Abstract
The sunray venus (sunray) clam, Macrocallista nimbosa, is an alternative clam species reared in hard clam hatcheries in Florida. Current feeding practices follow those used for hard clam culture. This study aimed to identify whether a hard clam bi-algal Tisochrysis lutea and Chaetoceros neogracile diet was an optimal diet for post-set sunray clams or whether other microalgal dietary combinations could improve production. Six dietary bi-, tri-, or tetra-algal combinations consisting of four microalgae species (Tisochrysis lutea, Diacronema lutheri, Chaetoceros neogracile, and Thalassiosira weissflogii) were fed for 6 weeks; the growth, survival, and fatty acid profiles of post-set clams were evaluated. Clams fed equal proportions of T. lutea, D. lutheri, C. neogracile, and T. weissflogii had higher growth, while those fed equal proportions of T. lutea and C. neogracile had higher survival. The poorest-performing diet consisted solely of diatoms. A contrasting polyunsaturated fatty acid (PUFA) profile was found in post-set clams fed flagellate- or diatom-only diets. Clams fed the bi-algal flagellate diet had a higher percentage of docosahexaenoic acid (DHA) but a lower percentage of (n-6) PUFA, whereas those fed the bi-algal diatom diet had a higher percentage of arachidonic acid (ARA) and eicosapentaenoic acid (EPA) but a lower percentage of DHA. The percentages were similar and neither very high nor very low in clams fed the remaining dietary treatments. The results of this study show that sunray venus post-set clams can be successfully produced when fed a typical hard clam bi-algal flagellate and diatom diet, but they indicate that growth may be accelerated by the addition of other microalgae species.
1. Introduction
Shellfish aquaculture in Florida is an established and economically viable industry driven mainly by the hard clam market. The northern quahog (Mercenaria mercenaria) represents 92% of total shellfish sales in Florida [1]. Industry diversification to increase economic stability has been noted, and feasibility studies have been conducted with alternative bivalve species, such as the angel wing clam (Cyrtopleura costata), bay scallop (Argopecten irradians), the blood ark (Anadara ovalis), the ponderous ark (Noetia ponderosa), and the sunray venus clam (Macrocallista nimbosa) [2,3,4,5,6,7].
The sunray venus (sunray) clam, Macrocallista nimbosa (Lightfoot, 1786), an attractive venerid clam that gets its name from the markings on its shell, is native to Atlantic waters ranging from North Carolina to Florida and the Gulf of Mexico [8]. Research conducted with this clam determined that it could be cultured using techniques similar to those used for hard clam culture [6,9]; however, little research has been conducted toward optimizing sunray culture.
Clam hatcheries are dependent on cultured microalgae as a food source for larval and post-set production [10,11,12,13]. Success at these early life stages is dependent on high-quality microalgae. Nutritional profile is the primary criteria for algal species selection although algal cell size, cell mobility and form, toxicity, and ease of culture may also be considered when choosing a microalgae species [10,11,14]. During early stages (first 7–10 days), larval clams are typically fed small flagellates, such as Isochrysis sp. Diatoms, such as Chaetoceros sp. are introduced during settlement, and a mixed diet of flagellates and diatoms is fed post settlement [13]. Due to the ease of culture, availability, and culture requirements, only a few species of microalgae are widely used in aquaculture. Species cultured in bivalve hatcheries include flagellates, such as Tisochrysis lutea, previously known as Isochrysis aff. galbana or T. Iso [15], Diacronema lutheri, Tetraselmis suecica, and diatoms, such as C. neogracile, C. muelleri, and Thalassiosira sp. [16], with most hatcheries culturing only one or two predominant flagellate and diatom species.
