Effects of Climate Variability on Two Commercial Tuna Species Abundance in the Indian Ocean
Abstract
1. Introduction
2. Materials and Methods
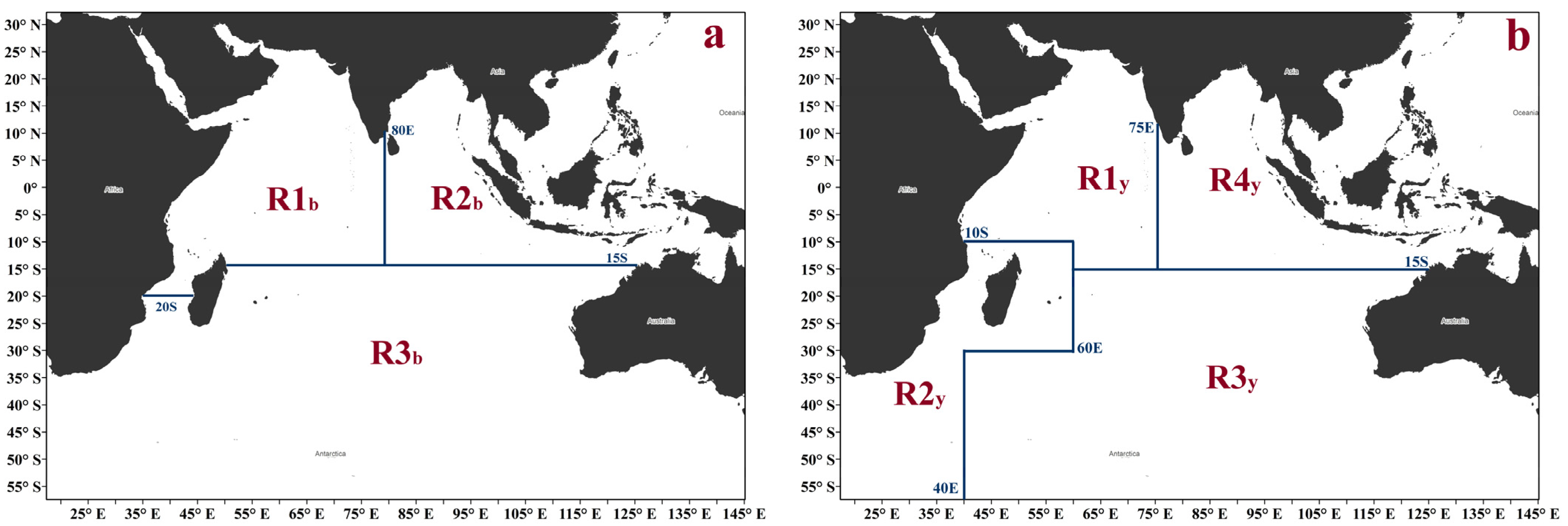
2.1. Spatial Structure
2.2. Standardized CPUE Data
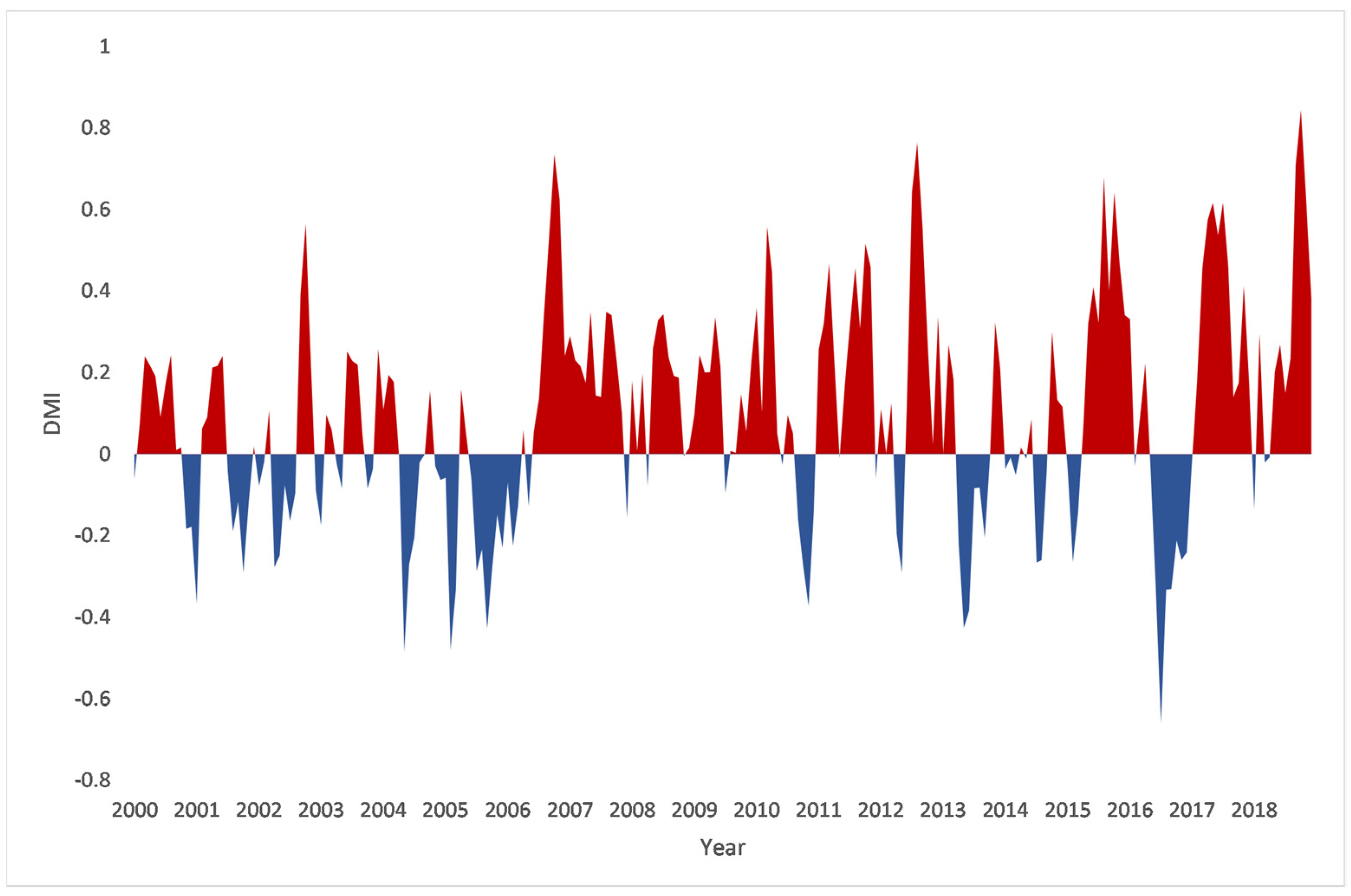
2.3. Indian Ocean Dipole
2.4. Statistical Analyses
3. Results
4. Discussion
4.1. The Reasons Why IOD Could Affect Tuna
4.2. The Effects of Climate Change Differ among Species and Regions
4.3. The Debates about the IOD Influence on Bigeye and Yellowfin Tuna in the Tropical Eastern Indian Ocean
4.4. Consider Climate Variabilities in Fishery Management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nye, J.A.; Link, J.S.; Hare, J.A.; Overholtz, W.J. Changing spatial distribution of fish stocks in relation to climate and population size on the Northeast United States continental shelf. Mar. Ecol. Prog. Ser. 2009, 393, 111–129. [Google Scholar] [CrossRef]
- Lehodey, P.; Bertignac, M.; Hampton, J.; Lewis, A.; Picaut, J. El Niño Southern Oscillation and tuna in the western Pacific. Nature 1997, 389, 715–718. [Google Scholar] [CrossRef]
- Dulvy, N.K.; Rogers, S.I.; Jennings, S.; Stelzenmüller, V.; Dye, S.R.; Skjoldal, H.R. Climate change and deepening of the North Sea fish assemblage: A biotic indicator of warming seas. J. Appl. Ecol. 2008, 45, 1029–1039. [Google Scholar] [CrossRef]
- Houde, E.; Hoyt, R. Fish early life dynamics and recruitment variability. Am. Fish. Soc. Symp. 1987, 2, 17–29. [Google Scholar]
- Rijnsdorp, A.D.; Peck, M.A.; Engelhard, G.H.; Möllmann, C.; Pinnegar, J.K. Resolving the effect of climate change on fish populations. ICES J. Mar. Sci. 2009, 66, 1570–1583. [Google Scholar] [CrossRef]
- Lehodey, P.; Chai, F.; Hampton, J. Modelling climate-related variability of tuna populations from a coupled ocean–biogeochemical-populations dynamics model. Fish. Oceanogr. 2003, 12, 483–494. [Google Scholar] [CrossRef]
