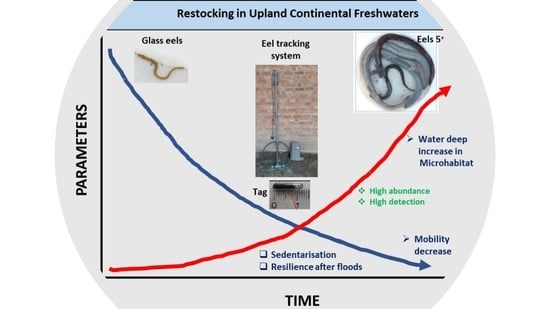
Space and Time Use of European Eel Restocked in Upland Continental Freshwaters, a Long-Term Telemetry Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Eel Capture and Tagging
2.3. Eel RFID Tracking
2.4. Behavioural Metrics Related to Tracking
- Total distance travelled (TD) is expressed as the sum of the net distance travelled, which is the straight-line distance between two consecutive positions of the tagged eel.
- Home range (HR) is the distance between the most upstream and downstream positions of the tagged eel.
- The exploitation index (EI) of the HR is the ratio calculated by dividing TD by HR.
- Longitudinal dispersal (LD) is the straight-line distance between two consecutive positions of the tagged eel, one before and one other after the floods.
- Net distance travelled (ND) under flood events is the position of eels 5+ on the longitudinal profile of the river, which is the straight-line distance between the glass eel release point in spring 2017 and the detection point of eels in autumn 2022.
2.5. Statistical Analyses
3. Results
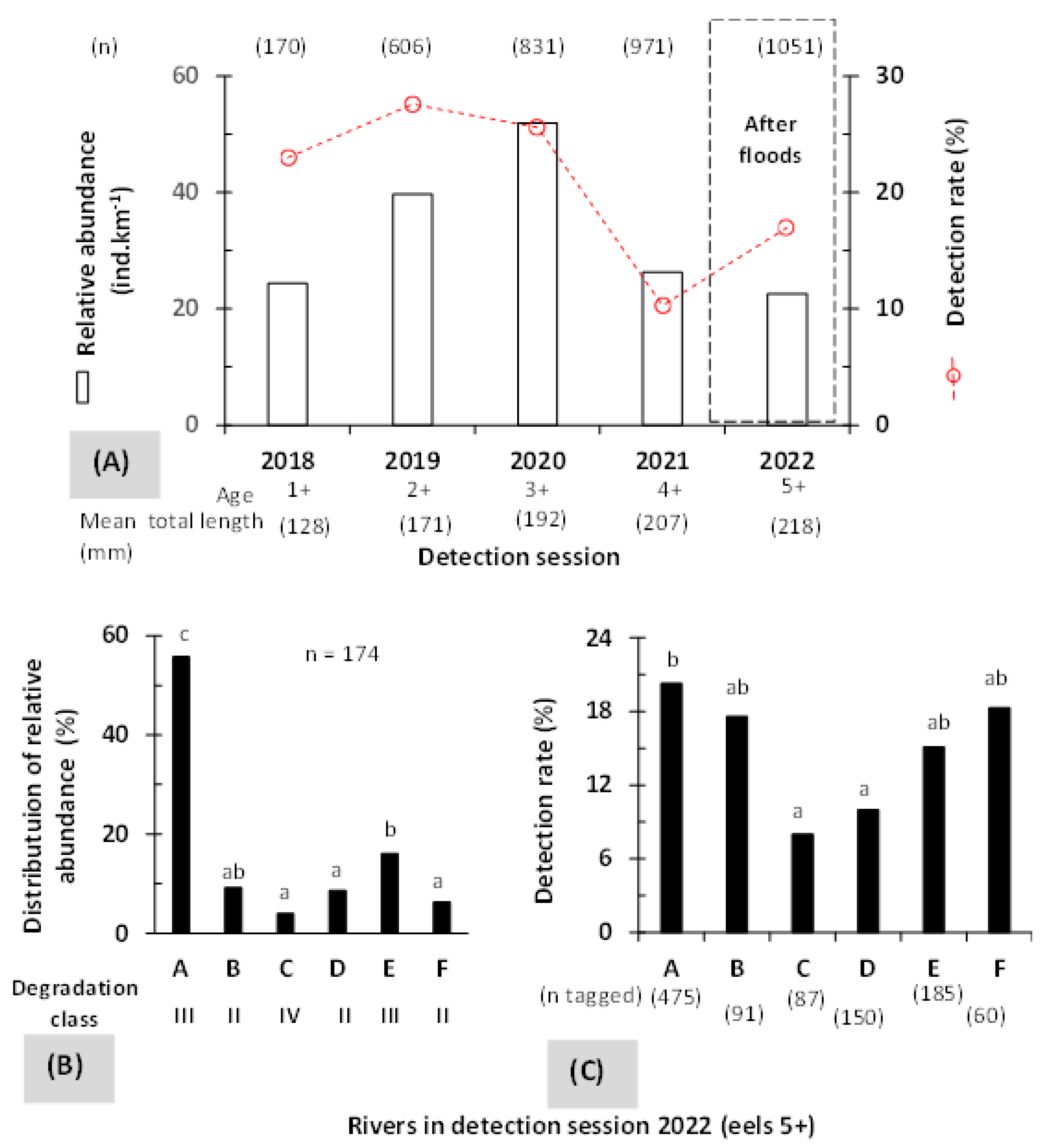
3.1. Relative Abundance and Detection Rate
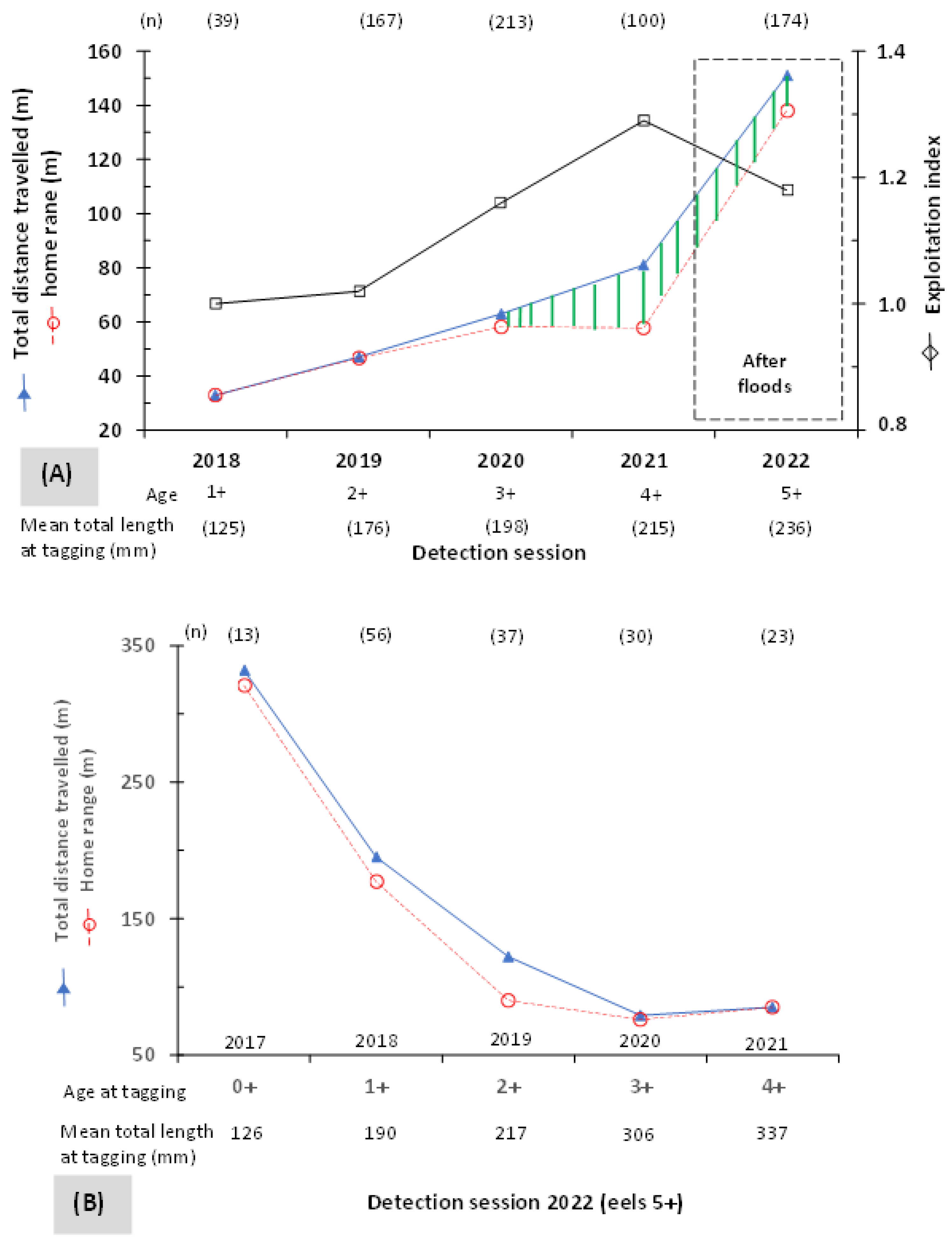
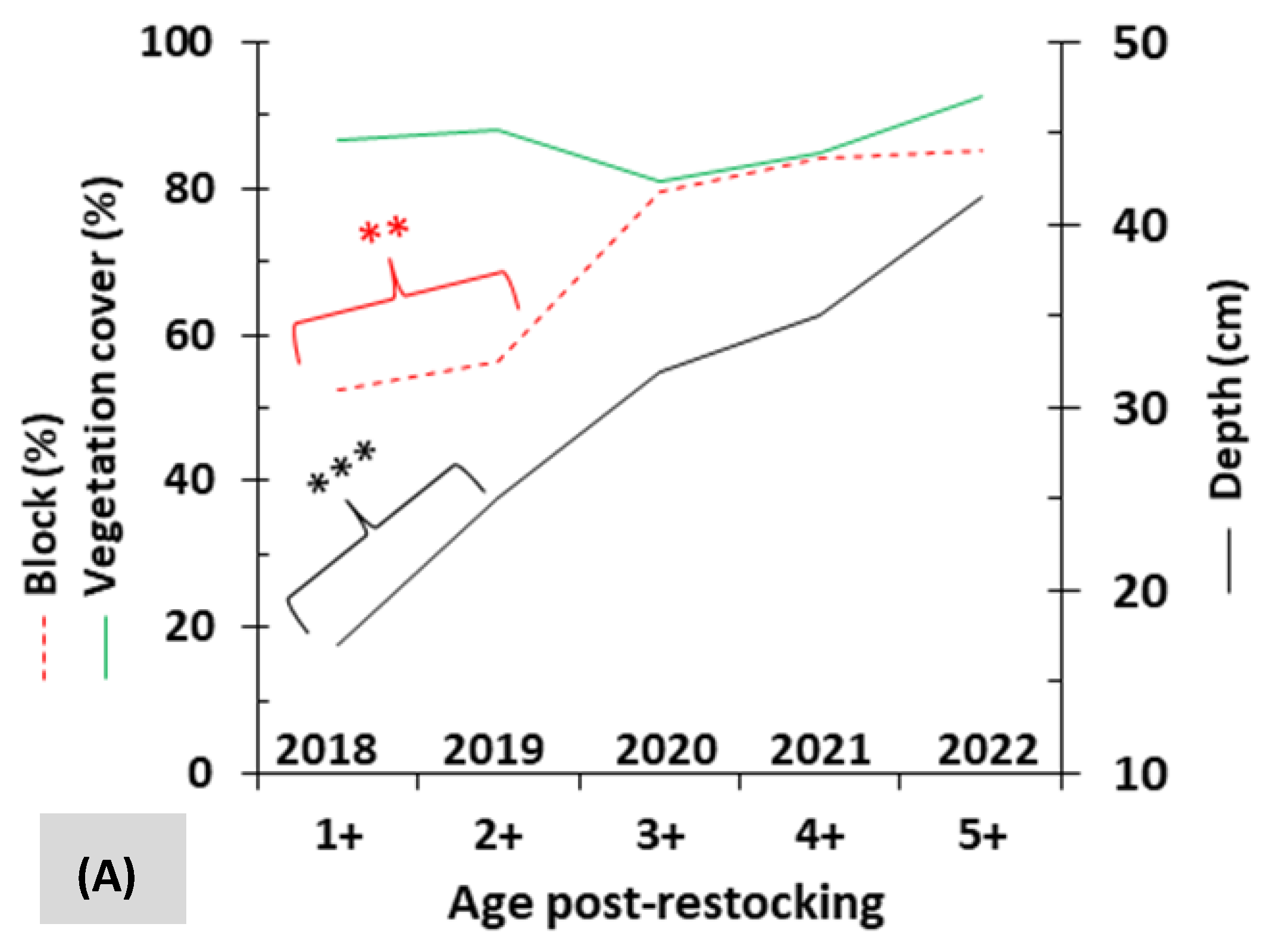
3.2. Mobility and Microhabitat Characteristics
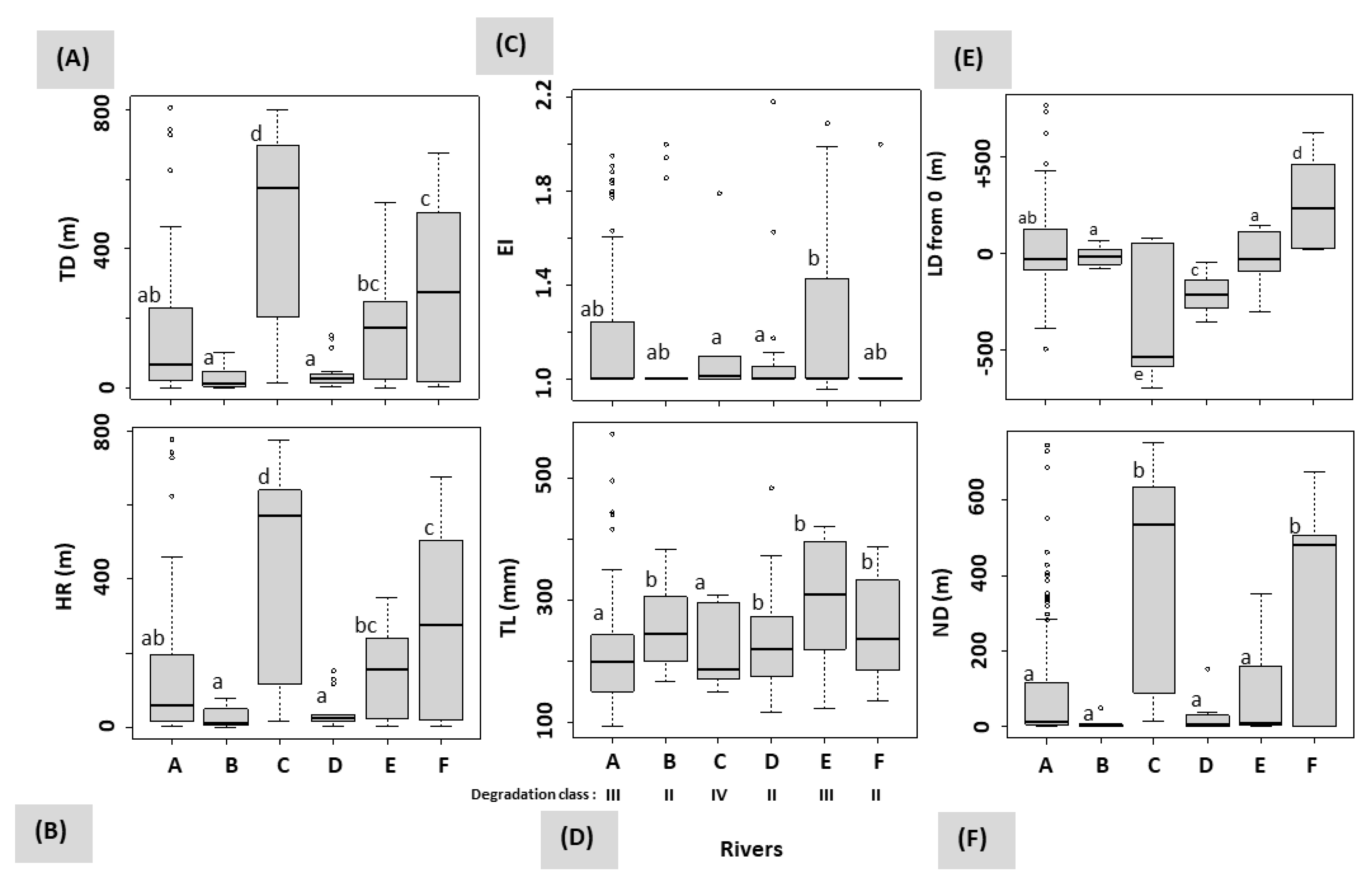
3.3. Influence of Rivers and Floods on Mobility
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pedersen, M.I. Long-term survival and growth of stocked eel, Anguilla anguilla (L.), in a small eutrophic lake. Dana 2000, 12, 71–76. [Google Scholar]
- Prigge, E.; Marohn, L.; Hanel, R. Tracking the migratory success of stocked European eels Anguilla anguilla in the Baltic Sea. J. Fish Biol. 2013, 82, 686–699. [Google Scholar] [CrossRef] [PubMed]
- Simon, J.; Dörner, H.; Scott, R.D.; Schreckenbach, K.; Knösche, R. Comparison of growth and condition of European eels stocked as glass and farm sourced eels in lakes in the first four years after stocking. J. Appl. Ichthyol. 2013, 29, 323–330. [Google Scholar] [CrossRef]
- Desprez, M.; Crivelli, A.J.; Lebel, I.; Massez, G.; Gimenez, O. Demographic assessment of a stocking experiment in European eels. Ecol. Freshw. Fish 2013, 22, 412–420. [Google Scholar] [CrossRef]
- Pedersen, M.I.; Rasmussen, C.H. Yield per recruit from stocking two different sizes of eel (Anguilla anguilla) in the brackish Roskilde Fjord. ICES J. Mar. Sci. 2016, 73, 158–164. [Google Scholar] [CrossRef]
- Kullmann, B.; Thiel, R. Bigger is better in eel stocking measures? Comparison of growth performance, body condition, and benefit-cost ratio of simultaneously stocked glass and farmed eels in a brackish fjord. Fish. Res. 2018, 205, 132–140. [Google Scholar] [CrossRef]
