The Responses of Sediment Bacterial Communities in Chinese Mitten Crab (Eriocheir sinensis) Culture Ponds to Changes in Physicochemical Properties Caused by Sediment Improvement
Abstract
:1. Introduction
2. Materials and Methods
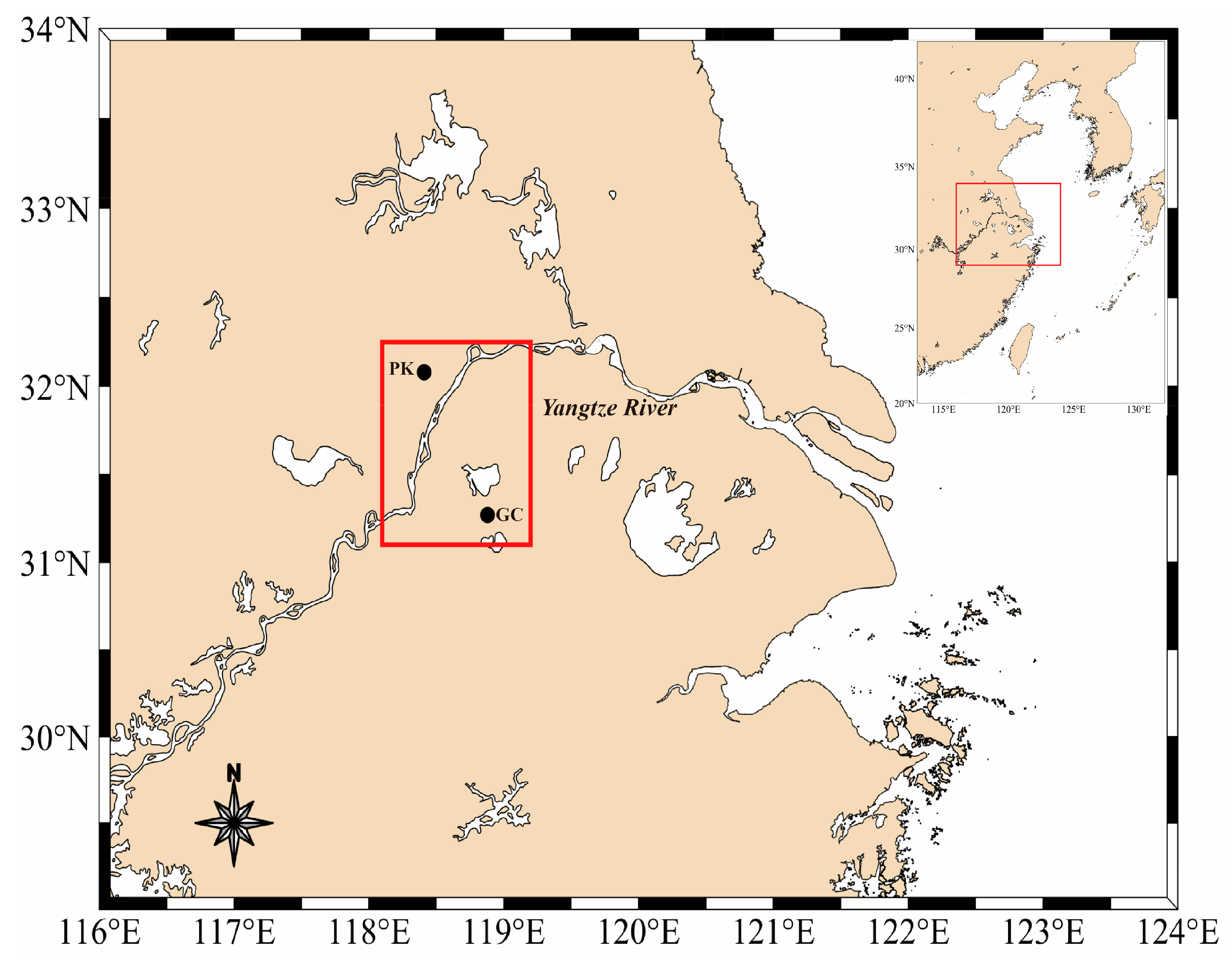
2.1. Study Site and Sampling
2.2. Determination of Physicochemical Properties and Metal Elements
2.3. DNA Extraction, PCR Amplification, and Sequencing Analysis
2.4. Bioinformatic Analysis
2.5. Statistical Analyses
3. Results
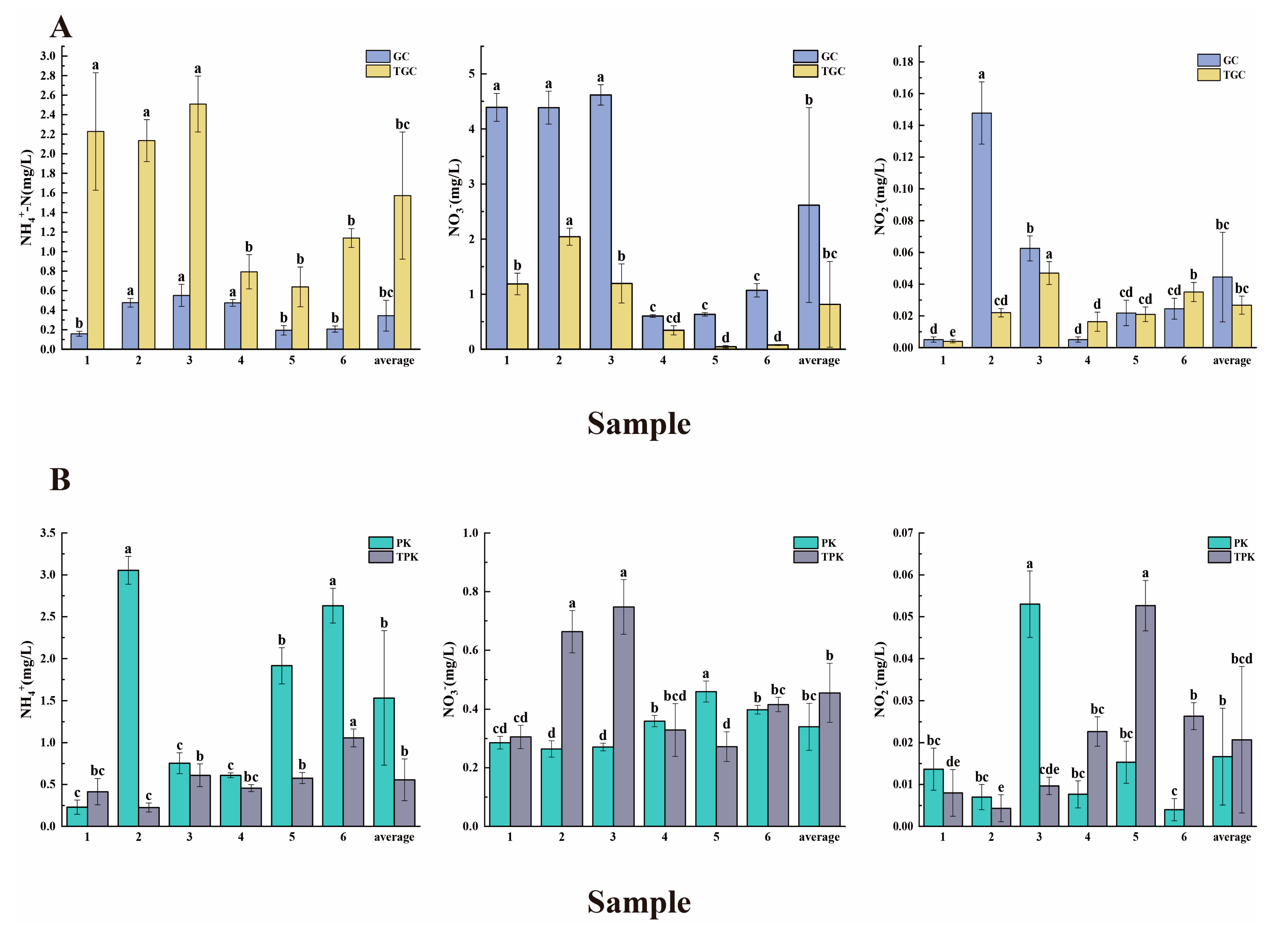
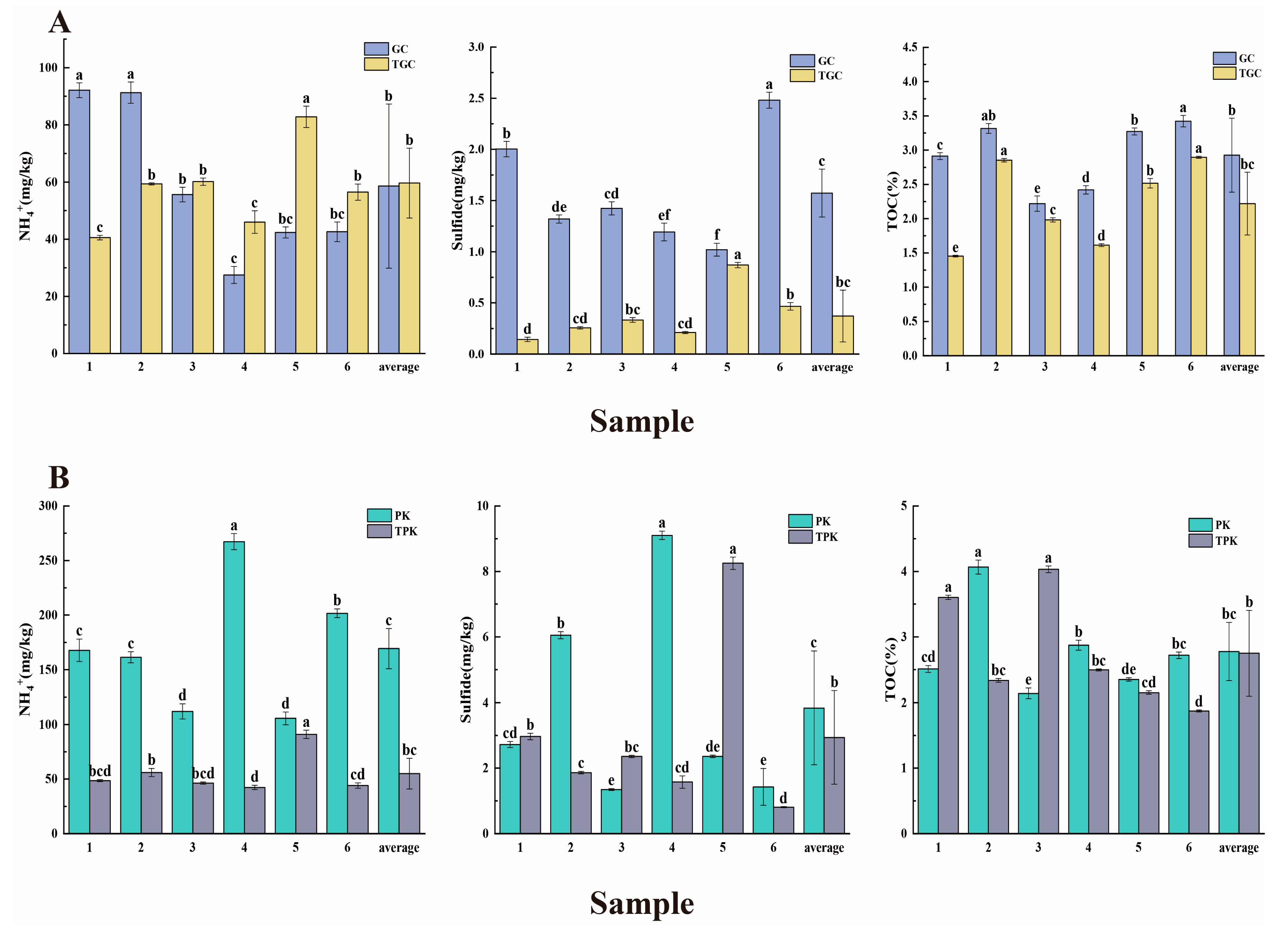
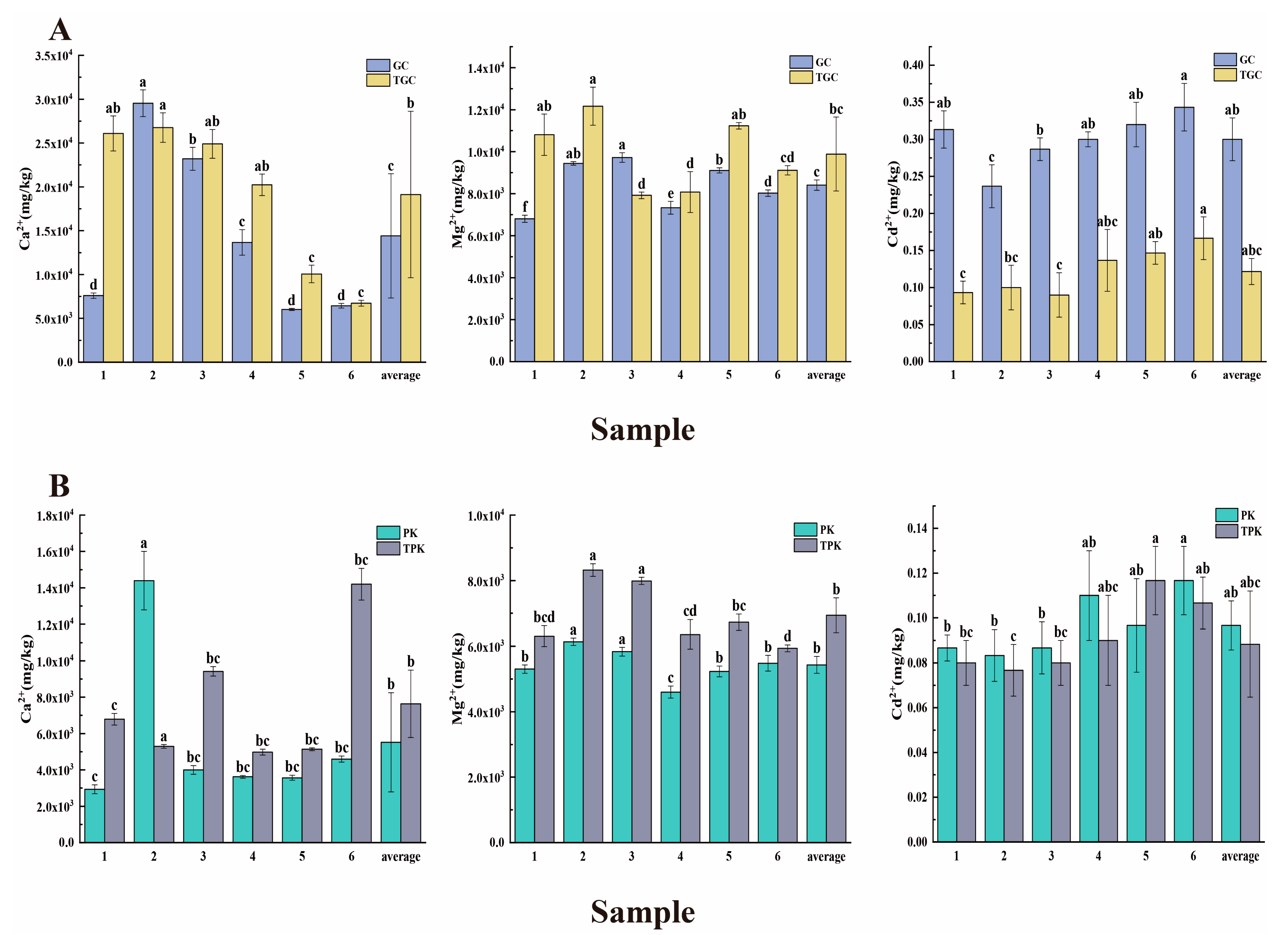
3.1. Physicochemical Characteristics of Water and Sediment Samples
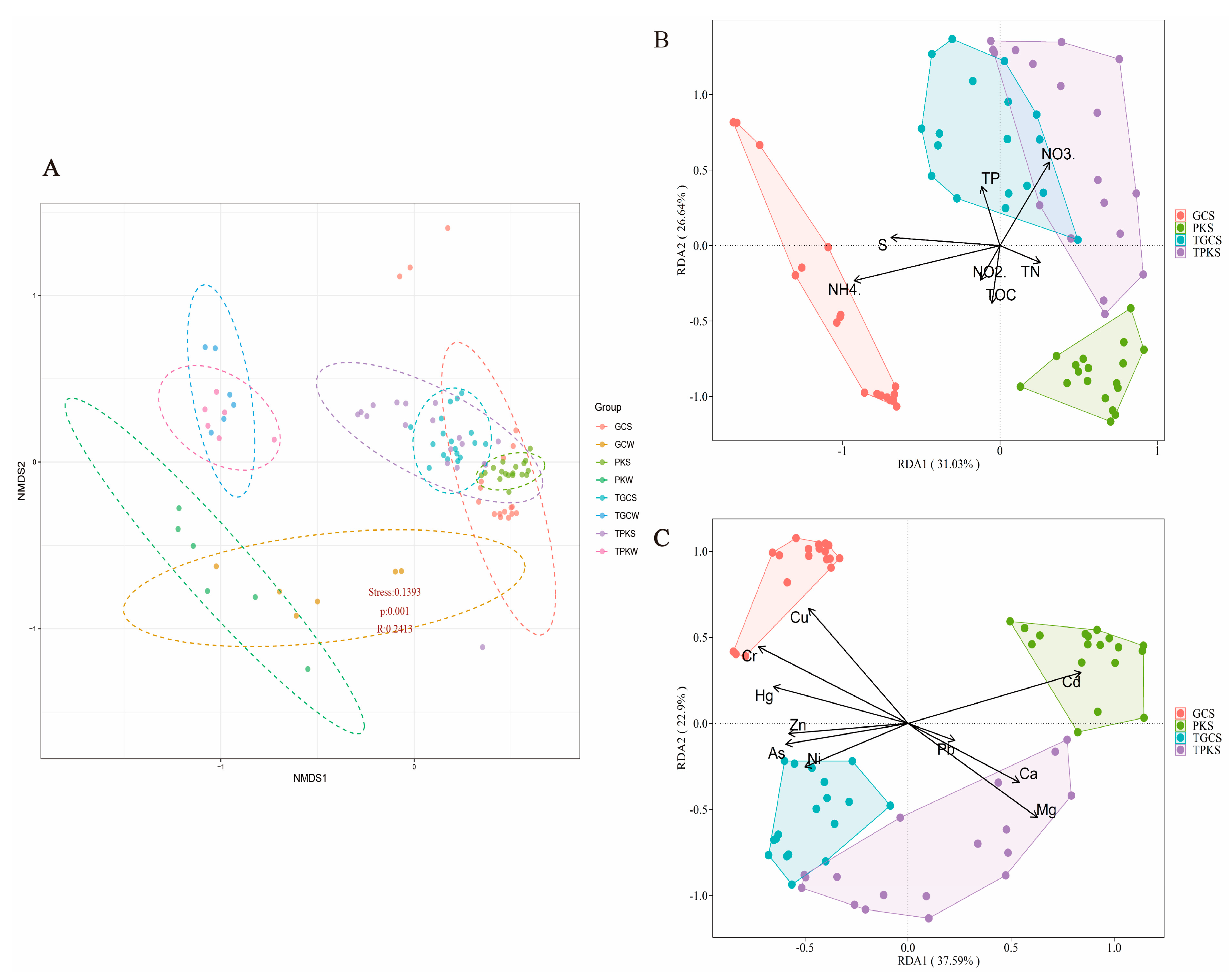
3.2. Diversity, Richness, and Structure of Microbial Communities
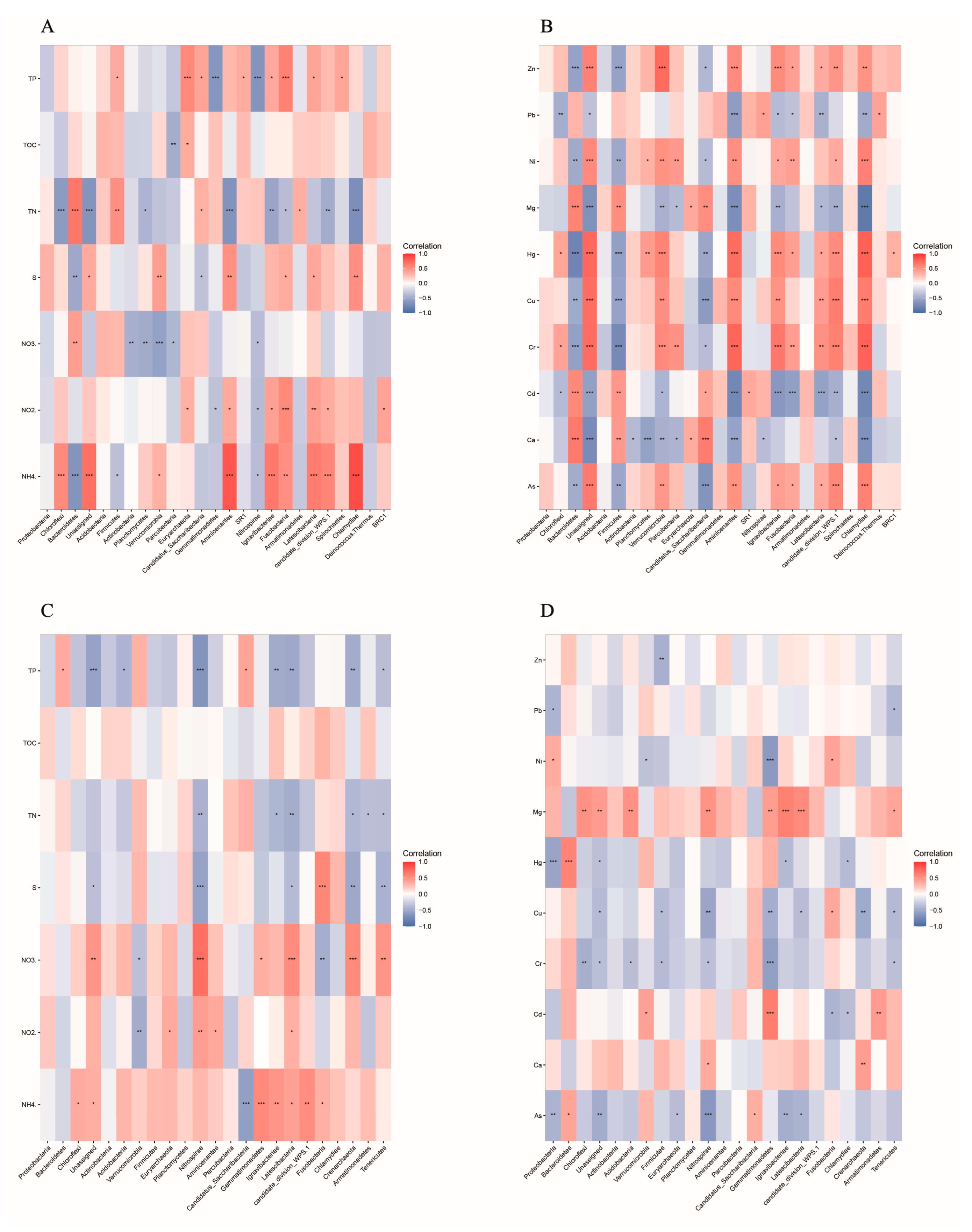
3.3. Relationships between Bacterial Community and Environmental Factors
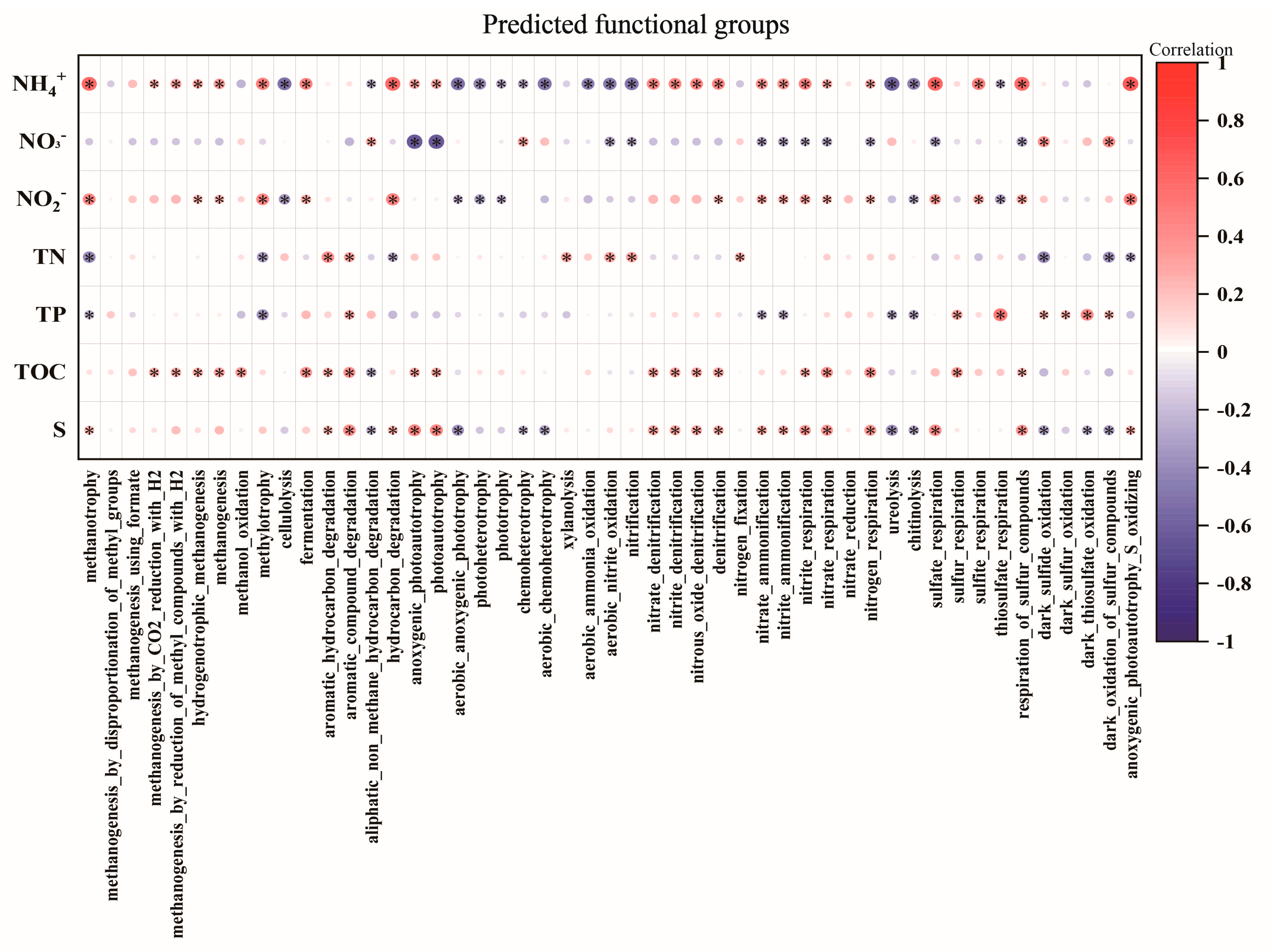
3.4. Predicted Function of Bacterial Community
4. Discussion
4.1. The Interconnection between Environmental Factors and Aquaculture Activities
4.2. Microbial Diversity in Response to Aquaculture Activities
4.3. Environmental Factors Affecting Bacterial Community and Function of Sediments
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Z.; Deng, Q.; Wan, L.; Cao, X.; Zhou, Y.; Song, C. Bacterial Communities and Enzymatic Activities in Sediments of Long-Term Fish and Crab Aquaculture Ponds. Microorganisms 2021, 9, 501. [Google Scholar] [CrossRef] [PubMed]
- Van Tung, T.; Tran, Q.; Thao, N.P.; Vi, L.; Hieu, T.; Le, S.; Tuan, N.; Sonne, C.; Lam, S.; Hai, L.; et al. Recycling of aquaculture wastewater and sediment for sustainable corn and water spinach production. Chemosphere 2021, 268, 129329. [Google Scholar] [CrossRef] [PubMed]
- Baskaran, V.; Patil, P.; Antony, M.; Avunje, S.; Nagaraju, V.; Ghate, S.; Nathamuni, S.; Dineshkumar, N.; Alavandi, S.; Vijayan, K. Microbial community profiling of ammonia and nitrite oxidizing bacterial enrichments from brackishwater ecosystems for mitigating nitrogen species. Sci. Rep. 2020, 10, 5201. [Google Scholar] [CrossRef] [PubMed]
- Rezk, R.; Galmed, A.; Abdelkreem, M.; Ghany, N.A.; Harith, M. Detachment of Cu (II) and Co (II) ions from synthetic wastewater via adsorption on Lates niloticus fish bones using LIBS and XRF. J. Adv. Res. 2018, 14, 1–9. [Google Scholar] [CrossRef]
