1. Introduction
Pabda (
O. pabda) is a strong candidate as a commercially important catfish species in the aquaculture industry of Bangladesh. The fast growth rate and high market demand are triggering Pabda catfish culture to gain rapid popularity in Bangladesh as well as many parts of South Asia. The species is thought to be the most delicious to eat due to its fine flesh with a soft meat texture. Alam et al. [
1] stated that Pabda flesh is a great source of amino acids, fatty acids, minerals, and vitamins. However, Pabda farming in captivity is sometimes problematic due to its cannibalistic nature [
2] and thus may require necessary approaches to overcome this problem, such as the maintenance of species homogeneity, optimum feed, and precise feeding intervals. Additionally, poor growth and survival rates, disease epidemics, high prices as well as a lack of good-quality feeds pose significant barriers to the sustainable production of Pabda. As a result, the search for alternative bioactive compounds and immunostimulants can greatly improve the growth performance and immune status of fish and also minimize the feed cost by replacing a highly valued fish meal in diets [
3,
4]. Siddik et al. [
5] reported that the supplementation of fish protein hydrolysate (FPH) in diets significantly boosts the productivity and defense mechanism of fish and also offers less susceptibility to disease occurrence.
It has been reported that the seafood processing industries generate a considerable amount of waste products annually [
6,
7,
8]. The conversion of these by-products into protein-based aqua-feed ingredients through enzymatic processes can potentially reduce the cost of waste disposal and also offer a strategy for waste management in the fish processing industry sector. Zamora-Sillero et al. [
9] documented that the enzymatic hydrolysis of such by-products produces very high-quality fish protein hydrolysate while preserving its nutritional value. Fish protein hydrolysate contains a very rich amount of soluble protein, making it more digestible and absorbable in comparison with whole protein [
10,
11]. According to previous findings, it has been proven that FPH promotes the nutrient intake and biological activity of fish by providing free amino acids, di-tri peptides, and medium-sized peptides [
12,
13]. In addition, the peptides found in FPH had antioxidant, antimicrobial, antihypertensive, immunomodulatory, and anticancer properties [
14,
15].
Numerous studies have evaluated the effects of low doses of FPH inclusion in diets on the growth performance, feed efficiency, immune mechanism, and specific disease resistance in various commercially important fish species such as
Paralichthys olivaceus [
16],
Lates calcarifer [
5,
17,
18],
Pagrus major [
19],
Acipenser persicus L. [
20],
Dicentrarchus labrax [
21],
Oncorhynchus mykiss [
22], and
Anguilla japonica [
23]. In contrast, the very high inclusion of dietary FPH in diets has detrimental effects on the growth performance of fish [
24,
25]. It is worth noting that the enzymatic process of protein hydrolysis produces some peptides and amino acids, causing a bitter taste to develop in fish diets [
26]. The elimination of hydrophobic peptides [
27,
28] and the selection of appropriate enzymes are possible solutions for the debittering of FPH [
29]. At present, FPH is effectively used in the diets of many fish, but there is little information available on the use of FPH in Pabda catfish diets. In an effort to diversify the application of FPH in the aquaculture industry sector, in this study, a method was designed to identify the suitable level of dietary FPH to maximize the growth performance, health status, and disease resistance for the feed formulation of Pabda (
O. pabda) catfish production.
2. Materials and Methods
2.1. Diet Formulation and Proximate Composition Analyses
The experimental feed ingredients and the proximate composition are presented in
Table 1. Except FPH, all the ingredients were bought from Bandar Bazar, Sylhet, Bangladesh. The commercially available liquid FPH was provided by Symrise Aqua Feed, Specialities Pet Food (226-FR-SPF), France. Four experimental diets (35% crude protein) were prepared with different FPH inclusion levels at 0% (control diet), 0.5%, 1%, and 2%. Briefly, all the ingredients were finely ground and mixed homogenously with FPH for 45 min and then extruded to make pellets 2 mm in size. The pelleted diets were oven-dried at 60 °C for 8 h, packed in pre-labeled airtight bags, and stored in a cool and dry place until the feeding trial commenced. Proximate composition analyses of test diets were performed according to AOAC [
30] with three replicates each.
2.2. Experimental Fish Collection and Husbandry Method
A total of 2000 Pabda catfish fry (mean weight: 2.00 ± 0.09 g) were purchased from a commercial fish farm. The fish were acclimatized for two weeks in a large hapa (8 feet × 6 feet × 4 feet, length × width × depth) and fed a basal diet (ACI Godrej Agrovet Private Limited, Bangladesh) containing 35% crude protein and 8% crude lipid. When the experimental feeding trial began, 1200 fish were individually weighed and distributed at random into 12 cages (1 m × 1 m × 1.5 m, length × width × depth) with 100 fish in each cage. All the cages were set up in the same pond to conduct the experiment. The experimental design was fully randomized, which consisted of 4 treatments with 3 replicates each. The feeding trial was conducted for 90 days, and the fish were fed twice daily at 9.15 a.m. and 5.15 p.m. until apparent satiation.