- Félix, P.M.; Costa, J.L.; Monteiro, R.; Castro, N.; Quintella, B.R.; Almeida, P.R.; Domingos, I. Can a restocking event with European (glass) eels cause early changes in local biological communities and its ecological status? Glob. Ecol. Conserv. 2020, 21, e00884. [Google Scholar] [CrossRef]
- Félix, P.M.; Costa, J.L.; Quintella, B.R.; Almeida, P.R.; Monteiro, R.; Santos, J.; Portela, T.; Domingos, I. Early settlement and growth of stocked European glass eels in a fragmented watercourse. Fish. Manag. Ecol. 2020, 28, 91–100. [Google Scholar] [CrossRef]
- Nzau Matondo, B.; Benitez, J.P.; Dierckx, A.; Renardy, S.; Rollin, X.; Colson, D.; Baltus, L.; Romain, V.R.M.; Ovidio, M. What are the best upland river characteristics for glass eel restocking practice? Sci. Total Environ. 2021, 784, 147042. [Google Scholar] [CrossRef]
- Nzau Matondo, B.; Delrez, N.; Bardonnet, A.; Vanderplasschen, A.; Joaquim-Justo, C.; Rives, J.; Benitez, J.P.; Dierckx, A.; Séleck, E.; Rollin, X.; et al. A complete check-up of European eel after eight years of restocking in an upland river: Trends in growth, lipid content, sex ratio and health status. Sci. Total Environ. 2022, 807, 151020. [Google Scholar] [CrossRef]
- Ovidio, M.; Tarrago-Bès, F.; Nzau Matondo, B. Short-term responses of glass eels transported from UK to small Belgian streams. Ann. Limnol. Int. J. Limnol. 2015, 51, 219–226. [Google Scholar] [CrossRef]
- Nzau Matondo, B.; Séleck, E.; Dierckx, A.; Benitez, J.-P.; Rollin, X.; Ovidio, M. What happens to glass eels after restocking in upland rivers? A long-term study on their dispersal and behavioural traits. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 374–388. [Google Scholar] [CrossRef]
- Tesch, F.W.; Thorpe, J.E. The Eel, 3rd ed.; Blackwell Science: Oxford, UK, 2003. [Google Scholar]
- Wright, M.R.; Piper, A.T.; Aarestrup, K.; Azevedo, J.M.N.; Cowan, G.; Don, A.; Gollock, M.; Ramallo, R.S.; Velterop, R.; Walker, A.; et al. First direct evidence of adult European eels migrating to their breeding place in the Sargasso Sea. Sci. Rep. 2022, 12, 15362. [Google Scholar] [CrossRef] [PubMed]
- Hanel, R.; Marohn, L.; Westerberg, H. No new evidence for anAtlantic eels spawning area outside the Sargasso Sea. Sci. Rep. 2022, 12, 11778. [Google Scholar] [CrossRef]
- Feunteun, E. Management and restoration of European eel population (Anguilla anguilla): An impossible bargain. Ecol. Eng. 2002, 18, 575–591. [Google Scholar] [CrossRef]