- Bere, T.; Dalu, T.; Mwedzi, T. Detecting the impact of heavy metal contaminated sediment on benthic macroinvertebrate communities in tropical streams. Sci. Total Environ. 2016, 572, 147–156. [Google Scholar] [CrossRef]
- Gan, Y.; Wang, L.; Yang, G.; Dai, J.; Wang, R.; Wang, W. Multiple factors impact the contents of heavy metals in vegetables in high natural background area of China. Chemosphere 2017, 184, 1388–1395. [Google Scholar] [CrossRef]
- Peng, F.; Pan, C.; Zhang, N.; Braak, C.T.; Salvito, D.; Selck, H.; Ying, G.; Van den Brink, P. Benthic invertebrate and microbial biodiversity in sub-tropical urban rivers: Correlations with environmental variables and emerging chemicals. Sci. Total Environ. 2020, 709, 136281. [Google Scholar] [CrossRef]
- Yu, Z.; He, Z.; Tao, X.; Zhou, J.; Yang, Y.; Zhao, M.; Zhang, X.; Zheng, Z.; Yuan, T.; Liu, P.; et al. The shifts of sediment microbial community phylogenetic and functional structures during chromium (VI) reduction. Ecotoxicology 2016, 25, 1759–1770. [Google Scholar] [CrossRef]
- Zhuang, M.; Sanganyado, E.; Li, P.; Liu, W. Distribution of microbial communities in metal-contaminated nearshore sediment from Eastern Guangdong, China. Environ. Pollut. 2019, 250, 482–492. [Google Scholar] [CrossRef]
- Fang, J.; Zhao, R.; Cao, Q.; Quan, Q.; Sun, R.; Liu, J. Effects of emergent aquatic plants on nitrogen transformation processes and related microorganisms in a constructed wetland in northern China. Plant Soil 2019, 443, 473–492. [Google Scholar] [CrossRef]
- Wu, H.; Li, Y.; Zhang, W.; Wang, C.; Wang, P.; Niu, L.; Du, J.; Gao, Y. Bacterial community composition and function shift with the aggravation of water quality in a heavily polluted river. J. Environ. Manag. 2019, 237, 433–441. [Google Scholar] [CrossRef]
- Wang, F.; Dong, W.; Zhao, Z.; Wang, H.; Li, W.; Chen, G.; Wang, F.; Zhao, Y.; Huang, J.; Zhou, T. Heavy metal pollution in urban river sediment of different urban functional areas and its influence on microbial community structure. Sci. Total Environ. 2021, 778, 146383. [Google Scholar] [CrossRef]