2.3. Physical Characteristics and Palatability Observation of Experimental Diets
2.3.1. Physical Parameters of Experimental Diets
The physiological properties of triplicate diets were determined according to the standard method and formulae described previously by Zulhisyam et al. [
31] with few modifications.
Expansion ratio (%) = 100 × (Mean of FPH treated pellet diameter—Mean of original commercial feed diameter)/Mean of original commercial feed diameter;
Bulk density (kg/m3) = Weight of feeds in measuring cylinder (kg)/Total volume occupy (m3);
PDI (%) = 100 × (Weight of feeds remaining on the sieve after tumbling over the period/Initial total weight of feeds);
Water stability (%) = 100 × (Weight of retained whole pellets/Initial weight of pelleted diet);
Floatability (%) = 100 × (Mean value of floating feed in the beaker after the given time/Mean initial numbers of feed).
2.3.2. Palatability Test of FPH-Treated Diets
The palatability of FPH diets was determined according to Zulhisyam et al.’s [
31] method with minor alterations. Briefly, observations on the palatability of the experimental diets were visually made by offering the feed sample on the surface of the water once, as far from the cage containing the experimental fish as possible. As soon as the pellets of FPH diets hit the water, the fish began to swim towards the diets. It was noticed that the response of the fish to the test diets was associated with a particular estimated product that the fish had a well-linked preference for. The experiment was conducted 3 times.
2.4. Analysis of Hydro-Ecological Variables
The cage water hydrological variables such as temperature, pressure, conductivity, TDS, salinity, and DO contents were monitored at 1-week intervals over the experimental period by employing a YSI Multiparameter probe (HI 9828, YSI Incorporation, Yellow Spring, OH, USA). Every week, the ammonia, nitrite, and nitrate levels in the holding cage water were examined with HACH test kits (HI 28049, HACH, Loveland, OH, USA).
2.5. Evaluation of Growth Performance
At the termination of the experimental feeding trial, all the fish from each replica cage were caught and immediately moved to the laboratory. After anesthesia with MS222, the total biomass of the fish was calculated. From this, about 30 fish per replication were randomly taken to measure the individual fish’s body weight and total length. The target inner organs of each fish were carefully removed after dissection, and the following formulae were used to determine the Pabdas’ growth performance:
Survival rate (%) = 100 × Number of fish survived/Total number of fish at the start of the experiment;
Weight gain (%) = 100 × (Final weight—Initial weight)/Initial weight;
Specific growth rate (%/day) = 100 × (ln (final weight)—ln (initial weight))/Days of experiment;
Total biomass gain (kg) = (Final biomass weight—Initial biomass weight);
Total yield (kg/m2) = Total biomass gain/Cage area;
Feed conversion ratio, FCR = Total feed intake/Live weight gain;
Feed conversion efficiency, FCE = Live weight gain/Total feed intake;
Protein efficiency ratio, PER = Live weight gain/Crude protein intake;
Hepatosomatic index, HSI = 100 × Liver weight/Final weight;
Visceral somatic index, VSI = 100 × Viscera weight/Final weight;
Intraperitoneal fat, IPF = 100 × Fat weight/Final weight;
Condition factor (CF) = 100 × Weight of fish body (g)/(Total length of fish, cm)3.
2.6. Biochemical Composition Analysis
The proximate composition of experimental diets, liver, intestine, and muscle was assessed according to AOAC [
30], and each sample was analyzed in triplicate. Briefly, 3 fish from each replica cage were randomly taken, and tissue samples were collected and then frozen at −20 °C until subsequent analysis. The crude protein (% N × 6.25) was estimated with the Kjeldahl method; the crude lipid was measured by n-hexane extraction employing the Soxhlet apparatus; ash content was evaluated using a Muffle furnace at 550 °C for 6 h; and moisture content was calculated by oven-drying the samples at 105 °C for 24 h.
2.7. Estimation of Total Bacteria in Diets and Fish Gut
The total bacteria in fish diets and intestines were estimated according to the modification of Nandi et al.’s [
32] method. In short, about 1 g of each sample was homogenized with 9 mL of sterile solution, and the suspension was then diluted serially to 10
−8. After that, 10 µL of each test solution was spread onto Tryptic Soy Agar (TSA, HiMedia, Mumbai, India) in triplicate plates to enumerate the total bacterial load. The plates were then incubated at 37 °C for 36 h, and the number of colonies was quantified.