- Pratchett, M.S.; Wilson, S.K.; Munday, P.L. Effects of Climate Change on Coral Reef Fishes; Cambridge University Press: Cambridge, UK, 2015; pp. 127–134. [Google Scholar]
- Guevara-Carrasco, R.; Lleonart, J. Dynamics and fishery of the Peruvian hake: Between nature and man. J. Mar. Syst. 2008, 71, 249–259. [Google Scholar] [CrossRef]
- Lehodey, P.; Bertrand, A.; Hobday, A.J.; Kiyofuji, H.; McClatchie, S.; Menkès, C.E.; Pilling, G.; Polovina, J.; Tommasi, D. ENSO impact on marine fisheries and ecosystems. In El Niño Southern Oscillation in a Changing Climate; American Geophysical Union: Washington, DC, USA, 2020; pp. 429–451. [Google Scholar] [CrossRef]
- Báez, J.C.; Real, R. The North Atlantic Oscillation affects landings of anchovy Engraulis encrasicolus in the Gulf of Cádiz (South of Spain). J. Appl. Ichthyol. 2011, 27, 1232–1235. [Google Scholar] [CrossRef]
- Saji, N.H.; Goswami, B.N.; Vinayachandran, P.N.; Yamagata, T. A dipole mode in the tropical Indian Ocean. Nature 1999, 401, 360–363. [Google Scholar] [CrossRef]
- Saji, N.H.; Yamagata, T. Possible impacts of Indian Ocean Dipole mode events on global climate. Clim. Res. 2003, 25, 151–169. [Google Scholar] [CrossRef]
- Feng, M.; Meyers, G. Interannual variability in the tropical Indian Ocean: A two-year time-scale of Indian Ocean dipole. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2003, 50, 2263–2284. [Google Scholar] [CrossRef]
- Brill, R.W.; Block, B.A.; Boggs, C.H.; Bigelow, K.A.; Freund, E.V.; Marcinek, D.J. Horizontal movements and depth distribution of large adult yellowfin tuna (Thunnus albacares) near the Hawaiian Islands, recorded using ultrasonic telemetry: Implications for the physiological ecology of pelagic fishes. Mar. Biol. 1999, 133, 395–408. [Google Scholar] [CrossRef]