- Akinwole, P.; Kan, J.; Kaplan, L.; Findlay, R. Spatial Variability in Streambed Microbial Community Structure across Two Watersheds. Microbiol. Spectr. 2021, 9, 159. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, J.; Li, H.; Yang, H.; Peng, C.; Peng, Z.; Lu, L. Shift in the microbial community composition of surface water and sediment along an urban river. Sci. Total Environ. 2018, 627, 600–612. [Google Scholar] [CrossRef]
- Shao, C.; Zhao, W.; Li, N.; Li, Y.; Zhang, H.; Li, J.; Xu, Z.; Wang, J.; Gao, T. Gut Microbiome Succession in Chinese Mitten Crab Eriocheir sinensis During Seawater-Freshwater Migration. Front. Microbiol. 2022, 13, 858508. [Google Scholar] [CrossRef]
- Liu, Y.; Qin, Y.; Chen, T.; Lu, M.; Qian, X.; Guo, X.; Bai, Y. A practical guide to amplicon and metagenomic analysis of microbiome data. Protein Cell 2021, 12, 315–330. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.; Tiedje, J.; Cole, J. Naive Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microbiol. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Louca, S.; Parfrey, L.; Doebeli, M. Decoupling function and taxonomy in the global ocean microbiome. Science 2016, 353, 1272–1277. [Google Scholar] [CrossRef]
- Louca, S.; Doebeli, M. Efficient comparative phylogenetics on large trees. Bioinformatics 2018, 34, 1053–1055. [Google Scholar] [CrossRef]
- Qin, F.; Shen, T.; Yang, H.; Qian, J.; Zou, D.; Li, J.; Liu, H.; Zhang, Y.; Song, X. Dietary nano cerium oxide promotes growth, relieves ammonia nitrogen stress, and improves immunity in crab (Eriocheir sinensis). Fish Shellfish Immunol. 2019, 92, 367–376. [Google Scholar] [CrossRef]
- Lake, P.; Palmer, M.; Biro, P.; Cole, J.; Covich, A.; Dahm, C.; Gibert, J.; Goedkoop, W.; Martens, K.; Verhoeven, J. Global change and the biodiversity of freshwater ecosystems: Impacts on linkages between above-sediment and sediment biota. Bioscience 2000, 50, 1099–1107. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.; Liu, P.; Sun, Y.; Song, Z.; Hu, X. Characteristics of bacterial community structure and function associated with nutrients and heavy metals in coastal aquaculture area. Environ. Pollut. 2021, 275, 116639. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Li, Y. Distributions and sources of heavy metals in sediments of the Bohai Sea, China: A review. Environ. Sci. Pollut. Res. Int. 2017, 24, 24753–24764. [Google Scholar] [CrossRef]