2.8. Assessment of Hematological and Biochemical Indices
The blood hematological and biochemical assays were performed according to some modifications of the methods by Abdul Kari et al. [
33] and Nandi et al. [
32]. After 90 days of feeding, 12 fish from each treatment were sampled and anesthetized using MS
222 at a dose of 0.1 g/L water. A volume of 150 μL of blood from each treatment was collected from the caudal puncture of fish by function with a 1 mm heparinized syringe and then kept separately in EDTA K
3 tubes. An automated hematology analyzer (Mythic 18 Vet, Corway diagnostics, USA) was employed to measure the hematological indices. Additionally, the plasma was separated by centrifuging the remaining blood sample at 3000 rpm for 15 min and then preserved at −20 °C until analysis. The biochemical parameters were determined by drawing plasma, 150 μL per treatment, onto pipette tips and dropping it into the cassettes, which contained the reagents for the measurement of each parameter (brand name: IDEXX, USA). A VetTest chemistry analyzer (Brand name: IDEXX, USA) was used to determine the biochemical parameters, except globulin. Globulin was calculated by subtracting the albumin content from the total protein in plasma.
2.9. Histopathology of Mid Gut and Liver
For the histopathological observation of fish tissues, a total of 4 fish per replication were taken at random and anesthetized with MS222. The fish were cut ventrally to take out the mid gut and liver portions and then fixed in a 10% neutral buffered formalin solution. After that, the collected samples were dehydrated in graded ethanol before being equilibrated in xylene, embedded in paraffin, sectioned into 5 to 8 μm slices, mounted on glass slides and stained in Hematoxylin and Eosin solution for histological examination under a light microscope (Leica DMIL-LED, Germany). Microphotographs of all fish intestine and liver sections were taken by employing an image capture analysis system (V1.18 64bit, Cellsens software, The Netherlands).
2.10. Experimental Infection with Aeromonas hydrophila
2.10.1. Collection and Maintenance of A. hydrophila
A. hydrophila (strain name: BDAH01, procured from Laboratory of Fish Disease Diagnosis and Pharmacology, Dept. of Fish Health Management, Sylhet Agricultural University, Bangladesh) was isolated from diseased catfish,
Clarias batrachus [
34], later confirmed by PCR and gene sequence (GB Accession number MZ046725.1). Subcultures were maintained on nutrient agar slants at 4°C. Bacteria were inoculated in tryptone soya broth (Himedia). The broth was incubated overnight in a shaker for 12 h at 37 °C and harvested at 0.8 optical density (OD) at 600 nm, and stock culture was preserved in 1.5% TSB with 20% glycerol at −20 °C until use.
2.10.2. LD50 of Aeromonas hydrophila
The mean lethal dose (LD
50) was determined in
O. pabda (15 ± 5.50 g) according to Reed and Muench [
35]. The Pabda fingerlings were maintained in a rectangular glass aquaria (73 × 35 × 38 cm) containing 70 L of water (10 fish/tank) with aeration. Waste was siphoned from the bottom of the tanks once in two days; subsequently, 20% of the water was replaced. Water quality parameters were measured using YSI professional multiparameter and were found to be suitable for fish growth (the temperature ranged from 28.20 to 29.10 °C, the pH ranged from 7.20 to 7.90, ammonia and nitrogen were less than 0.03 mg/L, and the DO ranged from 6.20 to 6.90 mg/L). The experiment was carried out in duplicate. The isolate was grown overnight on 1.5% tryptic soya broth (TSB, Himedia, India) medium at 37°C, and cell suspensions were prepared by centrifugation at 10,000 rpm for 5 min with phosphate-buffered saline (PBS). Later, serial 10-fold dilution was maintained in order to obtain 10
9 to 10
2 CFU/mL. Each fish was injected intraperitoneally with 0.1 mL of
A. hydrophila inoculate ranging from 10
2 to 10
9 CFU/mL. A control fish group was injected with 0.1 mL of phosphate-buffered saline (PBS). Mortality was recorded daily for ten days.
2.10.3. Challenge Study
At the end of the feeding trial, 30 fish from each treatment group, including the positive control, were injected with 0.1 mL of
A. hydrophila (LD
50–2.3 × 10
7 CFU/mL) and fed with a basal diet. Fishes injected with 0.1 mL of phosphate-buffered saline (PBS) served as a negative control. The survival rate was recorded daily for ten days. Fresh blood from moribund fish was collected, and streaking was carried out from kidneys on RS (Rimlar Shots) media (Specific for
A. hydrophila isolation) for the confirmation of fish death. The cumulative percent mortality of fish was calculated according to Rathore et al. [
36].
2.11. Economic Efficiency
Feed component, as an input, is usually the main cost in a farm, especially when investments in rearing facilities are moderate, as this is the case for Pabda farming in Bangladesh. Feed raw material costs were calculated by summing the costs of the different raw materials and ingredients making each experimental diet. Farm feed costs were then calculated per unit of produced biomass as follows:
FFC (USD/kg) = FCR × diet raw material cost.