- Cayré, P.; Marsac, F. Modelling the yellowfin tuna (Thunnus albacares) vertical distribution using sonic tagging results and local environmental parameters. Aquat. Living Resour. 1993, 6, 1–14. [Google Scholar] [CrossRef]
- Zhu, G.P.; Xu, L.X.; Zhou, Y.Q.; Song, L.M. Reproductive Biology of Yellowfin Tuna T. albacares in the West-Central Indian Ocean. J. Ocean. Univ. China 2008, 7, 327–332. [Google Scholar] [CrossRef]
- Lowe, T.E.; Brill, R.W.; Cousins, K.L. Blood oxygen-binding characteristics of bigeye tuna (Thunnus obesus), a high-energy-demand teleost that is tolerant of low ambient oxygen. Mar. Biol. 2000, 136, 1087–1098. [Google Scholar] [CrossRef]
- Brill, R.W.; Bigelow, K.A.; Musyl, M.K.; Fritsches, K.A.; Warrant, E.J. Bigeye tuna (Thunnus obesus) behavior and physiology and their relevance to stock assessments and fishery biology. Col. Vol. Sci. Pap. ICCAT 2005, 57, 142–161. [Google Scholar]
- Dagorn, L.; Holland, K.N.; Itano, D.G. Behavior of yellowfin (Thunnus albacares) and bigeye (T. obesus) tuna in a network of fish aggregating devices (FADs). Mar. Biol. 2007, 151, 595–606. [Google Scholar] [CrossRef]
- Holland, K.N. Horizontal and vertical movements of yellowfin and bigeye tuna associated with fish aggregating devices. Fish Bull. 1990, 88, 493–507. [Google Scholar]
- Sabarros, P.S.; Romanov, E.V.; Bach, P. Vertical Behavior and Habitat Preferences of Yellowfin and Bigeye Tuna in the South West Indian Ocean Inferred from PSAT Tagging Data. IOTC–2015–WPTT17–42. 2015. Available online: https://iotc.org/documents/vertical-behavior-and-habitat-utilization-yellowfin-and-bigeye-tuna-south-west-indian (accessed on 6 February 2022).
- Lan, K.-W.; Evans, K.; Lee, M.-A. Effects of climate variability on the distribution and fishing conditions of yellowfin tuna (Thunnus albacares) in the western Indian Ocean. Clim. Chang. 2012, 119, 63–77. [Google Scholar] [CrossRef]