- Lv, J.; Hu, R.; Wang, N.; Zhu, L.; Zhang, X.; Yuan, X.; Liu, B. Distribution and movement of heavy metals in sediments around the coastal areas under the influence of multiple factors: A case study from the junction of the Bohai Sea and the Yellow Sea. Chemosphere 2021, 278, 130352. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Zhang, X.; Wei, J.; Sun, X.; Yuan, J.; Li, F.; Xiang, J. Whole Transcriptome Analysis Provides Insights into Molecular Mechanisms for Molting in Litopenaeus vannamei. PLoS ONE 2015, 10, e0144350. [Google Scholar] [CrossRef]
- Liu, S.; Wang, X.; Bu, X.; Zhang, C.; Qiao, F.; Qin, C.; Li, E.; Qin, J.; Chen, L. Influences of dietary vitamin D3 on growth, antioxidant capacity, immunity and molting of Chinese mitten crab (Eriocheir sinensis) larvae. J. Steroid Biochem. Mol. Biol. 2021, 210, 105862. [Google Scholar] [CrossRef]
- Chen, H.; Dillaman, R.; Roer, R.; Watson, R. Stage-specific changes in calcium concentration in crustacean (Callinectes sapidus) Y-organs during a natural molting cycle, and their relation to the hemolymphatic ecdysteroid titer. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2012, 163, 170–173. [Google Scholar] [CrossRef]
- Weiner, A.; Chen, H.; Roegner, M.; Watson, R. Calcium signaling and regulation of ecdysteroidogenesis in crustacean Y-organs. Gen. Comp. Endocrinol. 2021, 314, 113901. [Google Scholar] [CrossRef]
- Dai, L.; Liu, C.; Peng, L.; Song, C.; Li, X.; Tao, L.; Li, G. Different distribution patterns of microorganisms between aquaculture pond sediment and water. J. Microbiol. 2021, 59, 376–388. [Google Scholar] [CrossRef]
- Xu, M.; Xu, R.; Shen, X.; Gao, P.; Xue, Z.; Huang, D.; Jin, G.; Li, C.; Cao, J. The response of sediment microbial communities to temporal and site-specific variations of pollution in interconnected aquaculture pond and ditch systems. Sci. Total Environ. 2022, 806 Pt 1, 150498. [Google Scholar] [CrossRef]
- Yi, Y.; Lin, C.; Wang, W.; Song, J. Habitat and seasonal variations in bacterial community structure and diversity in sediments of a Shallow lake. Ecol. Indic. 2021, 120, 1212. [Google Scholar] [CrossRef]
- Hou, D.; Zhou, R.; Zeng, S.; Wei, D.; Deng, X.; Xing, C.; Weng, S.; He, J.; Huang, Z. Stochastic processes shape the bacterial community assembly in shrimp cultural pond sediments. Appl. Microbiol. Biotechnol. 2021, 105, 5013–5022. [Google Scholar] [CrossRef]
