The Protective Effects of Korill Product on Carp Fingerlings Reared in High Densities and Challenged with Albendazole Treatment
Abstract
1. Introduction
2. Materials and Methods
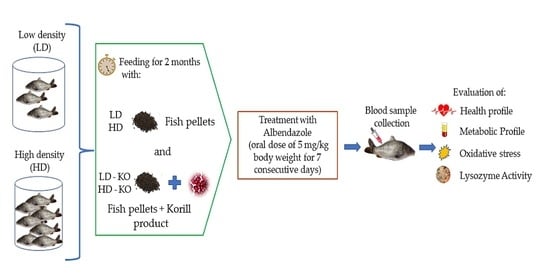
2.1. Experimental Design and Fish Maintenance
2.2. Water Quality Parameters
2.3. Sampling Protocol and Blood Analysis
2.4. Data Analysis
3. Results
3.1. Physicochemical Parameters of Water
3.2. Hematological and Biochemical Parameters
3.3. Serum Metabolic Profile
3.4. Oxidative Stress Parameters and Lysozyme Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naylor, R.L.; Hardy, R.W.; Buschmann, A.H.; Bush, S.R.; Cao, L.; Klinger, D.H.; Little, D.C.; Lubchenco, J.; Shumway, S.E.; Troell, M. A 20-year retrospective review of global aquaculture. Nature 2020, 591, 551–563. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, K.; Ogawa, K.; Nagae, M.; Ito, F. The influence of rearing density on stress response and disease susceptibility of ayu (Plecoglossus altivelis). Aquaculture 2003, 220, 515–523. [Google Scholar] [CrossRef]
- Yarahmadi, P.; Miandare, H.K.; Hoseinifar, S.H.; Gheysvandi, N.; Akbarzadeh, A. The effects of stocking density on hemato-immunological and serum biochemical parameters of rainbow trout (Oncorhynchus mykiss). Aquac. Int. 2015, 23, 55–63. [Google Scholar] [CrossRef]
- Barton, B.A. Stress in fishes: A diversity of responses with particular reference to changes in circulating corticosteroids. Integr. Comp. Biol. 2002, 42, 517–525. [Google Scholar] [CrossRef]
- Liu, B.L.; Fei, F.; Li, X.T.; Wang, X.Y.; Huang, B. Effects of stocking density on stress response, innate immune parameters, and welfare of turbot (Scophthalmus maximus). Aquac. Int. 2019, 27, 1599–1612. [Google Scholar] [CrossRef]
- Jia, R.; Wang, L.; Hou, Y.; Feng, W.; Li, B.; Zhu, J. Effects of Stocking Density on the Growth Performance, Physiological Parameters, Redox Status and Lipid Metabolism of Micropterus salmoides in Integrated Rice–Fish Farming Systems. Antioxidants 2022, 11, 1215. [Google Scholar] [CrossRef]
- Naderi, M.; Keyvanshokooh, S.; Salati, A.P.; Ghaedi, A. Effects of chronic high stocking density on liver proteome of rainbow trout (Oncorhynchus mykiss). Fish Physiol. Biochem. 2017, 43, 1373–1385. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, G.; Nie, Z.; Li, Q.; Shao, N.; Xu, P. Effect of Stocking Density on Growth, Serum Biochemical Parameters, Digestive Enzymes Activity and Antioxidant Status of Largemouth Bass, Micropterus salmoides. Pak. J. Zool. 2019, 51, 1509–1517. [Google Scholar] [CrossRef]
- Andrade, T.; Afonso, A.; Pérez-Jiménez, A.; Oliva-Teles, A.; de las Heras, V.; Mancera, J.M.; Serradeiro, R.; Costas, B. Evaluation of different stocking densities in a Senegalese sole (Solea senegalensis) farm: Implications for growth, humoral immune parameters and oxidative status. Aquaculture 2015, 438, 6–11. [Google Scholar] [CrossRef]
- Sahin, K.; Yazlak, H.; Orhan, C.; Tuzcu, M.; Akdemir, F.; Sahin, N. The effect of lycopene on antioxidant status in rainbow trout (Oncorhynchus mykiss) reared under high stocking density. Aquaculture 2014, 418–419, 132–138. [Google Scholar] [CrossRef]
- Adineh, H.; Naderi, M.; Nazer, A.; Yousefi, M.; Ahmadifar, E. Interactive effects of stocking density and dietary supplementation with Nano selenium and garlic extract on growth, feed utilization, digestive enzymes, stress responses, and antioxidant capacity of grass carp, Ctenopharyngodon idella. J. World Aquac. Soc. 2021, 52, 789–804. [Google Scholar] [CrossRef]
- Souza, C.D.F.; Baldissera, M.D.; Baldisserotto, B.; Heinzmann, B.M.; Martos-Sitcha, J.A.; Mancera, J.M. Essential oils as stress-reducing agents for fish aquaculture: A review. Front. Physiol. 2019, 10, 785. [Google Scholar] [CrossRef]