- Lumban-Gaol, J.; Leben, R.R.; Vignudelli, S.; Mahapatra, K.; Okada, Y.; Nababan, B.; Mei-Ling, M.; Amri, K.; Arhatin, R.E.; Syahdan, M. Variability of satellite-derived sea surface height anomaly, and its relationship with Bigeye tuna (Thunnus obesus) catch in the Eastern Indian Ocean. Eur. J. Remote Sens. 2015, 48, 465–477. [Google Scholar] [CrossRef]
- Vincent, M.T.; Hampton, J. Incorporation of updated growth information within the 2017 WCPO bigeye stock assessment grid, and examination of the sensitivity of estimates to alternative model spatial structures. In Proceedings of the 14th Regular Session of the Scientific Committee of the WCPFC, Busan, Republic of Korea, 8–16 August 2018; Available online: https://meetings.wcpfc.int/node/10717 (accessed on 6 February 2022).
- Fu, D. Preliminary Indian Ocean Bigeye Tuna Stock Assessment 1950–2018 (Stock Synthesis). IOTC–2019–WPTT21–61. 2019. Available online: https://iotc.org/documents/preliminary-indian-ocean-bigeye-tuna-stock-assessment-1950-2018-ss3 (accessed on 6 February 2023).
- Urtizberea, A.; Fu, D.; Merino, G.; Methot, R.; Cardinale, M.; Winker, H.; Walter, J.; Murua, H. Preliminary Assessment of Indian Ocean Yellowfin Tuna 1950–2018 (Stock Synthesis, v3. 30). IOTC-2019-WPTT21-50. 2019. Available online: https://iotc.org/documents/WPTT/21/50 (accessed on 6 February 2023).
- ISSF. Status of the World Fisheries for Tuna; ISSF Technical Report 2022-15; International Seafood Sustainability Foundation: Pittsburgh, PA, USA, 2022. [Google Scholar]
- Maunder, M.N.; Punt, A.E. Standardizing catch and effort data: A review of recent approaches. Fish. Res. 2004, 70, 141–159. [Google Scholar] [CrossRef]
- Holye, S.D.; Chang, S.T.; Fu, D.; Kim, D.N.; Lee, S.I.; Matsumoto, T.; Chassot, E.; Yeh, Y.M. Collaborative Study of Bigeye and Yellowfin Tuna CPUE from Multiple Indian Ocean Longline Fleets in 2019, with Consideration of Discarding. IOTC-2019-WPM10-16. 2019. Available online: https://iotc.org/documents/WPM/10/16 (accessed on 6 February 2023).
- Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control. 1974, 19, 716–723. [Google Scholar] [CrossRef]
- Cai, W.; Cowan, T.; Sullivan, A. Recent unprecedented skewness towards positive Indian Ocean Dipole occurrences and its impact on Australian rainfall. Geophys. Res. Lett. 2009, 36, 11. [Google Scholar] [CrossRef]
- Ratna, S.B.; Cherchi, A.; Osborn, T.J.; Joshi, M.; Uppara, U. The extreme positive Indian Ocean dipole of 2019 and associated indian summer monsoon rainfall response. Geophys. Res. Lett. 2021, 48, e2020GL091497. [Google Scholar] [CrossRef]
- Corbineau, A.; Rouyer, T.; Cazelles, B.; Fromentin, J.-M.; Fonteneau, A.; Menard, F. Time series analysis of tuna and swordfish catches and climate variability in the Indian Ocean (1968–2003). Aquat. Living Resour. 2008, 21, 277–285. [Google Scholar] [CrossRef]
- Horii, T.; Ueki, I.; Ando, K. Coastal upwelling events along the southern coast of Java during the 2008 positive Indian Ocean Dipole. J. Oceanogr. 2018, 74, 499–508. [Google Scholar] [CrossRef]
- Deshpande, A.; Chowdary, J.S.; Gnanaseelan, C. Role of thermocline-SST coupling in the evolution of IOD events and their regional impacts. Clim. Dyn. 2014, 43, 163–174. [Google Scholar] [CrossRef]
- Amri, K.; Suman, A.; Irianto, H.E.; Wudianto, W. Effects of dipole mode and El-nino events on catches of yellowfin tuna (Thunnus albacares) in the eastern Indian ocean off west java. Indones. Fish. Res. J. 2015, 21, 75–90. [Google Scholar] [CrossRef]
- Kumar, P.S.; Pillai, G.N.; Manjusha, U. El Nino Southern Oscillation (ENSO) impact on tuna fisheries in Indian Ocean. Springerplus 2014, 3, 591. [Google Scholar] [CrossRef]
- Lynam, C.P.; Hay, S.J.; Brierley, A.S. Interannual variability in abundance of North Sea jellyfish and links to the North Atlantic Oscillation. Limnol. Oceanogr. 2004, 49, 637–643. [Google Scholar] [CrossRef]
- Rubio, C.; Macías, D.; Camiñas, J.; Fernández, I.; Báez, J. Effects of the North Atlantic Oscillation on Spanish catches of albacore, Thunnus alalunga, and yellowfin tuna, Thunnus albacares, in the North–East Atlantic Ocean. Anim. Biodivers. Conserv. 2016, 39, 195–198. [Google Scholar] [CrossRef]
- Singh, A.A.; Sakuramoto, K.; Suzuki, N. Impact of Climatic Factors on Albacore Tuna Thunnus alalunga in the South Pacific Ocean. Am. J. Clim. Chang. 2015, 4, 295–312. [Google Scholar] [CrossRef]
- Lynam, C.P.; Hay, S.J.; Brierley, A.S. Jellyfish abundance and climatic variation: Contrasting responses in oceanographically distinct regions of the North Sea, and possible implications for fisheries. J. Mar. Biol. Assoc. UK 2005, 85, 435–450. [Google Scholar] [CrossRef]
- Wu, Y.-L.; Lan, K.-W.; Tian, Y. Determining the effect of multiscale climate indices on the global yellowfin tuna (Thunnus albacares) population using a time series analysis. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2020, 175, 104808. [Google Scholar] [CrossRef]
- Marsac, F. Outlook of Ocean Climate Variability in the West Tropical Indian Ocean, 1997–2008. IOTC-2008-WPTT-27. 2008. Available online: https://iotc.org/documents/outlook-ocean-climate-variability-tropical-indian-ocean-1997-2008 (accessed on 6 February 2023).