- Fan, L.; Barry, K.; Hu, G.; Meng, S.; Song, C.; Qiu, L.; Zheng, Y.; Wu, W.; Qu, J.; Chen, J.; et al. Characterizing bacterial communities in tilapia pond surface sediment and their responses to pond differences and temporal variations. World J. Microbiol. Biotechnol. 2017, 33, 1. [Google Scholar] [CrossRef]
- Nho, S.; Abdelhamed, H.; Paul, D.; Park, S.; Mauel, M.; Karsi, A.; Lawrence, M. Taxonomic and Functional Metagenomic Profile of Sediment from a Commercial Catfish Pond in Mississippi. Front. Microbiol. 2018, 9, 2855. [Google Scholar] [CrossRef]
- Kusunur, A.; Velayudhan, L.; Vaiyapuri, M.; Gaurav, R.; Tripathi, G.; Kurcheti, P.; Badireddy, M.; Joseph, T. Microbial diversity and composition in acidic sediments of freshwater finfish culture ponds fed with two types of feed: A metagenomic approach. Lett. Appl. Microbiol. 2022, 75, 171–181. [Google Scholar] [CrossRef]
- Hou, D.; Huang, Z.; Zeng, S.; Liu, J.; Weng, S.; He, J. Comparative analysis of the bacterial community compositions of the shrimp intestine, surrounding water and sediment. J. Appl. Microbiol. 2018, 125, 792–799. [Google Scholar] [CrossRef]
- Wei, D.; Xing, C.; Hou, D.; Zeng, S.; Zhou, R.; Yu, L.; Wang, H.; Deng, Z.; Weng, S.; He, J.; et al. Distinct bacterial communities in the environmental water, sediment and intestine between two crayfish-plant coculture ecosystems. Appl. Microbiol. Biotechnol. 2021, 105, 5087–5101. [Google Scholar] [CrossRef]
- Zeng, S.; Khoruamkid, S.; Kongpakdee, W.; Wei, D.; Yu, L.; Wang, H.; Deng, Z.; Weng, S.; Huang, Z.; He, J.; et al. Dissimilarity of microbial diversity of pond water, shrimp intestine and sediment in Aquamimicry system. AMB Express 2020, 10, 180. [Google Scholar] [CrossRef]
- Gan, Y.; Zhao, Q.; Ye, Z. Denitrification performance and microbial diversity of immobilized bacterial consortium treating nitrate micro-polluted water. Bioresour. Technol. 2019, 281, 351–358. [Google Scholar] [CrossRef]
- Cheng, W.; Zhang, J.; Wang, Z.; Wang, M.; Xie, S. Bacterial communities in sediments of a drinking water reservoir. Ann. Microbiol. 2014, 64, 875–878. [Google Scholar] [CrossRef]