Farm revenues were calculated on an expected farm gate price of 3.15 USD/kg of Pabda: FR (USD/m2): Total Yield × 3.15.
Farm raw margins were calculated as follows:
FRM (USD/m2): FR—Total Yield × FFC.
Return on Investment (ROI) was calculated as follows:
ROI (%) = 100 × FPRM/(Total Yield × FFC).
2.12. Statistical Analysis
All statistical data were analyzed using IBM SPSS software 20.1 for Windows. One-way analysis of variance (ANOVA) was performed to estimate the average of each variable, with the significant differences (p < 0.05) detected by using Duncan’s multiple range test. The results are presented as mean ± standard deviation (SD).
4. Discussion
Fish protein hydrolysate is one of the most promising feed ingredients in the aquaculture business sector due to its great nutritional profile, its status as a bioactive peptide, and its free amino acids. However, the identification of the optimum levels of FPH in diets is a critical issue that promotes the growth performance and health of fish. Therefore, this study examined the physical, biochemical, and bacteriological properties of FPH diets, intestinal microbiota, hematological and serum biochemical indices, liver and gut histopathology, and the disease resistance of O. pabda, aiming to provide more reliable data to identify the suitable FPH levels for practical application in the field of aquaculture.
The measurement of the physical properties of a diet is one of the prerequisites required to develop and commercialize any aqua-feed. Khater et al. [
37] stated that feed pellets with good physiological characteristics reduce dust production during transportation, handling, and pneumatic conveyance. The present results revealed that except PDI, other physical parameters of feeds were not influenced by the various levels of FPH. These outcomes might be due to the identical pellet sizes. However, the significantly lowest PDI in the 2% diet indicated that this FPH level improves the binding capacity of the diet and minimizes the dust and debris production during transportation. Previous reports noted that the feed expansion ratio, bulk density, and water stability tended to increase with increasing feed diameter [
31,
37,
38,
39], whereas PDI and floatability had an opposite relationship [
31,
37]. Additionally, fish preferred the 1 and 2% FPH diets as compared to the 0 and 0.5% diets due to their greater palatability. This result specified that the feed palatability was highly affected by the dietary FPH inclusion levels in diets. Similar outcomes were found by many previous authors [
40,
41,
42]. Small peptides and free amino acids in FPH make the feed more palatable and acceptable.
Successful aquaculture production greatly relies on the hydrological variables of water. In this research, water quality indices, including water pressure and DO content, significantly differed among the test groups but remained in the fishes’ comfort zone. However, other hydrological parameters were analogous among all treatments over the study period. As this experiment took place in the same pond, water variables did not vary to a great extent. In addition, the water quality standards recorded in this study met the optimum requirements for Pabda aquaculture [
43,
44,
45,
46].
From the current research, it was shown that the fish growth and feed efficiency indices were greatly affected by different levels of FPH. The results proved that increasing the levels of FPH from 0 to 2% in diets significantly enhanced the final weight, percent weight gain, SGR, total biomass, and condition factor and also improved the FCR, FCE, and PER values. Based on these parameters, it is summarized that the fish fed diets containing 2% FPH are suited better for outstanding growth and feed utilization. These results are in correspondence with many previous findings [
5,
17,
19,
20,
47,
48,
49,
50,
51,
52,
53]. As a result of protein hydrolysis, the large-sized polypeptide chain breaks down into smaller peptides and free amino acids that are readily digested and absorbed by fish, leading to an improvement in fish growth and feed intake. Many studies have suggested that the presence of biologically active peptides appears to increase the functional properties of FPH [
20,
54,
55]. Chotikachinda et al. [
40] also reported that protein hydrolysis produces peptides with small molecular weights that can serve as attractants for fish. Furthermore, the highest survival rate was recorded in 1%- and 2%-FPH-fed fish compared to other FPH diets in this study. Numerous scientists have claimed that FPH supplementation in diets also strengthens fishes’ defensive mechanisms [
5,
19,
52]. These could be the possible reasons why the survival of 1%- and 2%-FPH fed-fish was boosted. In general, HSI denotes fat and glycogen deposition in the liver [
32], whilst VSI reflects the meat content of fish [
33]. This is why the highest HSI and the lowest VSI in a 2% FPH diet indicate those fish livers and bodies contained greater fat and meat content, respectively. Contrary to our findings, numerous studies found no discernible differences in these parameters when fish were fed various FPH proportions [
52,
56,
57]. The IPF was significantly higher in the control fish even though no evident difference was identified in the IPF of 1% and 2% diets, showing that those fish had more fat deposited in their body than those fed a 0.5% FPH diet.