The nutritional value of algal species Is determined by biochemical composition, i.e., lipids, carbohydrates, and proteins [14,17]. Lipids provide the principal source of energy for bivalves, followed by protein; little energy comes from carbohydrates [18,19,20,21,22]. Growth and survival of bivalve larvae are particularly impacted by the fatty acid (FA) profile of the diet. Species rich in n-3 polyunsaturated fatty acids (PUFA), such as eicosapentaenoic acid (EPA, 20:5n-3) and docosahexaenoic acid (DHA, 22:6n-3), result in increased growth and survival of early-stage bivalves [23,24]. The n-6 PUFAs linoleic acid (LA, 18:2n-6) and arachidonic acid (ARA, 20:4n-6) are precursors of EPA and DHA synthesis in bivalves, and their ratios influence the quality of bivalve microalgal diets [25]. Bivalve shellfish must obtain these fAs from their diet as they have limited ability to synthesize essential fatty acids required for growth [26,27]. Mixed microalgae diets offer complementary nutritional benefits needed for bivalve physiological processes over that of mono-species diets and have been shown to enhance production [11,28]. The combination of flagellates and diatoms in the diet nutritionally is complementary, resulting in potentially enhanced larval development and growth [28]. Although FA profiles of algae species have been reported in the literature, they have been shown to vary on the basis of culture conditions, such as light intensity, temperature, and culture medium [29,30,31].
The cost of algal production in bivalve hatcheries is high, with the production of live algae comprising 30% of the operating costs [32,33,34]. As the Florida shellfish industry continues to grow and diversify, it is essential to determine whether the nutritional needs of these alternative bivalve species are similar to those of the hard clam. A better understanding of the nutritional profile of algal species cultured and their effects on clam production and nutritional profile may assist hatcheries in determining feed management strategies that result in increased seed production and increase their economic bottom line. To our knowledge, no research has been conducted to establish an optimal diet for the sunray clam. The objective of this study was to determine an effective live microalgal diet to maximize growth and survival for post-set sunray clams using bi-, tri-, and tetra-algal dietary combinations of Tisochrysis lutea, Diacronema lutheri, Chaetoceros neogracile, and Thalassosioria weissflogii. These species were chosen on the basis of their use in aquaculture operations, ease of culture, and the known differing FA profiles of diatoms and flagellates.
2. Methods
2.1. Spawning and Larval Production
This study was conducted at the Florida Atlantic University Harbor Branch Oceanographic Institute (FAU-HBOI) aquaculture facility. Post-set clams used in this study were obtained from sunray broodstock conditioned for 8 weeks at 21 °C and fed a mixed (50:50) diet of flagellates (T. lutea) and diatoms (C. neogracile) to satiation prior to spawning. Fertilized eggs were stocked in 700 L conical bottom larval rearing tanks filled with UV-treated 1 µm filtered seawater and maintained at 28 °C with light aeration. Larvae were fed T. lutea 25,000 algal cells/mL/day starting on day 1, with a gradual increase to 50,000 cells by day 7. Upon reaching the pediveliger stage (7 days) clams were transferred to floating setting containers (downwellers) (55 cm wide by 76 cm tall, 120 µm screen) immersed in shallow tanks. Clams were maintained on the downwellers for 2 weeks and fed a mixed diet of T. lutea and C. neogracile. Clams were then transferred to the experimental system.
2.2. Experimental System
The experimental setup consisted of 60 L round tanks each containing three mini-downwellers with airlifts (three replicate tanks per treatment). Thin-walled PVC pipes, 10 cm in diameter, were cut to 8 cm in length and placed horizontally at the bottom of the tank to support a plastic grid (5 × 5 cm wide cells) to act as a tank bottom. Downwellers were placed on top of the grid, providing a 10 cm space between the bottom of the downweller and the bottom of the tank. Downwellers were created using 15 cm wide thin-walled schedule 20 sewer-grade PVC pipes cut to 23 cm in length with a 150 µm screen secured to the bottom with a 15 cm thin-walled slip coupler (Figure 1). Each downweller was outfitted with an airlift made from 15 mm thin-walled PVC, a small air stone, and air tubing connected to an airline manifold. Airlifts were cut to 30 cm in length extended below the plastic grid bottom to ensure proper mixing and eliminate the possibility of dead zones with regard to water movement and algae settlement.