- Marsac, F. The Seychelles tuna fishery and climate change. In Climate Change Impacts on Fisheries and Aquaculture: A Global Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2017; Volume 2, pp. 523–568. [Google Scholar] [CrossRef]
- Karp, M.A.; Peterson, J.O.; Lynch, P.D.; Griffis, R.B.; Adams, C.F.; Arnold, W.S.; Barnett, L.A.K.; deReynier, Y.; DiCosimo, J.; Fenske, K.H.; et al. Accounting for shifting distributions and changing productivity in the development of scientific advice for fishery management. ICES J. Mar. Sci. 2019, 76, 1305–1315. [Google Scholar] [CrossRef]
- Edgar, G.J.; Stuart-Smith, R.D.; Willis, T.J.; Kininmonth, S.; Baker, S.C.; Banks, S.; Barrett, N.S.; Becerro, M.A.; Bernard, A.T.F.; Berkhout, J.; et al. Global conservation outcomes depend on marine protected areas with five key features. Nature 2014, 506, 216–220. [Google Scholar] [CrossRef]
- Roberts, C.M.; O’Leary, B.C.; McCauley, D.J.; Cury, P.M.; Duarte, C.M.; Lubchenco, J.; Pauly, D.; Sáenz-Arroyo, A.; Sumaila, U.R.; Wilson, R.W.; et al. Marine reserves can mitigate and promote adaptation to climate change. Proc. Natl. Acad. Sci. USA 2017, 114, 6167–6175. [Google Scholar] [CrossRef]
- Jameson, S.C.; Tupper, M.H.; Ridley, J.M. The three screen doors: Can marine “protected” areas be effective? Mar. Pollut. Bull. 2002, 44, 1177–1183. [Google Scholar] [CrossRef]
- Curnick, D.J.; Collen, B.; Koldewey, H.J.; Jones, K.E.; Kemp, K.M.; Ferretti, F. Interactions between a large marine protected area, pelagic tuna and associated fisheries. Front. Mar. Sci. 2020, 7, 318. [Google Scholar] [CrossRef]
- Kaplan, D.M.; Chassot, E.; Amandé, J.M.; Dueri, S.; Demarcq, H.; Dagorn, L.; Fonteneau, A. Spatial management of Indian Ocean tropical tuna fisheries: Potential and perspectives. ICES J. Mar. Sci. 2014, 71, 1728–1749. [Google Scholar] [CrossRef]