- Robinet, T.; Feunteun, E. Sublethal effects of exposure to chemical compounds: A cause for the decline in Atlantic eels? Ecotoxicology 2002, 11, 265–277. [Google Scholar] [CrossRef]
- Haenen, O.L.M.; Mladineo, I.; Konecny, R.; Yoshimizu, M.; Groman, D.; Munoz, P. Diseases of eels in an international perspective: Workshop on eel diseases at the 15th international conference on the diseases of fish and shellfish, Split, Croatia, 2011. Bull. Eur. Assoc. Fish Pathol. 2012, 32, 109–115. [Google Scholar]
- Muñoz, P.; Barcala, E.; Peñalver, J.; Romero, D. Can inorganic elements affect herpesvirus infections in European eels? Environ. Sci. Pollut. Res. 2019, 26, 35266–35269. [Google Scholar] [CrossRef]
- Delrez, N.; Zhang, H.; Lieffrig, F.; Mélard, C.; Farnir, F.; Boutier, M.; Donohoe, O.; Vanderplasschen, A. European eel restocking programs based on wild-caught glass eels: Feasibility of quarantine stage compatible with implementation of prophylactic measures prior to scheduled reintroduction to the wild. J. Nat. Conserv. 2021, 59, 125933. [Google Scholar] [CrossRef]
- Haro, D.; Gollock, M. Anguilla anguilla. The IUCNRed List of Threatened Species. Version 2014.2. 2014. Available online: www.iucnredlist.org (accessed on 28 May 2013).
- Ibbotson, A.; Smith, J.; Scarlett, P.; Aprahamian, M.W. Colonisation of freshwater habitats by the European eel Anguilla anguilla. Freshw. Biol. 2002, 47, 1696–1706. [Google Scholar] [CrossRef]
- Nzau Matondo, B.; Ovidio, M. Decreased stock entering the Belgian Meuse is associated with the loss of colonisation behaviour in yellow-phase European eels. Aquat. Living Resour. 2018, 31, 7. [Google Scholar] [CrossRef]
- Baras, E.; Philippart, J.C.; Salmon, B. Estimation of migrant yellow eels stock in large rivers through the survey of fish passes: A preliminary in the River Meuse (Belgium). In Stock Assessment in Inland Fisheries; Cowx, I.G., Ed.; Oxford Fishing News Books (Blackwell): London, UK, 1996; pp. 314–325. [Google Scholar]
- Philippart, J.C. L’érosion de la Biodiversité: Les Poissons; Université de Liège: Liège, Belgium, 2006; 306p. [Google Scholar]
- Philippart, J.C.; Ovidio, M.; Rimbaud, G.; Dierckx, A.; Poncin, P. Bilan des observations sur les populations de l’anguille dans les sous-bassins hydrographiques Meuse aval, Ourthe, Amblève et Vesdre; Commission provinciale de Liège du Fonds piscicole du Service Public de Wallonie: Liège, Belgium, 2010; 161p. [Google Scholar]
- Nzau Matondo, B.; Ovidio, M. Dynamics of upstream movements of the European eel Anguilla Anguilla in an inland area of the river Meuse over the last 20 years. Environ. Biol. Fish 2016, 99, 223–235. [Google Scholar] [CrossRef]