- Shourbela, R.M.; El-Hawarry, W.N.; Elfadadny, M.R.; Dawood, M.A.O. Oregano essential oil enhanced the growth performance, immunity, and antioxidative status of Nile tilapia (Oreochromis niloticus) reared under intensive systems. Aquaculture 2021, 542, 736868. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Taheri Mirghaed, A.; Iri, Y.; Hoseinifar, S.H.; Van Doan, H.; Reverter, M. Effects of dietary Russian olive, Elaeagnus angustifolia, leaf extract on growth, hematological, immunological, and antioxidant parameters in common carp, Cyprinus carpio. Aquaculture 2021, 536, 736461. [Google Scholar] [CrossRef]
- Lieke, T.; Meinelt, T.; Hoseinifar, S.H.; Pan, B.; Straus, D.L.; Steinberg, C.E. Sustainable aquaculture requires environmental-friendly treatment strategies for fish diseases. Rev. Aquac. 2020, 12, 943–965. [Google Scholar] [CrossRef]
- Mortuza, M.G.; Al-Misned, F.A. Prevalence of ectoparasites in carp fry and fingerlings of Rajshahi district, Bangladesh. J. Parasit. Dis. 2015, 39, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Mwainge, V.M.; Ogwai, C.; Aura, C.M.; Mutie, A.; Ombwa, V.; Nyaboke, H.; Oyier, K.N.; Nyaundi, J. An overview of fish disease and parasite occurrence in the cage culture of Oreochromis niloticus: A case study in Lake Victoria, Kenya. Aquat. Ecosyst. Health Manag. 2021, 24, 43–55. [Google Scholar] [CrossRef]
- Bártíková, H.; Vokřál, I.; Skálová, L.; Lamka, J.; Szotáková, B. In vitro oxidative metabolism of xenobiotics in the lancet fluke (Dicrocoelium dendriticum) and the effects of albendazole and albendazole sulphoxide ex vivo. Xenobiotica 2010, 40, 593–601. [Google Scholar] [CrossRef]
- Busatto, Z.; de França, W.G.; Cyrino, J.E.P.; Paschoal, J.A.R. Assessment of elimination profile of albendazole residues in fish. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2018, 35, 77–85. [Google Scholar] [CrossRef]
- Cordeiro, R.P.; Braga, P.A.D.C.; Jonsson, C.M.; Brandão, F.R.; Chagas, E.C.; Reyes, F.G.R. Therapeutic efficacy and bioaccumulation of albendazole in the treatment of tambaqui (Colossoma macropomum) parasitized by acanthocephalan (Neoechinorhynchus buttnerae). Aquac. Res. 2022, 53, 1446–1455. [Google Scholar] [CrossRef]
- Orobets, V.; Lisovets, E.; Zabashta, S.; Ermakov, A. Control of fish parasites in aquaculture. IOP Conf. Ser. Earth Envirn. Sci. 2019, 403, 012065. [Google Scholar] [CrossRef]
- Tojo, J.; Santamarina, M.; Ubeira, F.; Estevez, J.; Sanmartin, M. Anthelmintic activity of benzimidazoles against Gyiodactylus sp. infecting rainbow trout Oncorhynchus mykiss. Dis. Aquat. Org. 1992, 12, 185–189. [Google Scholar] [CrossRef]
- Carlsson, G.; Patring, J.; Ullerås, E.; Oskarsson, A. Developmental toxicity of albendazole and its three main metabolites in zebrafish embryos. Reprod. Toxicol. 2011, 32, 129–137. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, A.; Ullerås, E.; Patring, J.; Oskarsson, A. Albendazole causes stage-dependent developmental toxicity and is deactivated by a mammalian metabolization system in a modified zebrafish embryotoxicity test. Reprod. Toxicol. 2012, 34, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Nwani, C.D.; Odo, G.E.; Nwadinigwe, A.O.; Onyeke, C.C.; Atama, C.I.; Ngwu, G.; Oluah, S.N.; Ukonze, J.A.; Ezeibe, B.C.A. Short-term effects of albendazole on the oxidative stress markers and hematological parameters in tissues of African Catfish Clarias gariepinus. J. Aquat. Anim. Health 2016, 28, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Kanazawa, A. Effects of docosahexaenoic acid and phospholipids on stress tolerance of fish. Aquaculture 1997, 155, 129–134. [Google Scholar] [CrossRef]
- Gigliotti, J.C.; Davenport, M.P.; Beamer, S.K.; Tou, J.C.; Jaczynski, J. Extraction and characterisation of lipids from Antarctic krill (Euphausia superba). Food Chem. 2011, 125, 1028–1036. [Google Scholar] [CrossRef]