Fish tissue’s biochemical composition showed remarkable variation when the fish were fed with graded levels of FPH, except for some cases. The present outcomes also revealed that the fish muscle contained richer amounts of protein than the intestine and liver, which corroborate with the results obtained by previous researchers [
33,
58,
59]. Upon reaching maturity, the protein is mobilized to the muscle from the liver and intestine. Therefore, the protein deposition in the muscle becomes higher as compared to other tissues. Additionally, the protein percentage of each tissue followed a rising trend with increasing FPH levels and reached its highest levels at up to 2%. The lipid was deposited more in the liver, while muscle had a comparatively lower lipid level. Abdul Kari et al. [
33] reported a similar result in African catfish. Wee and Tacon [
60] stated that muscle with higher protein and lower fat is considered a food of excellent quality. Overall, the results indicated that graded FPH supplementation levels might have significant positive impacts on increasing and decreasing protein and lipid levels.
The bacteriological outcomes of this report demonstrated that FPH had a strong positive impact on raising the bacterial population in fish diets and guts. The dietary incorporation of FPH at levels from 0 to 2% increased the total bacterial counts in diets. These findings are in agreement with those obtained by Zulhisyam et al. [
31] and Nandi et al. [
32], who found similar results when fish feeds were formulated with microbial fermentations of plant-based ingredients. A similar increase in FPH in diets is also associated with an increase in fish guts’ total bacteria. Ebrahimnezhadarabi et al. [
61] reported parallel results while using canola protein hydrolysate at varying percentages in Beluga,
Huso huso diets. Correspondingly, the dietary administration of fermented plant protein at approximately 50% significantly improved gut microbiota in African catfish [
33,
62] and Stinging catfish [
32]. Kotzamanis et al. [
63] documented that FPH acts as a culture media for bacterial proliferation, and, therefore, it influences the bacterial load. A higher bacterial load in a 2%-FPH-fed fish gut might imply the improved growth, intestinal health, nutrient digestion, and disease resistance of fish against pathogenic bacteria.
This study’s findings demonstrated that dietary FPH supplementation levels had a considerable impact on red blood cell (RBC) but little impact on white blood cell (WBC) counts. Ebrahimnezhadarabi et al. [
61] cited that incorporating different amounts of canola protein hydrolysate in fish diets contributed to the increase and decrease in RBC and WBC numbers, respectively. Abdul Kari et al. [
33] stated that WBCs and RBCs are two major components of the blood, and their increased levels are associated with an improved health condition of fish. Additionally, higher neutrophil, lymphocyte, and monocyte levels in 2% FPH fish showed improved immune systems. Hemoglobin (HGB), mean corpuscular hemoglobin concentration (MCHC), and platelet distribution width (PDW) were greatly influenced by different FPH levels. These outcomes are corroborated by the study of Abdul Kari et al. [
33], Nandi et al. [
32], and Zakaria et al. [
64]. Acar and Türker [
65] also found similar alterations in HGB and MCHC values when peanut meal diets were used in rainbow trout aquaculture. Various levels of FPH have been shown to affect hematocrit (HCT) levels. Ribeiro et al. [
66] published results of a similar effect. Conversely, when fish were fed tuna hydrolysate in diets at different percentages, this had no effect on hemoglobin and hematocrit levels [
57]. The mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH) concentrations in the current study were unaffected by different FPH supplementation dosages.
Arapaima gigas fry fed with various FPH-treated diets showed identical results [
66]. Additionally, the procalcitonin (PCT) content significantly differed among tested fish. However, Abdul Kari et al. [
33] and Nandi et al. [
32] reported opposite results. In general, the overall variation in the blood hematological parameters of this catfish species reflects the changes in physiological and health conditions.
This study showed that increasing the FPH levels in diets resulted in a considerable rise in blood sugar and total protein. Recent studies also reported a similar increasing trend of glucose when the fish fed with fermented soy pulp [
33] and fermented water spinach meal-based diets [
32]. High blood glucose indicates environmental stressors in fish, while high content of total protein denotes a superior innate immune system [
67]. When the fish are properly nourished, their plasma total protein levels tend to be more consistent [
68]. Moreover, the creatinine, urea, and cholesterol levels in plasma varied widely among the treatments, although no obvious trend was followed. Nandi et al. [
32] found corresponding results in Stinging catfish. In contrast, Chaklader et al. [
18,
52] observed that varying degrees of tuna hydrolysate had no effects on these parameters. In parallel to our study, the fish fed the control diet had lower bilirubin in plasma [
18,
52]. Bilirubin is an important biomarker that reflects kidney disorders. That is why the lowest bilirubin in 2% FPH fish indicates better kidney health. Serum glutamic pyruvic transaminase, serum glutamic oxaloacetic transaminase, alkaline phosphatase, and globulin content are also indicators of health, and their remarkable variations in this study also indicate significant alterations in the health state of fish.