- Baki, M.; Hossain, M.; Akter, J.; Quraishi, S.; Shojib, M.H.; Ullah, A.A.; Khan, M. Concentration of heavy metals in seafood (fishes, shrimp, lobster and crabs) and human health assessment in Saint Martin Island, Bangladesh. Ecotoxicol. Environ. Saf. 2018, 159, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Ye, Y.; Hu, Y.; Shi, H. The variation in microbial community structure under different heavy metal contamination levels in paddy soils. Ecotoxicol. Environ. Saf. 2019, 180, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Yang, Y.; Wu, Z.; Feng, Q.; Xie, S.; Liu, Y. Spatiotemporal variation of planktonic and sediment bacterial assemblages in two plateau freshwater lakes at different trophic status. Appl. Microbiol. Biotechnol. 2016, 100, 4161–4175. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Chen, X.; Jiang, X.; Zheng, B. Characterization of sediment bacterial communities in plain lakes with different trophic statuses. Microbiologyopen 2017, 6, 732. [Google Scholar] [CrossRef]
- Mooshammer, M.; Hofhansl, F.; Frank, A.; Wanek, W.; Hammerle, I.; Leitner, S.; Schnecker, J.; Wild, B.; Watzka, M.; Keiblinger, K.; et al. Decoupling of microbial carbon, nitrogen, and phosphorus cycling in response to extreme temperature events. Sci. Adv. 2017, 3, 144. [Google Scholar] [CrossRef]
- Bottcher, M.; Hespenheide, B.; Brumsack, H.; Bosselmann, K. Stable isotope biogeochemistry of the sulfur cycle in modern marine sediments: I. Seasonal dynamics in a temperate intertidal sandy surface sediment. Isotopes Environ. Health Stud. 2004, 40, 267–283. [Google Scholar] [CrossRef]
- Sorensen, J. Nitrate reduction in marine sediment: Pathways and interactions with iron and sulfur cycling. Geomicrobiol. J. 1987, 5, 401–421. [Google Scholar] [CrossRef]
- Araújo, J.; Otero, X.; Marques, A.; Nóbrega, G.; Silva, J.; Ferreira, T. Selective geochemistry of iron in mangrove soils in a semiarid tropical climate: Effects of the burrowing activity of the crabs Ucides cordatus and Uca maracoani. Geo-Mar. Lett. 2011, 32, 289–300. [Google Scholar] [CrossRef]
- Buongiorno, J.; Herbert, L.; Wehrmann, L.; Michaud, A.; Laufer, K.; Roy, H.; Jorgensen, B.; Szynkiewicz, A.; Faiia, A.; Yeager, K.; et al. Complex Microbial Communities Drive Iron and Sulfur Cycling in Arctic Fjord Sediments. Appl. Environ. Microbiol. 2019, 85, 234. [Google Scholar] [CrossRef]
- Zhang, K.; Zheng, X.; He, Z.; Yang, T.; Shu, L.; Xiao, F.; Wu, Y.; Wang, B.; Li, Z.; Chen, P.; et al. Fish growth enhances microbial sulfur cycling in aquaculture pond sediments. Microb. Biotechnol. 2020, 13, 1597–1610. [Google Scholar] [CrossRef]