- Kaur, K.; Kortner, T.M.; Benitez-Santana, T.; Burri, L. Effects of Antarctic Krill Products on Feed Intake, Growth Performance, Fillet Quality, and Health in Salmonids. Aquac. Nutr. 2022, 2022, 3170854. [Google Scholar] [CrossRef]
- Berge, R.K.; Ramsvik, M.S.; Bohov, P.; Svardal, A.; Nordrehaug, J.E.; Rostrup, E.; Bjørndal, B. Krill oil reduces plasma triacylglycerol level and improves related lipoprotein particle concentration, fatty acid composition and redox status in healthy young adults-a pilot study. Lipids Health Dis. 2015, 14, 163. [Google Scholar] [CrossRef]
- Burri, L.; Berge, K.; Wibrand, K.; Berge, R.K.; Barger, J.L. Differential effects of krill oil and fish oil on the hepatic transcriptome in mice. Front. Gen. 2011, 2, 45. [Google Scholar] [CrossRef]
- Phleger, C.F.; Nelson, M.M.; Mooney, B.D.; Nichols, P.D. Interannual and between species comparison of the lipids, fatty acids and sterols of Antarctic krill from the US AMLR Elephant Island survey area. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2002, 131, 733–747. [Google Scholar] [CrossRef]
- Xie, D.; Gong, M.; Wei, W.; Jin, J.; Wang, X.; Wang, X.; Jin, Q. Antarctic krill (Euphausia superba) oil: A comprehensive review of chemical composition, extraction technologies, health benefits, and current applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 514–534. [Google Scholar] [CrossRef] [PubMed]
- Ramsvik, M.S.; Bjørndal, B.; Bruheim, I.; Bohov, P.; Berge, R.K. A phospholipid-protein complex from Krill with antioxidative and immunomodulating properties reduced plasma triacylglycerol and hepatic lipogenesis in rats. Mar. Drugs 2015, 13, 4375–4397. [Google Scholar] [CrossRef]
- Ali, K.H.; Al-Jawad, F.H.; Kadhim, H.M. The Possible Hepatoprotecive Effects of “Krill Oil and Silymarin against Carbon Tetrachloride (CCl4)-Induced Rats Model of Liver Fibrosis: In Vivo Study”. Res. J. Pharm. Technol. 2021, 14, 5953–5958. [Google Scholar] [CrossRef]
- Zadeh-Ardabili, P.M.; Rad, S.K. Anti-pain and anti-inflammation like effects of Neptune krill oil and fish oil against carrageenan induced inflammation in mice models: Current statues and pilot study. Biotechnol. Rep. 2019, 22, e00341. [Google Scholar] [CrossRef] [PubMed]
- Mellouk, Z.; Ramirez, M.; Pena, K.; Arivalo, J.; Agustina, M. P2-12: Cell-based anti-oxydant activity of dietary krill oil enrichment in high fat-fed rats target tissues. In Annales de Cardiologie et d’Angéiologie; Elsevier Masson: Amsterdam, The Netherlands, 2019; Volume 64, p. S27. [Google Scholar]
- Li, X.; Yuan, Y.; Jin, M.; Wang, X.; Hu, X.; Zhao, M.; Zhou, Q. Growth performance, antioxidant capacity, tissue fatty acid composition and lipid metabolism of juvenile green mud crab Scylla paramamosain in response to different dietary n-3 PUFA lipid sources. Aquac. Rep. 2021, 19, 100599. [Google Scholar] [CrossRef]
- Olsen, R.E.; Suontama, J.; Langmyhr, E.; Mundheim, H.; Ringø, E.; Melle, W.; Hemre, G.I. The replacement of fish meal with Antarctic krill, Euphausia superba in diets for Atlantic salmon, Salmo salar. Aquac. Nutr. 2006, 12, 280–290. [Google Scholar] [CrossRef]
- Choi, J.; Lee, K.W.; Han, G.S.; Byun, S.G.; Lim, H.J.; Kim, H.S. Dietary inclusion effect of krill meal and various fish meal sources on growth performance, feed utilization, and plasma chemistry of grower walleye pollock (Gadus chalcogrammus, Pallas 1811). Aquac. Rep. 2020, 17, 100331. [Google Scholar] [CrossRef]
- Mørkøre, T.; Moreno, H.M.; Borderías, J.; Larsson, T.; Hellberg, H.; Hatlen, B.; Krasnov, A. Dietary inclusion of Antarctic krill meal during the finishing feed period improves health and fillet quality of Atlantic salmon (Salmo salar L.). Br. J. Nutr. 2020, 124, 418–431. [Google Scholar] [CrossRef]