- Lehodey, P.; Senina, I.; Murtugudde, R. A spatial ecosystem and populations dynamics model (SEAPODYM)–Modeling of tuna and tuna-like populations. Prog. Oceanogr. 2008, 78, 304–318. [Google Scholar] [CrossRef]
- Bell, J.D.; Ganachaud, A.; Gehrke, P.C.; Griffiths, S.P.; Hobday, A.J.; Hoegh-Guldberg, O.; Johnson, J.E.; Le Borgne, R.; Lehodey, P.; Lough, J.M.; et al. Mixed responses of tropical Pacific fisheries and aquaculture to climate change. Nat. Clim. Chang. 2013, 3, 591–599. [Google Scholar] [CrossRef]
- Lehodey, P.; Senina, I.; Calmettes, B.; Hampton, J.; Nicol, S. Modelling the impact of climate change on Pacific skipjack tuna population and fisheries. Clim. Chang. 2013, 119, 95–109. [Google Scholar] [CrossRef]
- Lehodey, P.; Senina, I.; Nicol, S.; Hampton, J. Modelling the impact of climate change on South Pacific albacore tuna. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2015, 113, 246–259. [Google Scholar] [CrossRef]
- Loukos, H.; Monfray, P.; Bopp, L.; Lehodey, P. Potential changes in skipjack tuna (Katsuwonus pelamis) habitat from a global warming scenario: Modelling approach and preliminary results. Fish. Oceanogr. 2003, 12, 474–482. [Google Scholar] [CrossRef]
- Cheung, W.W.L.; Lam, V.W.Y.; Sarmiento, J.L.; Kearney, K.; Watson, R.; Pauly, D. Projecting global marine biodiversity impacts under climate change scenarios. Fish Fish. 2009, 10, 235–251. [Google Scholar] [CrossRef]
- Blanchard, J.L.; Jennings, S.; Holmes, R.; Harle, J.; Merino, G.; Allen, J.I.; Holt, J.; Dulvy, N.K.; Barange, M. Potential consequences of climate change for primary production and fish production in large marine ecosystems. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 2979–2989. [Google Scholar] [CrossRef]
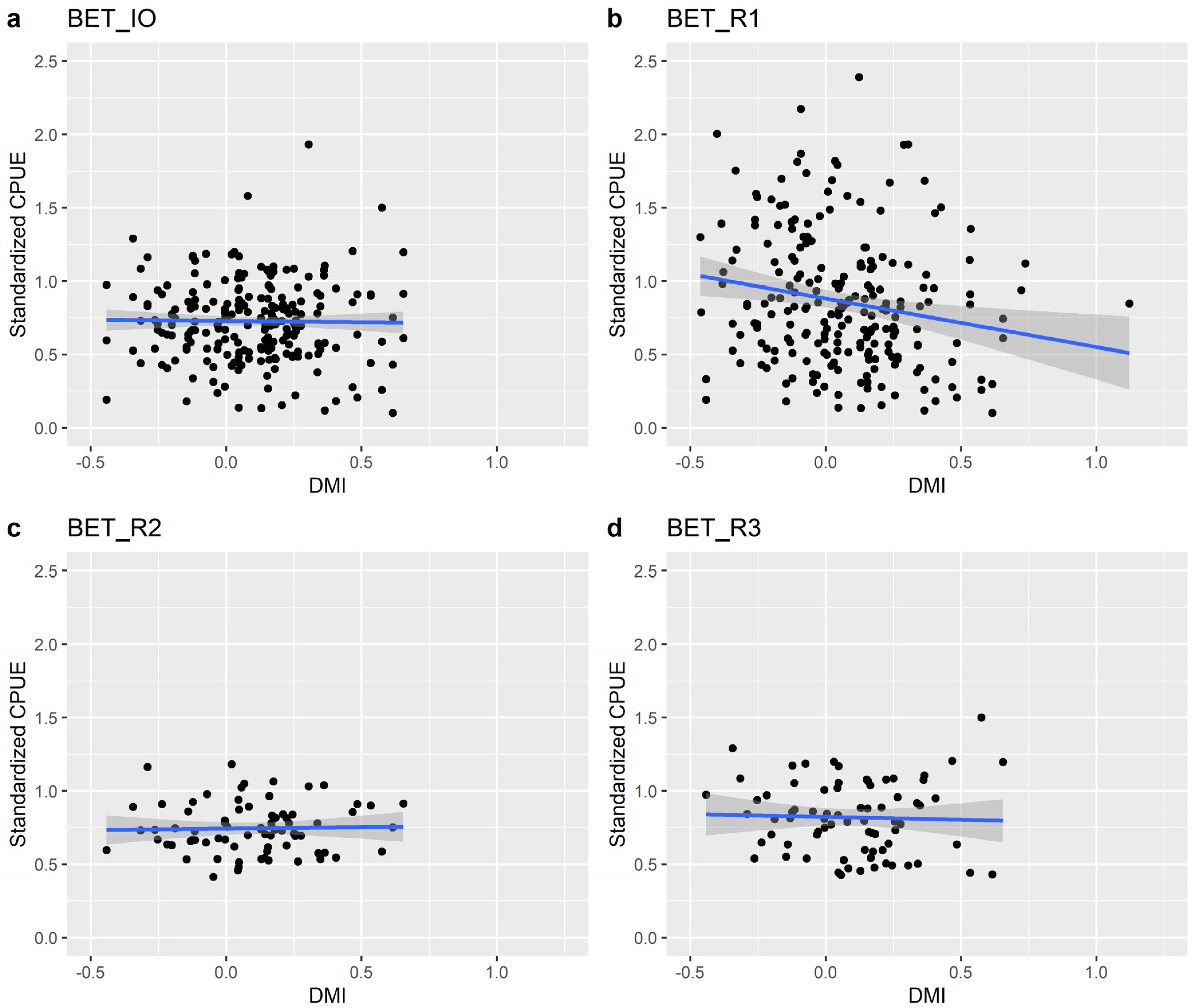

| Estimate | Std. Error | t-Value | p-Value | Adjusted R-Squared | |||
|---|---|---|---|---|---|---|---|