- Nzau Matondo, B.; Benitez, J.P.; Dierckx, A.; Rollin, X.; Ovidio, M. An evaluation of restocking practice and demographic stock assessment methods for cryptic juvenile European eel in upland rivers. Sustainability 2020, 12, 1124. [Google Scholar] [CrossRef]
- ICES. Joint EIFAAC/ICES/GFCM Working Group on Eels (WGEEL); ICES Scientific Reports: Copenhagen, Denmark, 2020; 223p. [Google Scholar]
- Council of the European Communities. Council regulation (EC) no 1100/207 of 18 September 2007 establishing measures for the recovery of the stock of European eel. O. J. Eur. Union 2007, 248, 17–23. [Google Scholar]
- Shiao, J.C.; Lozys, L.; Iizuka, Y.; Tzeng, W.N. Migratory patterns and contribution of stocking to the population of European eel in Lithuanian waters as indicated by otolith Sr:Ca ratios. J. Fish Biol. 2006, 69, 749–769. [Google Scholar] [CrossRef]
- Simon, J.; Dörner, H. Survival and growth of European eels stocked as glass- and farm-sourced eels in five lakes in the first years after stocking. Ecol. Freshw. Fish 2014, 23, 40–48. [Google Scholar] [CrossRef]
- Sadler, K. Effects of temperature on the growth and survival of the European eel, Anguilla anguilla L. J. Fish Biol. 1979, 15, 499–507. [Google Scholar] [CrossRef]
- Morin, A.; Lamoureux, W.; Busnarda, J. Empirical models predicting primary productivity from chlorophyll and water temperature for stream periphyton and lake and ocean phytoplankton. J. N. Am. Benthol. Soc. 1999, 18, 299–307. [Google Scholar] [CrossRef]
- Huet, M. Aperçu de la relation entre la pente et les populations piscicoles des eaux courantes. Schweiz. Z. Hydrol. 1949, 11, 332–351. [Google Scholar] [CrossRef]
- Jepsen, N.; Koed, A.; Thorstad, E.B.; Baras, E. Surgical implantation of telemetry transmitters in fish: How much have we learned? Hydrobiologia 2002, 483, 239–248. [Google Scholar] [CrossRef]
- Wentworth, C.K. A scale of grade and class terms for clastic sediments. J. Geol. 1922, 30, 377–392. [Google Scholar] [CrossRef]
- Baras, E.; Jeandrain, D.; Serouge, B.; Philippart, J.C. Seasonal variations in time and space utilization by radio-tagged yellow eels Anguilla anguilla (L.) in A Small Stream. Hydrobiologia 1998, 371/372, 187–198. [Google Scholar] [CrossRef]
- Ovidio, M.; Seredynski, A.; Philippart, J.C.; Nzau Matondo, B. A bit of quiet between the migrations: The resting life of the European eel during their freshwater growth phase in a small stream. Aquat. Ecol. 2013, 47, 291–301. [Google Scholar] [CrossRef]
- R Development Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. 2016. Available online: https://www.R-project.org/ (accessed on 20 December 2022).