- Arai, S.; Mori, T.; Miki, W.; Yamaguchi, K.; Konosu, S.; Satake, M.; Fujita, T. Pigmentation of juvenile coho salmon with carotenoid oil extracted from Antarctic krill. Aquaculture 1987, 66, 255–264. [Google Scholar] [CrossRef]
- Taylor, J.F.; Martinez-Rubio, L.; del Pozo, J.; Walton, J.M.; Tinch, A.E.; Migaud, H.; Tocher, D.R. Influence of dietary phospholipid on early development and performance of Atlantic salmon (Salmo salar). Aquaculture 2015, 448, 262–272. [Google Scholar] [CrossRef]
- Li, Y.; Zeng, Q.H.; Liu, G.; Peng, Z.; Wang, Y.; Zhu, Y.; Wang, J.J. Effects of ultrasound-assisted basic electrolyzed water (BEW) extraction on structural and functional properties of Antarctic krill (Euphausia superba) proteins. Ultrason. Sonochem. 2021, 71, 105364. [Google Scholar] [CrossRef]
- Dediu, L.; Docan, A.; Crețu, M.; Grecu, I.; Mogodan, A.; Maereanu, M.; Oprea, L. Effects of stocking density on growth performance and stress responses of bester and bester♀× beluga♂ juveniles in recirculating aquaculture systems. Animals 2021, 11, 2292. [Google Scholar] [CrossRef] [PubMed]
- Jahanbakhshi, A.; Shaluei, F.; Baghfalaki, M.; Ramazi, F.G.; Ahmadvand, S. Efficacy of 2-phenoxyethanol as an Anesthetic for Two Size of Persian Sturgeon, Acipenser persicus. J. Walailak. 2012, 9, 31–36. [Google Scholar] [CrossRef]
- Blaxhall, P.C.; Daisley, K.W. Routine hematological methods for use with fish blood. J. Fish Biol. 1973, 5, 771–781. [Google Scholar] [CrossRef]
- Svobodova, Z.; Pravda, D.; Modra, H. Metody hematologickeho vysetrovani ryb. In Unified Methods of Fish Haematological Investigations, Edice Metodik; Research Institute of Fish Culture and Hydrobiology: Vodňany, Czech Republic, 2012; 29p. [Google Scholar]
- Hesser, E.F. Methods for routine fish hematology. Progress. Fish Cult. 1960, 22, 164–171. [Google Scholar] [CrossRef]
- Ghergariu, S.; Pop, A.; Kadar, L. Veterinary Clinical Laboratory Guide; Ceres Publishing House: Bucharest, Romania, 1985; pp. 82–90. (In Romanian) [Google Scholar]
- Svobodova, Z. Stress in fishes (a review). Bull Vurh Vodnany 2001, 4, 169–191. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef] [PubMed]
- Roberta, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Goran, S.M.A.; Omar, S.S.; Anwer, A.A. Water Quality and Physiological Parameters of Common Carp Fingerling Fed on Jerusalem artichoke Tubers. Polytechnic 2016, 3, 502–516. [Google Scholar]
- Ortuño, J.; Esteban, M.A.; Meseguer, J. Effects of short-term crowding stress on the gilthead seabream (Sparus aurata L.) innate immune response. Fish Shellfish Immunol. 2001, 11, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Tort, L. Stress and immune modulation in fish. Dev. Comp. Immunol. D 2011, 35, 1366–1375. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, J.L.; Rachinas-Lopes, P.; Arechavala-Lopez, P. Finding the “golden stocking density”: A balance between fish welfare and farmers’ perspectives. Front. Vet. Sci. 2022, 9, 1099. [Google Scholar] [CrossRef]
- Baldwin, L. The effects of stocking density on fish welfare. Plymouth Stud. Sci. 2010, 4, 372–383. [Google Scholar]
- Ahmed, I.; Reshi, Q.M.; Fazio, F. The influence of the endogenous and exogenous factors on hematological parameters in different fish species: A review. Aquac. Int. 2020, 28, 869–899. [Google Scholar] [CrossRef]
- Montero, D.; Izquierdo, M.S.; Tort, L.; Robaina, L.; Vergara, J.M. High stocking density produces crowding stress altering some physiological and biochemical parameters in gilthead seabream, Sparus aurata, juveniles. Fish Physiol. Biochem. 1999, 20, 53–60. [Google Scholar] [CrossRef]
- Dai, W.; Wang, X.; Guo, Y.; Wang, Q.; Ma, J. Growth performance, hematological and biochemical responses of African catfish (Clarias gariepinus) reared at different stocking densities. Afr. J. Agric. Res. 2011, 6, 6177–6182. [Google Scholar] [CrossRef]