| Sample Type | Sediment | Water | ||||||
|---|---|---|---|---|---|---|---|---|
| Sampling period | Before sediment improvement | After sediment improvement | Before sediment improvement | After sediment improvement | ||||
| Sampling location | Gaochun District | Pukou District | Gaochun District | Pukou District | Gaochun District | Pukou District | Gaochun District | Pukou District |
| Groups | GCS | PKS | TGCS | TPKS | GCW | PKW | TGCW | TPKW |
| Sample | Zn (mg/kg) | Cr (mg/kg) | Ni (mg/kg) | Cu (mg/kg) | As (mg/kg) | Pb (mg/kg) | Hg (mg/kg) |
|---|---|---|---|---|---|---|---|
| PK1 | 92.33 ± 1.53 | 81.33 ± 2.52 | 42.33 ± 3.06 | 51.33 ± 3.51 | 11.80 ± 1.13 | 21.33 ± 2.06 | 0.20 ± 0.02 |
| PK2 | 97.67 ± 3.06 | 86.33 ± 2.89 | 38.67 ± 3.79 | 53.67 ± 4.16 | 16.60 ± 1.48 | 21.40 ± 3.20 | 0.16 ± 0.01 |
| PK3 | 93.67 ± 3.06 | 90.67 ± 0.58 | 40.67 ± 0.58 | 52.33 ± 3.21 | 26.80 ± 1.40 | 20.70 ± 2.45 | 0.15 ± 0.01 |
| PK4 | 84.67 ± 1.53 | 79.00 ± 4.36 | 37.00 ± 2.65 | 54.00 ± 2.65 | 11.57 ± 1.46 | 21.37 ± 1.78 | 0.15 ± 0.01 |
| PK5 | 90.33 ± 2.31 | 82.00 ± 4.58 | 41.67 ± 4.16 | 50.67 ± 0.58 | 12.40 ± 0.92 | 23.63 ± 1.31 | 0.15 ± 0.03 |
| PK6 | 90.67 ± 2.08 | 90.00 ± 3.00 | 41.67 ± 3.79 | 53.33 ± 3.06 | 9.18 ± 0.34 | 24.80 ± 1.93 | 0.18 ± 0.02 |
| GC1 | 88.33 ± 2.52 | 63.33 ± 4.93 | 38.33 ± 2.52 | 43.00 ± 4.58 | 8.97 ± 0.48 | 30.53 ± 1.67 | 0.10 ± 0.01 |
| GC2 | 72.00 ± 3.61 | 38.67 ± 4.51 | 22.67 ± 3.21 | 27.67 ± 3.79 | 5.53 ± 0.40 | 20.83 ± 2.49 | 0.09 ± 0.02 |
| GC3 | 80.33 ± 4.04 | 46.67 ± 3.21 | 28.33 ± 4.73 | 30.33 ± 2.08 | 5.89 ± 0.31 | 23.93 ± 2.10 | 0.09 ± 0.01 |
| GC4 | 77.00 ± 2.00 | 49.33 ± 3.06 | 32.67 ± 2.52 | 45.00 ± 1.73 | 8.97 ± 0.21 | 25.23 ± 0.95 | 0.09 ± 0.01 |
| GC5 | 85.00 ± 4.00 | 42.67 ± 1.53 | 35.33 ± 4.51 | 53.00 ± 1.73 | 11.67 ± 1.82 | 31.67 ± 1.16 | 0.12 ± 0.02 |
| GC6 | 91.33 ± 1.53 | 50.33 ± 2.52 | 33.67 ± 4.73 | 46.33 ± 2.08 | 8.22 ± 0.31 | 31.13 ± 0.67 | 0.11 ± 0.00 |
| TPK1 | 92.67 ± 3.06 | 72.00 ± 8.19 | 45.00 ± 3.46 | 45.00 ± 1.00 | 12.43 ± 1.50 | 23.27 ± 4.80 | 0.09 ± 0.01 |
| TPK2 | 94.67 ± 2.08 | 71.33 ± 6.43 | 51.00 ± 1.73 | 45.00 ± 1.00 | 12.90 ± 1.75 | 22.07 ± 3.81 | 0.10 ± 0.01 |