- Fox, J. Using the R Commander: A Point-and-Click Interface for R; Chapman and CRC Press: Boca Raton, FL, USA, 2017; Available online: http://socserv.mcmaster.ca/jfox/Books/RCommander/ (accessed on 20 December 2022).
- Harrell, F.E., Jr. Hmisc: Harrell miscellaneous (Version 4.1-1). Cran. 2018. Available online: http://CRAN.Rproject.org/package=Hmisc (accessed on 20 December 2022).
- Naismith, I.A.; Knights, B. Studies of sampling methods and of techniques for estimating populations of eels, Anguilla anguilla L. Aquac. Res. 1990, 21, 357–368. [Google Scholar] [CrossRef]
- White, E.M.; Knights, B. Environmental factors affecting migration of the European eel in the Rivers Severn and Avon, England. J. Fish Biol. 1997, 50, 1104–1116. [Google Scholar] [CrossRef]
- Pedersen, M.I. Recapture rate, growth and sex of stocked cultured eels Anguilla anguilla (L.). Bull. Français Pêche Piscic. 1998, 349, 153–162. [Google Scholar] [CrossRef]
- Nzau Matondo, B.; Benitez, J.P.; Dierckx, A.; Philippart, J.C.; Ovidio, M. Assessment of the entering stock, migration dynamics and fish pass fidelity of European eel in the Belgian Meuse River. River Res. Appl. 2017, 33, 292–301. [Google Scholar] [CrossRef]
- Kennedy, M.; Fitzmaurice, P. Growth and food of brown Trout Salmo trutta (L.) in Irish waters. P. Roy. Irish Acad. B 1971, 71, 269–352. [Google Scholar]
- Laffaille, P.; Acou, A.; Guillouet, J. The yellow European eel (Anguilla anguilla L.) may adopt a sedentary lifestyle in inland freshwaters. Ecol. Freshw. Fish 2005, 14, 191–196. [Google Scholar] [CrossRef]
- Oehm, J.; Thalinger, B.; Eisenkölbl, S.; Traugott, M. Diet analysis in piscivorous birds: What can the addition of molecular tools offer? Ecol. Evol. 2017, 7, 1984–1995. [Google Scholar] [CrossRef]
- Baisez, A. Optimisation des Suivis des Indices D’abondances et des Structure de Taille de L’anguille Européenne (Anguilla anguilla, L.) dans un Marais Endigué de la Côte Atlantique: Relations Espèce Habitat. PhD Thesis, University of Toulouse, Toulouse, France, 2001; 396p. [Google Scholar]
- Feunteun, E.; Acou, A.; Laffaille, P.; Legault, A. European eel (Anguilla anguilla): Prediction of spawner escapement from continental population parameters. Can. J. Fish. Aquat. Sci. 2000, 57, 1627–1635. [Google Scholar] [CrossRef]
- Baras, E.; Jeandrain, D. Evaluation of surgery procedures for tagging eel Anguilla anguilla (L.) with biotelemetry transmitters. Hydrobiologia 1998, 371, 107–111. [Google Scholar] [CrossRef]
- Helfman, G.S.; Facey, D.E.; Stanton Hales, L.; Bozeman, E.L. Reproductive ecology of the American eel. Am. Fish. Soc. Symp. 1987, 1, 42–56. [Google Scholar]
- Righton, D.; Piper, A.; Aarestrup, K.; Amilhat, E.; Belpaire, C.; Casselman, J.; Castonguay, M.; Díaz, E.; Dörner, H.; Faliex, E.; et al. Important questions to progress science and sustainable management of anguillid eels. Fish Fish. 2021, 22, 1–27. [Google Scholar] [CrossRef]
- Greene, K.E.; Zimmerman, J.L.; Laney, R.W.; Thomas-Blate, J.C. Atlantic Coast Diadromous Fish Habitat: A Review of Utilization, Threats, Recommendations for Conservation, and Research Needs; Atlantic States Marine Fisheries Commission Habitat Management: Washington, DC, USA, 2009; 68p. Available online: https://www3.epa.gov/region1/npdes/merrimackstation/pdfs/ar/AR-56.pdf (accessed on 10 January 2023).