- Farias, C.F.S.; Brandão, F.R.; de Alexandre Sebastião, F.; de Melo Souza, D.C.; Monteiro, P.C.; Majolo, C.; Chagas, E.C. Albendazole and praziquantel for the control of Neoechinorhynchus buttnerae in tambaqui (Colossoma macropomum). Aqua. Int. 2021, 29, 1495–1505. [Google Scholar] [CrossRef]
- Lim, K.C.; Yusoff, F.M.; Shariff, M.; Kamarudin, M.S.; Nagao, N. Dietary supplementation of astaxanthin enhances hemato-biochemistry and innate immunity of Asian seabass, Lates calcarifer (Bloch, 1790). Aquaculture 2019, 512, 734339. [Google Scholar] [CrossRef]
- Witeska, M.; Kondera, E.; Ługowska, K.; Bojarski, B. Hematological methods in fish—Not only for beginners. Aquaculture 2022, 47, 73749. [Google Scholar] [CrossRef]
- Ambili, T.R.; Saravanan, M.; Ramesh, M.; Abhijith, D.B.; Poopal, R.K. Toxicological Effects of the Antibiotic Oxytetracyclineto an Indian Major Carp Labeo rohita. Arch. Environ. Contam. Toxicol. 2013, 64, 494–503. [Google Scholar] [CrossRef]
- Nwani, C.D.; Omah, M.C.; Ivoke, N.; Nwamba, H.O.; Ani, C.; Ogbonna, S.U. Biochemical, haematological and morphological variations in juvenile Clarias gariepinus exposed to Carbendazim® fungicide. Afr. J. Aquat. Sci. 2015, 40, 63–71. [Google Scholar] [CrossRef]
- Baghizadeh, E.; Khara, H. Variability in hematology and plasma indices of common carp Cyprinus carpio, associated with age, sex and hormonal treatment. Iran. J. Fish. Sci. 2015, 14, 99–111. [Google Scholar]
- Tahmasebi-Kohyani, A.; Keyvanshokooh, S.; Nematollahi, A.; Mahmoudi, N.; Pasha-Zanoosi, H. Effects of Dietary Nucleotides Supplementation on Rainbow Trout (Oncorhynchus mykiss) Performance and Acute Stress Response. Fish Physiol. Biochem. 2012, 38, 431–440. [Google Scholar] [CrossRef]
- Brucka-Jastrzębska, E.; Kawczuga, D. Antioxidant Status and Lipid Peroxidation in Blood of Common Carp (Cyprinus carpio L.). Pol. J. Environ. Stud. 2011, 20, 541–550. [Google Scholar]
- Swain, H.S.; Das, B.K.; Upadhyay, A.; Ramteke, M.H.; Kumar, V.; Meena, D.K.; Sarkar, U.K.; Chadha, N.K.; Rawat, K.D. Stocking density mediated stress modulates growth attributes in cage reared Labeo rohita (Hamilton) using multifarious biomarker approach. Sci. Rep. 2022, 12, 9869. [Google Scholar] [CrossRef] [PubMed]
- Tammam, M.S.; Wassef, E.A.; Toutou, M.M.; El-Sayed, A.F.M. Combined effects of surface area of periphyton substrates and stocking density on growth performance, health status, and immune response of Nile tilapia (Oreochromis niloticus) produced in cages. J. Appl. Phycol. 2020, 32, 3419–3428. [Google Scholar] [CrossRef]
- Kpundeh, M.D.; Xu, P.; Yang, H.; Qiang, J.; He, J. Stocking densities and chronic zero culture water exchange stress’ effects on biological performances, hematological and serum biochemical indices of gift tilapia juveniles (Oreochromis niloticus). J. Aquac. Res. Dev. 2013, 4, 2. [Google Scholar] [CrossRef]
- Abdel-Tawwab, M.; Hagras, A.E.; Elbaghdady, H.A.M.; Monier, M.N. Dissolved oxygen level and stocking density effects on growth, feed utilization, physiology, and innate immunity of Nile Tilapia, Oreochromis niloticus. J. Appl. Aquac. 2014, 26, 340–355. [Google Scholar] [CrossRef]
- Mahmoud, H.K.; Reda, F.M.; Alagawany, M.; Farag, M.R. Ameliorating deleterious effects of high stocking density on Oreochromis niloticus using natural and biological feed additives. Aquaculture 2021, 26, 340–355. [Google Scholar] [CrossRef]
- Opiyo, M.A.; Jumbe, J.; Ngugi, C.C.; Charo-Karisa, H. Dietary administration of probiotics modulates non-specific immunity and gut microbiota of Nile tilapia (Oreochromis niloticus) cultured in low input ponds. Int. J. Vet. Sci. 2019, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Soltanian, S.; Vazirzadeh, A.; Akbary, P. Effect of Praziquantel on Hemato-Immunological Indices in Common Carp (Cyprinus carpio). Iran. J. Sci. Technol. Trans. A Sci. 2018, 42, 1015–1025. [Google Scholar] [CrossRef]