| IO | BET | (Intercept) DMI | 0.73 | 0.016 | |||
| −0.02 | 0.063 | −0.248 | 0.804 | −0.003 | |||
| YFT | (Intercept) | 0.619 | 0.021 | ||||
| DMI | −0.17 | 0.086 | −2.057 | 0.041 | 0.011 | ||
| WIO | BET(R1b) | (Intercept) | 0.891 | 0.029 | |||
| DMI | −0.392 | 0.104 | −3.758 | 0.0002 | 0.054 | ||
| YFT(R1y) | (Intercept) | 0.612 | 0.033 | ||||
| DMI | −0.348 | 0.132 | −2.624 | 0.0106 | 0.072 | ||
| EIO | BET(R2b) | (Intercept) | 0.742 | 0.021 | |||
| DMI | 0.02 | 0.085 | 0.237 | 0.813 | −0.013 | ||
| YFT(R4y) | (Intercept) | 0.388 | 0.032 | ||||
| DMI | −0.2 | 0.127 | −1.59 | 0.116 | 0.019 | ||
| SIO | BET(R3b) | (Intercept) | 0.822 | 0.031 | |||
| DMI | −0.038 | 0.123 | −0.315 | 0.754 | −0.012 | ||
| YFT(R2y) | (Intercept) | 0.872 | 0.028 | ||||
| DMI | −0.0068 | 0.1121 | −0.061 | 0.925 | −0.013 | ||
| YFT(R3y) | (Intercept) | 0.604 | 0.051 | ||||
| DMI | −0.15 | 0.203 | −0.745 | 0.458 | −0.006 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Zhang, F.; Geng, Z.; Zhang, Y.; Zhu, J.; Dai, X. Effects of Climate Variability on Two Commercial Tuna Species Abundance in the Indian Ocean. Fishes 2023, 8, 99. https://doi.org/10.3390/fishes8020099
Wang Y, Zhang F, Geng Z, Zhang Y, Zhu J, Dai X. Effects of Climate Variability on Two Commercial Tuna Species Abundance in the Indian Ocean. Fishes. 2023; 8(2):99. https://doi.org/10.3390/fishes8020099
Chicago/Turabian StyleWang, Yang, Fan Zhang, Zhe Geng, Yuying Zhang, Jiangfeng Zhu, and Xiaojie Dai. 2023. "Effects of Climate Variability on Two Commercial Tuna Species Abundance in the Indian Ocean" Fishes 8, no. 2: 99. https://doi.org/10.3390/fishes8020099
APA StyleWang, Y., Zhang, F., Geng, Z., Zhang, Y., Zhu, J., & Dai, X. (2023). Effects of Climate Variability on Two Commercial Tuna Species Abundance in the Indian Ocean. Fishes, 8(2), 99. https://doi.org/10.3390/fishes8020099