- Laffaille, P.; Feunteun, E.; Baisez, A.; Robinet, T.; Acou, A.; Legault, A.; Lek, S. Spatial organisation of European eel (Anguilla anguilla L.) in a small catchment. Ecol. Freshw. Fish 2003, 12, 254–264. [Google Scholar] [CrossRef]
- Laffaille, P.; Baisez, A.; Rigaud, C.; Feunteun, E. Habitat preferences of different European eel size classes in a reclaimed marsh: A contribution to species and ecosystem conservation. Wetlands 2004, 24, 642–651. [Google Scholar] [CrossRef]
- Jellyman, D.J.; Arai, T. Juvenile eels: Upstream migration and habitat use. In Biology and Ecology of Anguillid Eels; Arai, T., Ed.; CRC Press: Boca Raton, FL, USA, 2016; pp. 171–191. [Google Scholar]
- Herrera, M.; Moreno-Valcárcel, R.; Rubio, R.D.M.; Fernández-Delgado, C. From Transient to Sedentary? Changes in the Home Range Size and Environmental Patterns of Movements of European Eels (Anguilla anguilla) in a Mediterranean River. Fishes 2019, 4, 43. [Google Scholar] [CrossRef]
- Edeline, E.; Dufour, S.; Elie, P. Proximate and Ultimate Control of Eel Continental Dispersal. In Spawning Migration of the European Eel; Van den Thillart, G., Dufour, S., Rankin, J.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 433–461. [Google Scholar]
- Braithwhaite, V.A.; De Perera, B. Short-range orientation in fish: How fish map space. Mar. Fresh. Behav. Physiol. 2006, 39, 37–47. [Google Scholar] [CrossRef]
- Jellyman, D.J.J. Diet of two species of freshwater eel (Anguilla spp.) in Lake Pounui. New Zealand. N. Z. J. Mar. Freshwater Res. 1989, 23, 1–10. [Google Scholar] [CrossRef]
- Fjellheim, A.; Raddum, G.G. Acidification and liming of River Vikedal, western Norway. A 20 year study of responses in the benthic invertebrate fauna. Water Air Soil Pollut. 2001, 130, 1379–1384. [Google Scholar] [CrossRef]
- Larsen Bjørn, M.; Hesthagen, T.; Thorstad, E.B.; Diserud, O.H. Increased abundance of European eel (Anguilla anguilla) in acidified Norwegian rivers after liming. Ecol. Freshw. Fish 2015, 24, 575–583. [Google Scholar] [CrossRef]
- Haro, A.; Richkus, W.; Whalen, K.; Hoar, A.; Busch, W.-D.; Lary, S.; Brush, T.; Dixon, D. Population decline of the American eel: Implications for research and management. Fisheries 2000, 25, 7–16. [Google Scholar] [CrossRef]
- Jacoby, D.M.P.; Casselman, J.M.; Crook, V.; DeLucia, M.-B.; Ahn, H.; Kaifu, K.; Kurwie, T.; Sasal, P.; Silfvergrip, A.M.C.; Smith, K.G.; et al. Synergistic patterns of threat and the challenges facing global anguillid eel conservation. Glob. Ecol. Conserv. 2015, 4, 321–333. [Google Scholar] [CrossRef]
- Jellyman, D.J. An enigma: How can freshwater eels (Anguilla spp.) be such a successful genus yet be universally threatened? Rev. Fish Biol. Fish. 2022, 32, 701–718. [Google Scholar] [CrossRef]
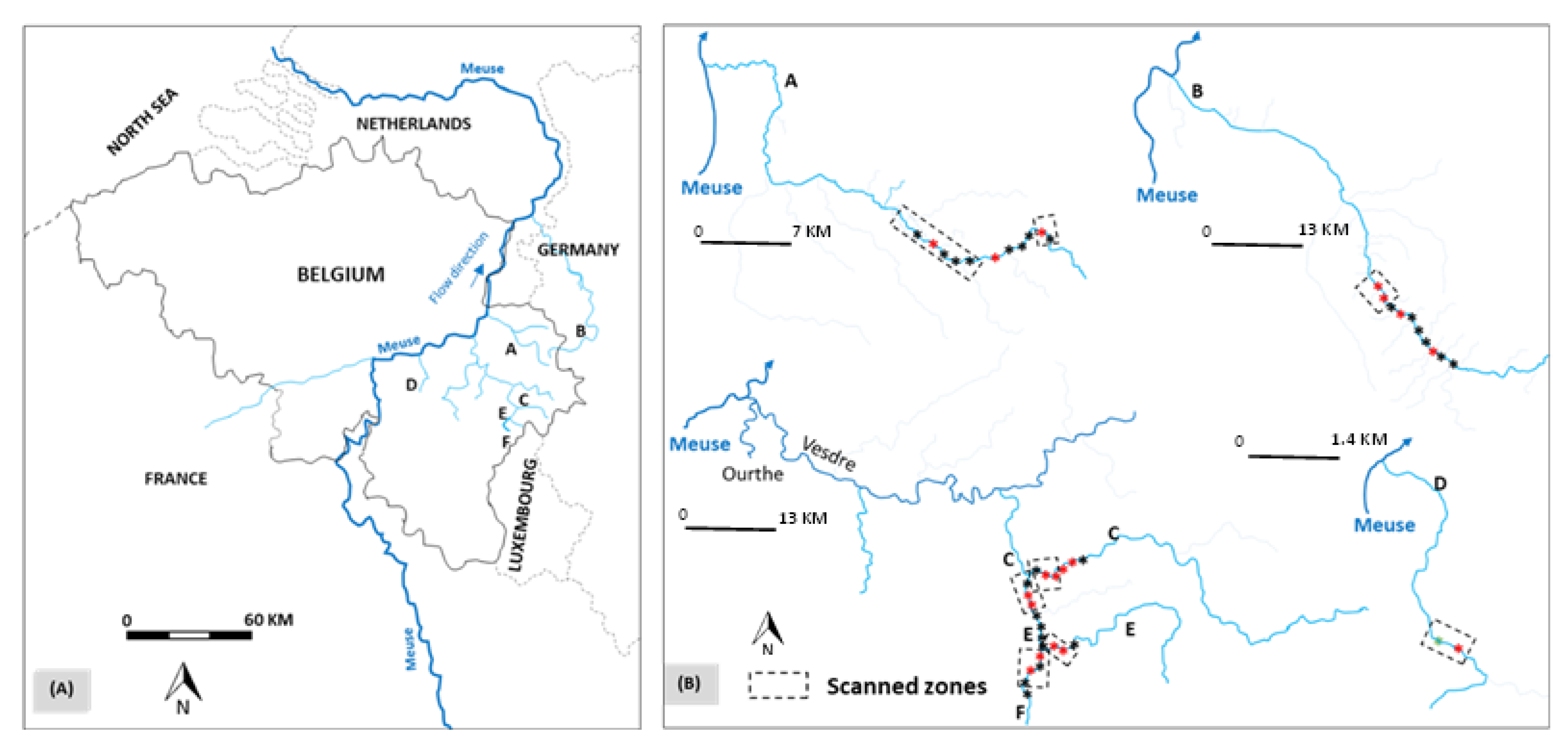

| Rivers | ||||||
|---|---|---|---|---|---|---|
| Parameters | A | B | C | D | E | F |
| Altitude (m) | 144 | 155 | 178 | 100 | 172 | 221 |
| Catchment area (km²) | 118.0 | 380.0 | 128.9 | 45.3 | 97.0 | – |
| Direct tributary | Meuse | Meuse | Vesdre | Meuse | Hoegne | Wayai |
| River length (km) | 25 | 56 | 34 | 12 | 15 | 8 |
| Distance from the North Sea (km) | 341 | 324 | 376 | 366 | 376 | 381 |
| Distance from the River Meuse (km) | 18 | 43 | 36 | 3 | 34 | 38 |
| Distance from the River mouth (km) | 18 | 43 | 11.5 | 3 | 0.35 | 0.09 |
| Fish zone | Brown trout | Lower Grayling | Upper Brown trout | Brown trout | Lower Brown trout | Brown trout |