| TPK3 | 94.00 ± 7.55 | 66.67 ± 10.69 | 48.33 ± 6.66 | 48.33 ± 1.53 | 14.50 ± 0.96 | 23.63 ± 5.08 | 0.12 ± 0.02 |
| TPK4 | 98.33 ± 1.15 | 91.00 ± 6.08 | 52.00 ± 3.46 | 45.33 ± 2.31 | 18.87 ± 3.25 | 26.07 ± 5.22 | 0.16 ± 0.03 |
| TPK5 | 88.67 ± 3.21 | 50.00 ± 3.00 | 34.33 ± 2.08 | 38.33 ± 1.53 | 30.63 ± 5.16 | 29.90 ± 3.12 | 0.19 ± 0.01 |
| TPK6 | 82.00 ± 1.00 | 63.67 ± 5.86 | 37.67 ± 5.51 | 41.33 ± 1.15 | 21.43 ± 3.42 | 28.20 ± 2.16 | 0.15 ± 0.01 |
| TGC1 | 78.67 ± 2.08 | 51.33 ± 5.03 | 37.67 ± 4.51 | 29.33 ± 1.53 | 9.89 ± 1.16 | 22.00 ± 4.57 | 0.09 ± 0.01 |
| TGC2 | 90.33 ± 0.58 | 45.00 ± 5.00 | 44.00 ± 4.00 | 36.33 ± 0.58 | 8.68 ± 1.22 | 26.40 ± 5.15 | 0.08 ± 0.01 |
| TGC3 | 79.67 ± 0.58 | 51.00 ± 2.00 | 37.67 ± 2.89 | 34.67 ± 2.31 | 12.60 ± 0.92 | 22.87 ± 3.86 | 0.12 ± 0.01 |
| TGC4 | 88.67 ± 3.06 | 49.67 ± 3.51 | 37.33 ± 2.52 | 34.00 ± 1.00 | 11.27 ± 1.36 | 25.00 ± 1.23 | 0.12 ± 0.01 |
| TGC5 | 91.00 ± 3.46 | 44.67 ± 4.93 | 38.67 ± 4.16 | 37.00 ± 0.00 | 11.97 ± 1.01 | 20.90 ± 1.84 | 0.14 ± 0.02 |
| TGC6 | 95.00 ± 1.00 | 65.67 ± 5.03 | 35.00 ± 4.36 | 37.33 ± 1.15 | 13.90 ± 2.40 | 24.97 ± 5.72 | 0.19 ± 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, T.; Li, N.; Xue, W.; Hu, Y.; Lin, H. The Responses of Sediment Bacterial Communities in Chinese Mitten Crab (Eriocheir sinensis) Culture Ponds to Changes in Physicochemical Properties Caused by Sediment Improvement. Fishes 2023, 8, 98. https://doi.org/10.3390/fishes8020098
Gao T, Li N, Xue W, Hu Y, Lin H. The Responses of Sediment Bacterial Communities in Chinese Mitten Crab (Eriocheir sinensis) Culture Ponds to Changes in Physicochemical Properties Caused by Sediment Improvement. Fishes. 2023; 8(2):98. https://doi.org/10.3390/fishes8020098
Chicago/Turabian StyleGao, Tianheng, Nannan Li, Wenlei Xue, Yuning Hu, and Hai Lin. 2023. "The Responses of Sediment Bacterial Communities in Chinese Mitten Crab (Eriocheir sinensis) Culture Ponds to Changes in Physicochemical Properties Caused by Sediment Improvement" Fishes 8, no. 2: 98. https://doi.org/10.3390/fishes8020098
APA StyleGao, T., Li, N., Xue, W., Hu, Y., & Lin, H. (2023). The Responses of Sediment Bacterial Communities in Chinese Mitten Crab (Eriocheir sinensis) Culture Ponds to Changes in Physicochemical Properties Caused by Sediment Improvement. Fishes, 8(2), 98. https://doi.org/10.3390/fishes8020098