- Aly, S.; Abd-Allah, O.; Mahmoud, A.; Gafer, H. Efficiency of Levamisole in Improving the Immune Response of Catfish (Clarias gariepenus) to Aeromonas hydrophila Vaccine: Clinico- Pathological Studies. Mediterr. Aquac. J. 2010, 3, 8–17. [Google Scholar] [CrossRef]
- Martínez-Porchas, M.; Rafael Martínez-Córdova, L.; Ramos-Enriquez, R. Cortisol and Glucose: Reliable indicators of fish stress? Pan-Am. J. Aquat. Sci. 2009, 4, 158–178. [Google Scholar]
- Wu, F.; Wen, H.; Tian, J.; Jiang, M.; Liu, W.; Yang, C.; Yu, L.; Lu, X. Effect of stocking density on growth performance, serum biochemical parameters, and muscle texture properties of genetically improved farm tilapia, Oreochromis niloticus. Aquac. Int. 2018, 26, 1247–1259. [Google Scholar] [CrossRef]
- Dong, Y.; Jia, R.; Hou, Y.; Diao, W.; Li, B.; Zhu, J. Effects of stocking density on the growth performance, mitophagy, endocytosis and metabolism of Cherax quadricarinatus in integrated rice–crayfish farming systems. Front. Physiol. 2022, 13, 2517. [Google Scholar] [CrossRef]
- Onxayvieng, K.; Piria, M.; Fuka, M.M.; Gavrilović, A.; Liang, X.; Liu, L.; Tang, R.; Li, L.; Li, D. High stocking density alters growth performance, blood biochemical profiles, and hepatic antioxidative capacity in gibel carp (Carassius gibelio). Fish Physiol. Biochem. 2021, 47, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Refaey, M.M.; Li, D.; Tian, X.; Zhang, Z.; Zhang, X.; Li, L.; Tang, R. High stocking density alters growth performance, blood biochemistry, intestinal histology, and muscle quality of channel catfish Ictalurus punctatus. Aquaculture 2018, 492, 73–81. [Google Scholar] [CrossRef]
- Qi, C.; Xie, C.; Tang, R.; Qin, X.; Wang, D.; Li, D. Effect of stocking density on growth, physiological responses, and body composition of juvenile blunt snout bream, Megalobrama amblycephala. J. World Aquac. Soc. 2016, 47, 358–368. [Google Scholar] [CrossRef]
- Suárez, M.D.; Trenzado, C.E.; García-Gallego, M.; Furné, M.; García-Mesa, S.; Domezain, A.; Sanz, A. Interaction of dietary energy levels and culture density on growth performance and metabolic and oxidative status of rainbow trout (Oncorhynchus mykiss). Aquac. Eng. 2015, 67, 59–66. [Google Scholar] [CrossRef]
- Yang, Q.; Guo, L.; Liu, B.S.; Guo, H.Y.; Zhu, K.C.; Zhang, N.; Jiang, S.G.; Zhang, D.C. Effects of stocking density on the growth performance, serum biochemistry, muscle composition and HSP70 gene expression of juvenile golden pompano Trachinotus ovatus (Linnaeus, 1758). Aquaculture 2020, 518, 734841. [Google Scholar] [CrossRef]
- Coz-Rakovac, R.; Smuc, T.; Topic Popovic, N.; Strunjak-Perovic, I.; Hacmanjek, M.; Jadan, M. Novel methods for assessing fish blood biochemical data. J. Appl. Ichthyol. 2008, 24, 77–80. [Google Scholar] [CrossRef]
- Bernet, D.; Schmidt, H.; Wahli, T.; Burkhardt-Holm, P. Effluent from a sewage treatment works causes changes in serum chemistry of brown trout (Salmo trutta L.). Ecotoxicol. Environ. Saf. 2001, 48, 140–147. [Google Scholar] [CrossRef]
- Fang, L.S.; Bada, J.L. Biliverdin reductase activity in marine fishes. Mar. Biol. Lett. 1982, 3, 121–130. [Google Scholar]
- Tripathi, N.K.; Latimer, K.S.; Lewis, T.L.; Burnley, V.V. Biochemical reference intervals for koi (Cyprinus carpio). Comp. Clin. Path. 2003, 12, 160–165. [Google Scholar] [CrossRef]
- Ming, J.H.; Ye, J.Y.; Zhang, Y.X.; Xu, P.; Xie, J. Effects of dietary reduced glutathione on growth performance, non-specific immunity, antioxidant capacity and expression levels of IGF-I and HSP70 mRNA of grass carp (Ctenopharyngodon idella). Aquaculture 2015, 438, 39–46. [Google Scholar] [CrossRef]