| Width (mean ± SE, in m) | 4.7 ± 1.4 | 7.5 ± 1.0 | 7.4 ± 1.3 | 4.8 ± 1.1 | 6.7 ± 1.2 | 4.8 ± 1.0 |
| Depth (mean ± SE, in m) | 18.4 ± 10.0 | 36.6 ± 19.9 | 22.2 ± 12.2 | 16.8 ± 8.4 | 21.4 ± 11.6 | 20.7 ± 12,9 |
| Species richness (number) | 12 | 18 | 9 | 8 | 10 | 7 |
| Density of brown trout (mean ± SE, in ind. m−2) | 0.185 ± 0.081 | 0.995 ± 0.555 | 4.243 ± 1.457 | 2.048 ± 0.770 | 3.971 ± 2.132 | 3.893 ± 0.373 |
| Vegetation cover (mean ± SE, in %) | 56.3 ± 35.0 | 36.3 ± 25.8 | 84.0 ± 19.8 | 87.1 ± 16.3 | 59.8 ± 31.6 | 64.7 ± 34.0 |
| Daily water temperature (mean ± SE, in °C) | 11.3 ± 3.6 | 11.2 ± 3.5 | 10.5 ± 3.9 | 10.7 ± 3.4 | 11.0 ± 3.5 | 10.3 ± 4.0 |
| Monthly pH (mean ± SE) | 7.8 ± 0.3 | 8.0 ± 0.3 | 6.8 ± 0.4 | 8.0 ± 0.3 | 7.4 ± 0.3 | 6.8 ± 0.4 |
| Monthly calcium carbonate (mean ± SE, in mg L−1) | 108 ± 26 | 94 ± 27 | 22 ± 9 | 105 ± 25 | 31 ± 20 | 21 ± 5 |
| Monthly conductivity (mean ± SE, μs cm−1) | 600 ± 125 | 572 ± 67 | 161 ± 50 | 541 ± 55 | 221 ± 75 | 116 ± 24 |
| Monthly total hardness (mean ± SE, °GH) | 14 ± 2 | 13 ± 2 | 3 ± 1 | 13 ± 2 | 4 ± 2 | 2 ± 1 |
| Monthly carbonate hardness (mean ± SE, °KH) | 8 ± 3 | 10 ± 2 | 2 ± 1 | 9 ± 2 | 4 ± 2 | 2 ± 1 |
| Bottom substrates (in decreasing abundance order) | ||||||
| Predominant substratum | Large stone + Block | Block + Large stone + Boulder | Block + Large stone + Boulder | Large stone + Fine stone + Coarse pebble | Boulder + Large stone + Coarse gravel + Block | Boulder + Coarse pebble |
| Abundance (%) | 43.9 + 20.9 = 64.4 | 30.1 + 29.0 + 20.0 = 79.1 | 30.1 + 29.0 + 20.0 = 79.1 | 30.6 + 26.6 + 14.0 = 71.2 | 28.8 + 18.6 + 16.4 + 14.4 = 78.3 | 46.8 + 36.7 = 83.5 |
| Flow features (in decreasing abundance order) | ||||||
| Predominant flow features | Run, pool and Riffle | Run, Lentic Channel and Riffle | Run and Rapid | Run, pool and Riffle | Run and Rapid | Run and pool |
| Abundance (%) | 48.5 + 32.0+17.0 = 97.5 | 38.6 + 27.9 + 13.2 = 79.7 | 58.7 + 32.5 = 91.2 | 64.0 + 16.9 + 12.5 = 93.4 | 58.8 + 28.6 = 87.4 | 72.0 + 16.4 = 88.4 |
| Trophic status | Eutrophic | Eutrophic | Oligotrophic | Oligotrophic | Oligotrophic | Oligotrophic |
| Age at Tagging | River | Sector | n detected | Total Length at Tagging (mm) | Net Distance Travelled after Floods (m) | Total Distance Travelled (m) | Home Range (m) | Exploitation Index of Home Range | Microhabitat | |
|---|---|---|---|---|---|---|---|---|---|---|
| Bottom Substrate | Water Depth (cm) | |||||||||
| 0+ (2017) | A | 2 | 11 | 125 ± 15 | 290 ± 291 | 344 ± 225 | 321 ± 230 | 1.07 ± 0.13 | 11B | 44.3 ± 19.2 |
| E | 3 | 1 | 134 ± 0 | 6 ± 0 | 349 ± 0 | 349 ± 0 | 1.00 ± 0.00 | 1B | 50.0 ± 0.0 | |
| 8 | 1 | 123 ± 0 | 349 ± 0 | 191 ± 0 | 150 ± 0 | 1.27 ± 0.00 | 1B | 24.0 ± 0.0 | ||
| 1+ (2018) | A | 2 | 30 | 192 ± 63 | 140 ± 194 | 188 ± 207 | 176 ± 197 | 1.10 ± 0.15 | 30B | 39.7 ± 22.3 |
| 10 | 10 | 187 ± 41 | 9 ± 11 | 67 ± 82 | 50 ± 66 | 1.50 ± 0.35 | 9B+1PG | 34.1 ± 12.8 | ||
| B | 1 | 1 | 193 ± 0 | 4 ± 0 | 27 ± 0 | 27 ± 0 | 1.00 ± 0.00 | 1RA | 26.0 ± 0.0 | |
| 2 | 1 | 167 ± 0 | 6 ± 0 | 6 ± 0 | 6 ± 0 | 1.00 ± 0.00 | 1RA | 58.0 ± 0.0 | ||
| C | 3 | 4 | 173 ± 18 | 508 ± 290 | 585 ± 263 | 541 ± 292 | 1.23 ± 0.38 | 4B | 33.5 ± 14.6 | |
| D | 2 | 4 | 159 ± 29 | 18 ± 18 | 62 ± 54 | 51 ± 52 | 1.37 ± 0.55 | 4B | 36.0 ± 9.1 | |
| E | 8 | 1 | 199 ± 0 | 1 ± 0 | 145 ± 0 | 144 ± 0 | 1.00 ± 0.00 | 1B | 24.0 ± 0.0 | |
| 9 | 3 | 188 ± 28 | 138 ± 123 | 278 ± 123 | 194 ± 37 | 1.40 ± 0.39 | 3B | 44.7 ± 28.9 | ||