The liver and intestine are two important accessory body organs involved in nutrient digestion and absorption [
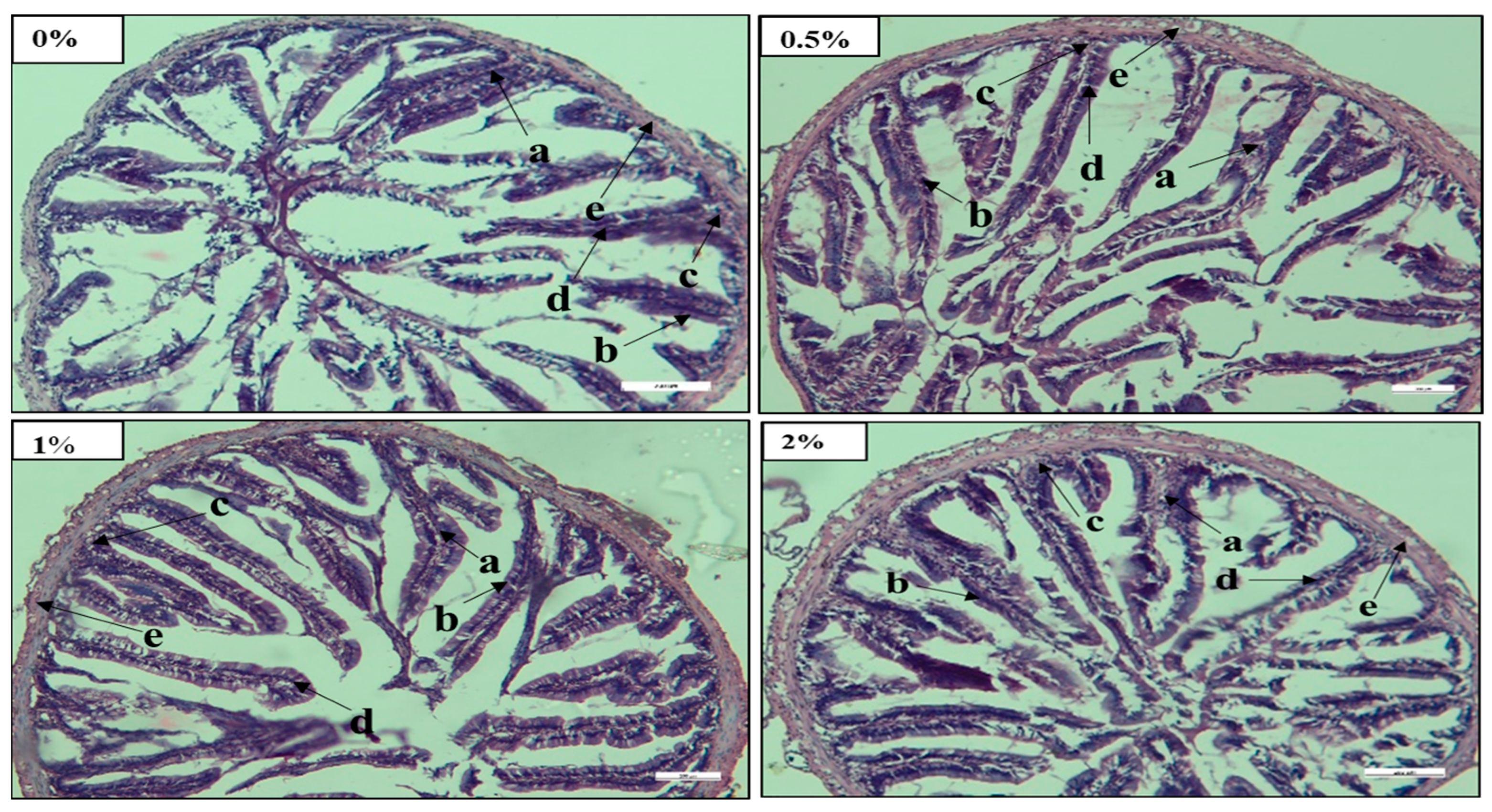
69]. In this study, the mid intestine of fish fed with 2% FPH had a well-anchored epithelial wall with well-arranged goblet cells, a large villi structure, and tunica muscularis compared to those fed other FPH diets (
Figure 1). This result indicated that fish fed a 2% FPH diet showed better nutrient absorption capacity and improved gut health compared to all other fish groups. Similar outcomes were investigated when various levels of FPH were incorporated into fish diets [
5,
17,
52,
70]. Siddik et al. [
17] noted that the inclusion of up to 75% poultry by-product meals in diets as an alternative to FM greatly increased intestinal microvilli density. Another study discovered that when gilthead sea bream were fed protein hydrolysate or autolyzed yeast instead of FM, they had considerably more goblet cells in their gut [
49]. Similar histological changes were demonstrated in the gut of striped catfish [
58] and female Stinging catfish [
32].