- de la Rosa, L.C.; Goicoechea, L.; Torres, S.; Garcia-Ruiz, C.; Fernandez-Checa, J.C. Role of Oxidative Stress in Liver Disorders. Livers 2022, 2, 283–314. [Google Scholar] [CrossRef]
- Liang, X.; Luo, X.; Lin, H.; Han, F.; Qin, J.G.; Chen, L.; Xu, C.; Li, E. Effects and Mechanism of Different Phospholipid Diets on Ovary Development in Female Broodstock Pacific White Shrimp, Litopenaeus vannamei. Front. Nutr. 2022, 9, 830934. [Google Scholar] [CrossRef]
- Saurabh, S.; Sahoo, P.K. Lysozyme: An important defence molecule of fish innate immune system. Aquac. Res. 2008, 39, 223–239. [Google Scholar] [CrossRef]
- Costas, B.; Aragao, C.; Dias, J.; Afonso, A.; Conceicao, L.E.C. Interactive effects of a high-quality protein diet and high stocking density on the stress response and some innate immune parameters of Senegalese sole Solea senegalensis. Fish Physiol. Biochem. 2013, 39, 1141–1151. [Google Scholar] [CrossRef]
| Parameters | Before ABZ Challenge | After ABZ Challenge | ||||||
|---|---|---|---|---|---|---|---|---|
| LD | LD-KO | HD | HD-KO | LD | LD-KO | HD | HD-KO | |
| RBC (×106/µL) | 1.24 ± 0.14 a* | 1.31 ± 0.16 a* | 1.14 ± 0.14 a* | 1.28 ± 0.05 a* | 1.40 ± 0.12 a* | 1.67 ± 0.15 a* | 1.36 ± 0.21 a* | 1.41 ± 0.11 a* |
| Hb (g/dL) | 8.42 ± 0.63 a* | 8.83 ± 0.69 a* | 9.71 ± 0.51 a* | 9.34 ± 0.27 a* | 8.44 ± 0.31 c* | 9.48 ± 0.39 d* | 7.09 ± 0.73 a** | 7.28 ± 0.72 b** |
| PCV (%) | 28.22 ± 2.10 a* | 29,13 ± 1.31 a* | 31.59 ± 1.80 a* | 27.93 ± 2.65 a* | 31.30 ± 2.17 c* | 31.80 ± 1.77 c* | 23.00 ± 2.62 a** | 28.87 ± 2.02 b* |
| MCV (fl) | 238.59 ± 36.97 a* | 233.77 ± 34.33 a* | 286.63 ± 12.3 a* | 220.36 ± 14.88 a* | 231.23 ± 12.30 a* | 196.57 ± 9.74 a* | 194.52 ± 16.87 a** | 213.77 ± 11.01 a* |
| MCH (pg) | 71.71 ± 11.45 a* | 70.68 ± 10.97 a* | 88.01 ± 10.31 a* | 73.46 ± 4.46 a* | 62.40 ± 6.37 a* | 58.68 ± 6.07 a* | 56.48 ± 9.06 a** | 53.01 ± 7.38 a* |
| MCHC (g/dL) | 30.02 ± 2.05 a* | 30.21 ± 1.21 a* | 30.93 ± 2.02 a* | 34.42 ± 3.44 a* | 27.33 ± 1.57 a* | 30.35 ± 2.52 a* | 30.55 ± 5.63 a* | 25.56 ± 2.71 a** |
| Parameters | Experimental Groups | Interaction | ||||
|---|---|---|---|---|---|---|
| LD | LD-KO | HD | HD-KO | Feed × Density | ||
| Before ABZ challenge | ||||||
| ALB (g/dl) | 1.09 ± 0.03 a* | 1.00 ± 0.05 a* | 1.13 ± 0.10 a* | 1.03 ± 0.07 a* | p = 0.084 | |
| GLOB(g/dl) | 2.76 ± 0.25 c* | 2.96 ± 0.10 c* | 2.33 ± 0.06 a* | 2.56 ± 0.15 b* | p = 0.034 | |
| A/G | 0.39 ± 0.03 b* | 0.33 ± 0.02 a* | 0.48 ± 0.03 c* | 0.40 ± 0.01 b* | p = 0.020 | |
| TP (g/dl) | 3.83 ± 0.18 b* | 3.96 ± 0.25 b* | 3.36 ± 0.05 a* | 3.59 ± 0.10 b* | p = 0.235 | |
| GLU (mg/dl) | 48.33 ± 3.79 a* | 43.67 ± 2.42 a* | 68.67 ± 3.21 c* | 51.50 ± 5.03 b* | p = 0.967 | |
| ALT (U/L) | 81.33 ± 3.59 a* | 54.33 ± 3.46 a* | 110.00 ± 4.94 c* | 96.00 ± 3.58 b* | p = 0.560 | |
| AST (U/L) | 120.67 ± 8.36 a* | 106.67 ± 5.16 a* | 189.67 ± 4.98 c* | 150.33 ± 6.53 b* | p = 0.872 | |
| ALP (U/l) | 129.20 ± 4.44 a* | 103.60 ± 4.50 a* | 135.33 ± 4.31 a* | 113.50 ± 6.79 a* | p = 0.403 | |
| GGT (U/L) | 0.50 ± 0.71 a* | 0.50 ± 0.71 a* | 3.00 ± 0.83 c* | 1.33 ± 1.15 b* | p = 0.457 | |
| BIL D(mg/dl) | 0.20 ± 0.07 a* | 0.22 ± 0.08 a* | 0.20 ± 0.05 a* | 0.18 ± 0.04 a* | p = 0.213 | |
| BIL T (mg/dl) | 0.29 ± 0.10 a* | 0.25 ± 0.11 a* | 0.32 ± 0.09 a* | 0.27 ± 0.06 a* | p = 0.187 | |
| HDL (mg/dl) | 79.73 ± 6.85 a* | 90.07 ± 6.38 b* | 74.63 ± 5.03 a* | 87.73 ± 8.24 b* | p = 0.578 | |
| LDL (mg/L) | 27.67 ± 6.00 a* | 26.33 ± 2.65 a* | 25.67 ± 4.85 a* | 27.67 ± 12.22 a* | p = 0.858 | |
| CHOL (mg/dl) | 211.67 ± 15.57 a* | 204.67 ± 8.50 a* | 203.50 ± 5.69 a* | 181.33 ± 8.02 a* | p = 0.961 | |
| TG (mg/dl) | 449.33 ± 9.29 a* | 435.00 ± 33.51 a* | 440.00 ± 4.43 a* | 421.33 ± 27.74 a* | p = 0.642 | |
| TL (mg/dl) | 1026.33 ± 63.01 a* | 983.00 ± 74.64 a* | 999.00 ± 9.81 a* | 931.33 ± 43.25 a* | p = 0.734 | |
| After ABZ challenge | ||||||