| F | 1 | 2 | 162 ± 35 | 493 ± 18 | 493 ± 18 | 493 ± 18 | 1.00 ± 0.00 | 1RA+1RO | 49.0 ± 4.2 | |
| 2+ (2019) | A | 2 | 16 | 233 ± 75 | 99 ± 139 | 147 ± 136 | 126 ± 130 | 1.24 ± 0.31 | 16B | 40.6 ± 15.0 |
| 10 | 8 | 229 ± 58 | 11 ± 13 | 47 ± 62 | 34 ± 39 | 1.3 ± 0.41 | 8B | 27.5 ± 7.7 | ||
| B | 1 | 2 | 207 ± 33 | 0.5 ± 0.7 | 26 ± 36 | 26 ± 36 | 1.00 ± 0.00 | 2RA | 46.5 ± 26.2 | |
| 2 | 3 | 229 ± 31 | 1.3 ± 0.6 | 64 ± 46 | 45 ± 35 | 1.60 ± 0.52 | 3RA | 57.3 ± 9.2 | ||
| E | 8 | 3 | 238 ± 45 | 62 ± 92 | 453 ± 127 | 234 ± 57 | 1.92 ± 0.09 | 3B | 22 ± 6.9 | |
| 9 | 1 | 214 ± 0 | 2 ± 0 | 2 ± 0 | 2 ± 0 | 1.00 ± 0.00 | 1B | 62.0 ± 0.0 | ||
| D | 2 | 4 | 207 ± 39 | 41 ± 74 | 43 ± 72 | 42 ± 73 | 1.16 ± 0.31 | 1B+3RA | 32.0 ± 8.5 | |
| 3+ (2020) | A | 2 | 10 | 302 ± 123 | 46 ± 120 | 48 ± 119 | 47 ± 119 | 1.06 ± 0.18 | 9B+1RA | 36.6 ± 15.6 |
| 10 | 2 | 249 ± 8 | 13 ± 17 | 18 ± 10 | 16 ± 14 | 1.42 ± 0.59 | 2B | 42.5 ± 3.5 | ||
| B | 1 | 1 | 297 ± 0 | 48 ± 0 | 48 ± 0 | 48 ± 0 | 1.00 ± 0.00 | 1RA | 94.0 ± 0.0 | |
| 2 | 4 | 306 ± 72 | 5 ± 1 | 6 ± 3 | 5 ± 0,5 | 1.25 ± 0.50 | 1B+3RA | 63.8 ± 18.9 | ||
| C | 3 | 2 | 302 ± 8 | 262 ± 349 | 262 ± 349 | 262 ± 349 | 1.00 ± 0.00 | 2B | 33.0 ± 12.7 | |
| D | 2 | 2 | 378 ± 149 | 4 ± 6 | 31 ± 4 | 31 ± 4 | 1.00 ± 0.00 | 2B | 56.0 ± 11.3 | |
| E | 8 | 4 | 318 ± 21 | 13 ± 21 | 29 ± 21 | 25 ± 23 | 1.31 ± 0.53 | 4B | 27.5 ± 9.6 | |
| 9 | 2 | 328 ± 88 | 135 ± 27 | 197 ± 33 | 164 ± 14 | 1.21 ± 0.30 | 2B | 21.5 ± 7.8 | ||
| F | 1 | 3 | 269 ± 65 | 225 ± 390 | 233 ± 385 | 231 ± 384 | 1.33 ± 0.58 | 2B+1RA | 29.7 ± 15.9 | |
| 4+ (2021) | A | 2 | 6 | 310 ± 100 | - | 104 ± 107 | 104 ± 107 | 1.00 ± 0.00 | 6B | 42.5 ± 26.4 |
| B | 2 | 2 | 328 ± 46 | - | 14 ± 5 | 14 ± 5 | 1.00 ± 0.00 | 2B | 75.0 ± 10.8 | |
| D | 2 | 5 | 286 ± 65 | - | 39 ± 44 | 39 ± 44 | 1.00 ± 0.00 | 5B | 35.4 ± 12.0 | |
| E | 8 | 5 | 364 ± 59 | 19 ± 21 | 19 ± 21 | 1.00 ± 0.00 | 5B | 29.8 ± 7.9 | ||
| 9 | 4 | 406 ± 11 | - | 239 ± 16 | 239 ± 16 | 1.00 ± 0.00 | 2B+1RA | 29.6 ± 6.8 | ||
| F | 1 | 1 | 387 ± 0 | - | 71 ± 0 | 71 ± 0 | 1.00 ± 0.00 | 1B | 22.0 ± 0.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nzau Matondo, B.; Backory, L.; Dupuy, G.; Amoussou, G.; Oumarou, A.A.; Gelder, J.; Renardy, S.; Benitez, J.-P.; Dierckx, A.; Dumonceau, F.; et al. Space and Time Use of European Eel Restocked in Upland Continental Freshwaters, a Long-Term Telemetry Study. Fishes 2023, 8, 137. https://doi.org/10.3390/fishes8030137
Nzau Matondo B, Backory L, Dupuy G, Amoussou G, Oumarou AA, Gelder J, Renardy S, Benitez J-P, Dierckx A, Dumonceau F, et al. Space and Time Use of European Eel Restocked in Upland Continental Freshwaters, a Long-Term Telemetry Study. Fishes. 2023; 8(3):137. https://doi.org/10.3390/fishes8030137
Chicago/Turabian StyleNzau Matondo, Billy, Léa Backory, Guillaume Dupuy, Gildas Amoussou, Ali Abdou Oumarou, Justine Gelder, Séverine Renardy, Jean-Philippe Benitez, Arnaud Dierckx, Frédéric Dumonceau, and et al. 2023. "Space and Time Use of European Eel Restocked in Upland Continental Freshwaters, a Long-Term Telemetry Study" Fishes 8, no. 3: 137. https://doi.org/10.3390/fishes8030137
APA StyleNzau Matondo, B., Backory, L., Dupuy, G., Amoussou, G., Oumarou, A. A., Gelder, J., Renardy, S., Benitez, J.-P., Dierckx, A., Dumonceau, F., Rollin, X., & Ovidio, M. (2023). Space and Time Use of European Eel Restocked in Upland Continental Freshwaters, a Long-Term Telemetry Study. Fishes, 8(3), 137. https://doi.org/10.3390/fishes8030137