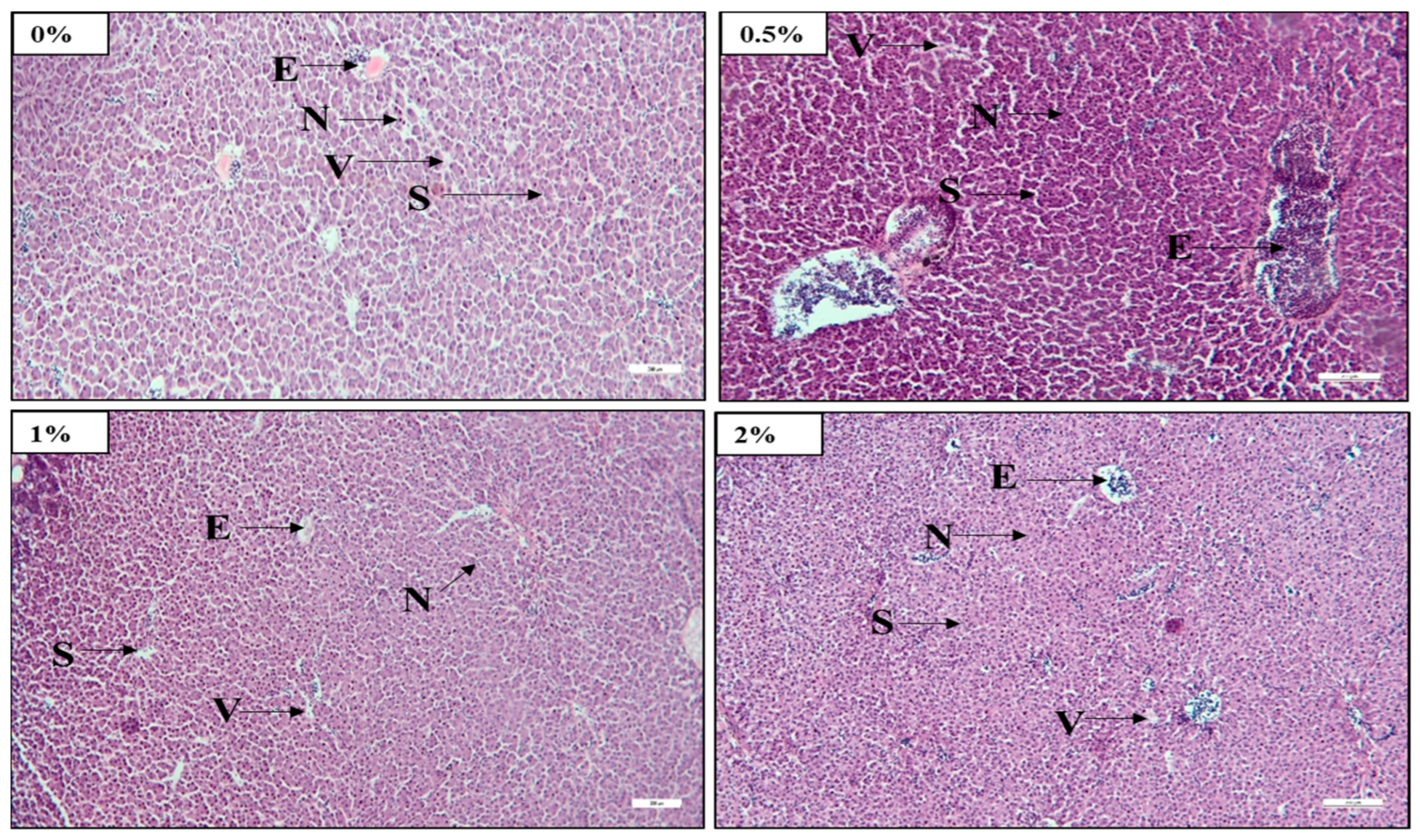
The liver is an excellent indicator of a fish’s nutritional status [
71]. The light microscopic observation of histological slides showed that raising the FPH levels in test diets resulted in a notable increase in nuclei and erythrocyte numbers and decreased vacuolar cytoplasm in the liver. This result specified that 2%-FPH-fed fish groups had improved liver health compared to other treatment fish. When FM was substituted with fermented soy pulp at levels of 50% in the diet of African catfish, a similar observation was recorded by Abdul Kari et al. [
33]. Pham et al. [
72] similarly observed a commensurate reduction in the vacuole numbers when using tuna hydrolysate at 60 g/kg in a poultry by-product meal diet. However, another finding has shown that a very high amount of FPH increased liver tissue necrosis, fat deposition, and vacuolar cytoplasm [
57].
