampicillin | ||||||||
| 1 | 80 ± 1 | 11 | 84 ± 1 | 11 | 80 ± 2 | 5 | 87 ± 6 | 8 |
| 5 | -a | -a | 97 ± 3 | 8 | -a | -a | 95 ± 1 | 5 |
| 10 | 88 ± 1 | 9 | 88 ± 1 | 11 | 85 ± 3 | 8 | 87 ± 4 | 8 |
| 50 | -a | -a | 95 ± 3 | 7 | -a | -a | 96 ± 4 | 8 |
| 100 | 99 ± 9 | 11 | 100 ± 9 | 9 | 94 ± 8 | 8 | 96 ± 6 | 6 |
| Dapsone | ||||||||
| 1 | 85 ± 1 | 13 | 97 ± 1 | 18 | 79 ± 1 | 14 | 82 ± 1 | 14 |
| 5 | -a | -a | 96 ± 2 | 5 | -a | -a | 97 ± 2 | 4 |
| 10 | 85 ± 1 | 8 | 88 ± 2 | 13 | 87 ± 7 | 7 | 92 ± 3 | 11 |
| 50 | -a | -a | 89 ± 2 | 5 | -a | -a | 90 ± 2 | 5 |
| 100 | 79 ± 5 | 14 | 88 ± 8 | 10 | 80 ± 3 | 16 | 81 ± 7 | 10 |
| Erythromycin | ||||||||
| 1 | 83 ± 1 | 3 | 92 ± 4 | 5 | 79 ± 1 | 14 | 91 ± 5 | 8 |
| 5 | -a | -a | 91 ± 2 | 6 | -a | -a | 91 ± 3 | 7 |
| 10 | 83 ± 1 | 5 | 89 ± 1 | 14 | 91 ± 7 | 10 | 92 ± 1 | 13 |
| 50 | -a | -a | 81 ± 4 | 9 | -a | -a | 83 ± 4 | 9 |
| 100 | 91 ± 7 | 19 | 87 ± 9 | 11 | 95 ± 7 | 18 | 89 ± 8 | 10 |
| Penicilloic acid of Amoxicillin | ||||||||
| 1 | 98 ± 2 | 3 | 99 ± 4 | 5 | 92 ± 5 | 10 | 98 ± 5 | 5 |
| 5 | -a | -a | 97 ± 1 | 3 | -a | -a | 96 ± 4 | 9 |
| 10 | 89 ± 1 | 13 | 82 ± 3 | 4 | 81 ± 4 | 3 | 82 ± 1 | 4 |
| 50 | -a | -a | 87 ± 3 | 6 | -a | -a | 88 ± 3 | 6 |
| 100 | 90 ± 5 | 6 | 88 ± 5 | 6 | 87 ± 4 | 11 | 89 ± 5 | 6 |
| Penicillin G | ||||||||
| 1 | 85 ± 1 | 17 | 85 ± 1 | 17 | 82 ± 2 | 15 | 87 ± 1 | 18 |
| 5 | -a | -a | 90 ± 1 | 4 | -a | -a | 87 ± 1 | 3 |
| 10 | 85 ± 2 | 5 | 75 ± 1 | 7 | 87 ± 4 | 4 | 79 ± 4 | 6 |
| 50 | -a | -a | 87 ± 7 | 15 | -a | -a | 84 ± 6 | 14 |
| 100 | 101 ± 9 | 18 | 86 ± 11 | 13 | 92 ± 9 | 10 | 83 ± 8 | 10 |
| Samples | Sulfacetamide (µg kg−1) | Sulfapyridine (µg kg−1) | Sulfamethoxypyridazine (µg kg−1) | Sulfadoxine (µg kg−1) |
|---|---|---|---|---|
| F-1 | _ | _ | 0.75 ± 0.15 | _ |
| F-2 | _ | _ | _ | _ |
| F-3 | 4.31 ± 0.70 | _ | _ | _ |
| F-4 | _ | _ | _ | _ |
| F-5 | _ | _ | _ | _ |
| F-6 | _ | _ | _ | _ |
| S-1 | _ | 0.35 ± 0.02 | _ | 0.35 ± 0.03 |
| S-2 | _ | 0.21 ± 0.01 | _ | 0.36 ± 0.02 |
| S-3 | _ | 0.56 ± 0.16 | _ | 1.44 ± 0.12 |
| S-4 | _ | 0.26 ± 0.03 | _ | 0.57 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayasinghe, G.D.T.M.; Szpunar, J.; Lobinski, R.; Edirisinghe, E.M.R.K.B. Determination of Multi-Class Antibiotics Residues in Farmed Fish and Shrimp from Sri Lanka by Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry (UPLC-MS/MS). Fishes 2023, 8, 154. https://doi.org/10.3390/fishes8030154
Jayasinghe GDTM, Szpunar J, Lobinski R, Edirisinghe EMRKB. Determination of Multi-Class Antibiotics Residues in Farmed Fish and Shrimp from Sri Lanka by Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry (UPLC-MS/MS). Fishes. 2023; 8(3):154. https://doi.org/10.3390/fishes8030154
Chicago/Turabian StyleJayasinghe, G. D. T. M., Joanna Szpunar, Ryszard Lobinski, and E. M. R. K. B. Edirisinghe. 2023. "Determination of Multi-Class Antibiotics Residues in Farmed Fish and Shrimp from Sri Lanka by Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry (UPLC-MS/MS)" Fishes 8, no. 3: 154. https://doi.org/10.3390/fishes8030154
APA StyleJayasinghe, G. D. T. M., Szpunar, J., Lobinski, R., & Edirisinghe, E. M. R. K. B. (2023). Determination of Multi-Class Antibiotics Residues in Farmed Fish and Shrimp from Sri Lanka by Ultra Performance Liquid Chromatography-Tandem Mass Spectrometry (UPLC-MS/MS). Fishes, 8(3), 154. https://doi.org/10.3390/fishes8030154