| ALB (g/dl) | 1.28 ± 0.06 b** | 1.18 ± 0.06 a* | 1.42 ± 0.05 c** | 1.21 ± 0.03 a** | p = 0.044 | |
| GLOB (g/dl) | 3.02 ± 0.04 b** | 3.22 ± 0.22 c** | 2.75 ± 0.18 a** | 3.11 ± 0.13 b** | p = 0.026 | |
| A/G | 0.40 ± 0.02 a* | 0.36 ± 0.03 a* | 0.51 ± 0.01 b** | 0.38 ± 0.01 a* | p = 0.312 | |
| TP (g/dl) | 4.30 ± 0.15 b** | 4.40 ± 0.22 c** | 4.17 ± 0.32 a** | 4.32 ± 0.15 b** | p = 0.031 | |
| GLU (mg/dl) | 86.80 ± 4.95 b** | 75.20 ± 3.11 a** | 97.60 ± 3.80 c** | 85.60 ± 4.73 b** | p = 0.751 | |
| ALT (U/L) | 92.40 ± 2.40 b** | 65.00 ± 2.47 a** | 148.50 ± 1.44 d** | 122.00 ± 2.99 c** | p = 0.011 | |
| AST (U/L) | 233.50 ± 10.32 b** | 197.75 ± 6.79 a** | 367.25 ± 7.25 d** | 281.00 ± 7.06 c** | p = 0.012 | |
| ALP (U/l) | 53.20 ± 0.73 a* | 69.60 ± 4.33 a** | 74.80 ± 2.19 a* | 75.53 ± 3.95 a** | p = 0.494 | |
| GGT (U/L) | 2.40 ± 2.30 a** | 2.50 ± 1.29 a** | 4.00 ± 1.00 b** | 2.40 ± 1.14 a** | p = 0.148 | |
| BIL D(mg/dl) | 0.11 ± 0.04 a** | 0.11 ± 0.05 a** | 0.13 ± 0.04 a** | 0.12 ± 0.03 a** | p = 0.830 | |
| BIL T (mg/dl) | 0.19 ± 0.06 a** | 0.19 ± 0.09 a** | 0.21 ± 0.06 b** | 0.24 ± 0.05 c** | p = 0.860 | |
| HDL (mg/dl) | 75.40 ± 3.15 a** | 82.10 ± 6.50 a** | 70.16 ± 7.45 a** | 80.00 ± 6.08 a** | p = 0.571 | |
| LDL (mg/L) | 89.00 ± 3.76 a** | 81.60 ± 5.66 a** | 118.40 ± 9.07 c** | 95.20 ± 2.52 b** | p = 0.459 | |
| CHOL (mg/dl) | 228.40 ± 9.58 a* | 214.80 ± 7.29 a* | 247.60 ± 6.89 b** | 229.00 ± 4.16 a** | p = 0.771 | |
| TG (mg/dl) | 263.80 ± 8.94 a** | 266.60 ± 7.04 a** | 334.40 ± 6.89 b** | 285.80 ± 9.05 a** | p = 0.252 | |
| TL (mg/dl) | 899.00 ± 10.23 a** | 959.40 ± 7.87 b** | 871.60 ± 12.46 a** | 903.00 ± 15.23 a** | p = 0.268 | |
| Parameters | Before ABZ Challenge | After ABZ Challenge | ||||||
|---|---|---|---|---|---|---|---|---|
| LD | LD-KO | HD | HD-KO | LD | LD-KO | HD | HD-KO | |
| MDA (nmol/mL) | 2.10 ± 0.09 b* | 1.67 ± 0.13a* | 2.67 ± 0.16 c* | 2.00 ± 0.15 b* | 2.44 ± 0.11 b** | 1.88 ± 0.17 a** | 2.98 ± 0.15 c** | 2.28 ± 0.13 b** |
| MDA (nmol/g liver) | 8.12 ± 0.17 b* | 5.49 ± 0.10 a* | 8.47 ± 0.21 c* | 8.12 ± 0.22 b* | 10.77 ± 0.19 c** | 6.61 ± 0.19 a** | 11.84 ± 0.18 c** | 9.12 ± 0.14 b** |
| TAC (mM Trolox) | 21.78 ± 0.25 b* | 24.64 ± 0.29 c* | 19.31 ± 0.26 a* | 21.48 ± 0.31 b* | 18.97 ± 0.22 a** | 20.95 ± 0.24 a** | 14.37 ± 0.21 b** | 19.21 ± 0.23 a** |
| LZM (U/mL) | 7.70 ± 0.29 b* | 9.10 ± 0.12 c* | 7.33 ± 0.14 a* | 9.03 ± 0.11 c* | 9.18 ± 0.18 b** | 9.33 ± 0.16 c* | 8.54 ± 0.11 a** | 9.58 ± 0.14 d** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Năstac, L.; Dediu, L.; Crețu, M.; Rîmniceanu, C.; Docan, A.; Grecu, I.; Dima, F.M.; Stroe, M.D.; Vizireanu, C. The Protective Effects of Korill Product on Carp Fingerlings Reared in High Densities and Challenged with Albendazole Treatment. Fishes 2023, 8, 153. https://doi.org/10.3390/fishes8030153
Năstac L, Dediu L, Crețu M, Rîmniceanu C, Docan A, Grecu I, Dima FM, Stroe MD, Vizireanu C. The Protective Effects of Korill Product on Carp Fingerlings Reared in High Densities and Challenged with Albendazole Treatment. Fishes. 2023; 8(3):153. https://doi.org/10.3390/fishes8030153
Chicago/Turabian StyleNăstac, Lacrămioara (Grădinariu), Lorena Dediu, Mirela Crețu, Cristian Rîmniceanu, Angelica Docan, Iulia Grecu, Floricel Maricel Dima, Maria Desimira Stroe, and Camelia Vizireanu. 2023. "The Protective Effects of Korill Product on Carp Fingerlings Reared in High Densities and Challenged with Albendazole Treatment" Fishes 8, no. 3: 153. https://doi.org/10.3390/fishes8030153
APA StyleNăstac, L., Dediu, L., Crețu, M., Rîmniceanu, C., Docan, A., Grecu, I., Dima, F. M., Stroe, M. D., & Vizireanu, C. (2023). The Protective Effects of Korill Product on Carp Fingerlings Reared in High Densities and Challenged with Albendazole Treatment. Fishes, 8(3), 153. https://doi.org/10.3390/fishes8030153