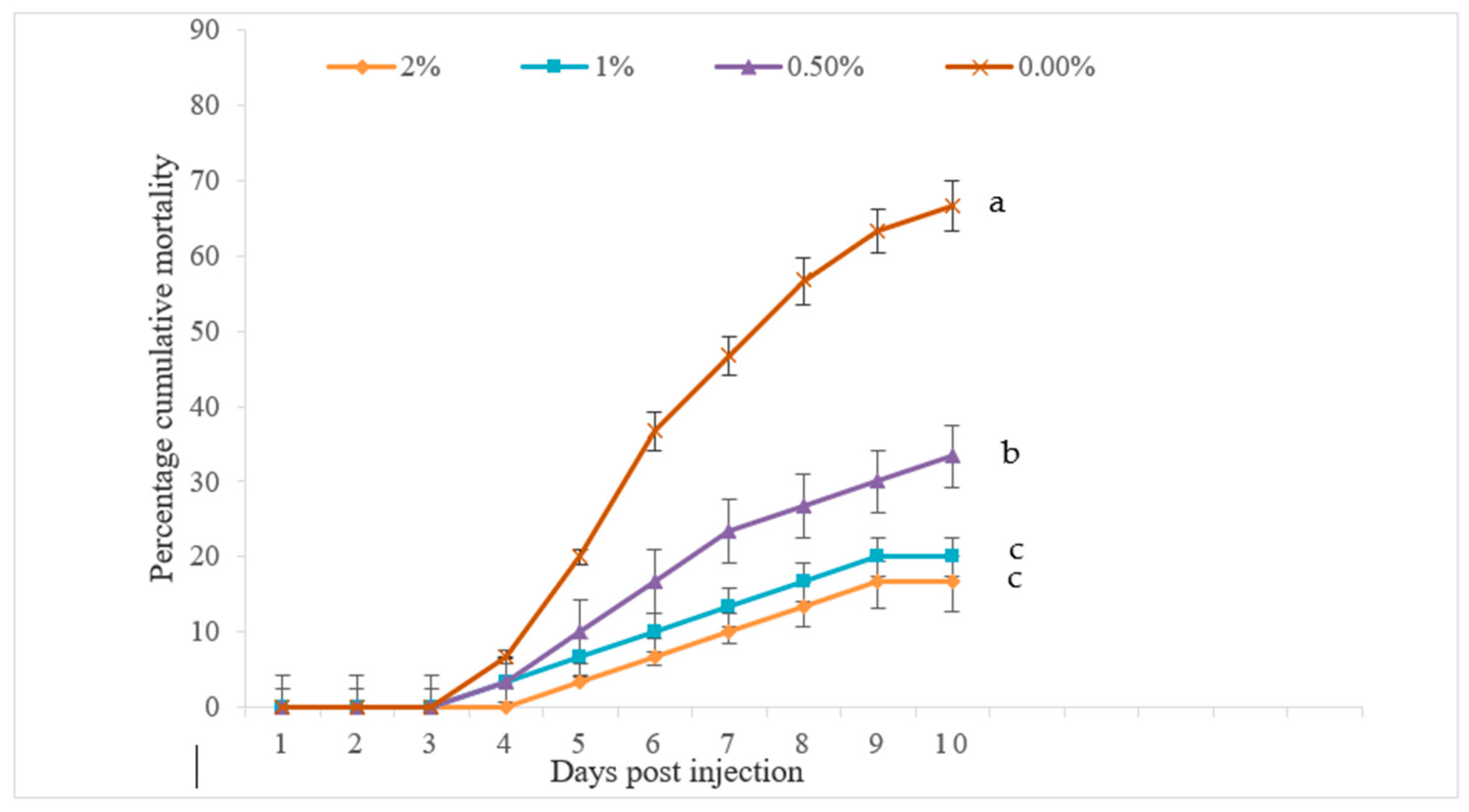
The challenge study discovered that the cumulative percent mortality (CPM) of Pabda against
A. hydrophila was significantly decreased as dietary FPH levels increased, with a 2% FPH diet resulting in the lowest CPM value (
Figure 3). Thus, the basal-diet-fed fish experienced significantly higher CPMs than other treatments. These findings indicated that the dietary supplementation of fish protein hydrolysate could enhance the specific disease resistance of fish by improving defense mechanisms. Siddik et al. [
5] reported similar observations with the disease resistance of
Lates calcarifer following a challenge trial with
S. iniae, which considerably increased in 10% tuna-hydrolysate-fed fish in comparison with other tested fish. Likewise, multiple previous studies observed that the supplementation of FPH in diets at various percentages increased the specific disease resistance of fish, including red sea bream,
Pagrus major against
E. tarda [
19,
56] and barramundi juveniles,
Lates calcarifer against
Vibrio harveyi [
17].
This study demonstrated the commercial farm benefit in supplementing Pabda feeds with protein hydrolysate. By enhancing the fish growth rate and feed assimilation, farm economic data are widely improved at higher levels than those observed for zootechnical data. For instance, while fish SGR was maximally improved by 21% and FCR reduced by 35%, the farm raw margin was folded by 2.13 and ROI by 2.09 with 2% dietary supplementation of protein hydrolysate.