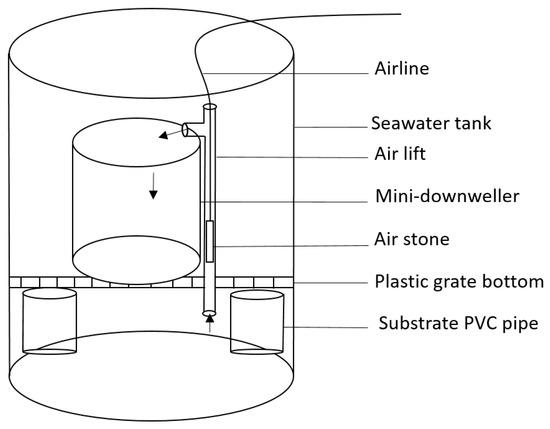
Figure 1.
Diagram of experimental post-set rearing system used in both feed studies. Arrows indicate the direction of seawater flow through airlift and downweller (figure reproduced from [35]).
Following initial measurement, 0.5 mL of post-set clams (1074 ± 39 individuals) were stocked into each downweller (n = 36). Water quality was measured to maintain optimal growing parameters for post-set clam culture (pH 7.5–8.5, DO > 5 mg/L:, temperature 27–29 °C, salinity 26–30 ppt, and total ammonia nitrogen < 0.25). Tanks were cleaned on alternate days, and 100% of the water was changed.
2.3. Algae Culture and Standardization
The four species of microalgae (T. lutea (TL), Diacronema lutheri (DL), C. neogracile (CN), and Thalassiosira weissflogii (TW)) used in this study were produced in batch culture (flasks, 18 L carboys, 200 L fiberglass suntubes) in the indoor microalgae culture facility at FAU-HBOI using UV-treated, 1 μm filtered salt well water. Temperature was maintained at 20 °C, along with salinity at 27–30 ppt and pH at 8.0–8.2, through CO2 injection. Microalgae were cultured with f/2 nutrient media [36], with metasilicates added to diatom cultures.
Size variations of microalgae species were standardized according to dry weight [37]. A total of 100 mL of each species was filtered using pre-weighted 1.2 μm GF filters in triplicate and washed with 50 mL of 0.5 M ammonium formate to remove salt residue [38]. The filters were then dried in an oven at 70 °C for 18 h. Microalgae cell concentrations were determined using a hemocytometer, and the dry weight of one algal cell was calculated on the basis of algae concentration and total dry weight of the filtered algae. The algae feed ration was calculated as a function of the clam biomass and standardized on the basis of the dry weight of each species according to the following formula: F = (S × R)/7, where F is the algae dry weight per day (mg), R is the ration as dry weight of algae per mg clam per week, and S is the weight of algae at the start of each week. As is standard practice when calculating a ration for bivalve diets incorporating two or more species, the representation of each species in the ration was calculated on a cell volume equivalency basis [26]. The dry weights (picogram/cell) of microalgae used were as follows: T. lutea, 13.1 pg; D. lutheri, 13.4 pg; C. neogracile, 24.6 pg; T. weissflogii, 75 pg.
2.4. Experimental Design
The study consisted of feeding six dietary treatments (bi-, tri-, and tetra-algal species combinations), with three replicates per treatment for 43 days (Table 1). Juveniles were starved for 24 h prior to the start of the experiment. Each tank was fed at a rate of 1,000,000 algal cells of T. lutea per clam (or equivalent cell number based on dry weight of other microalgae species), and feed rates were increased by 20% weekly.

Table 1.
Microalgae dietary treatments fed to post-set sunray venus clams (Macrocallista nimbosa).
2.5. Clam Growth and Survival
Shell lengths of post-set clams were measured prior to stocking using an Olympus S2X7 microscope (Olympus Corporation, Tokyo, Japan) outfitted with an Olympus DP71 camera and Olympus cellSens Standard imaging software (Olympus cellSens Standard, version 1.11). The initial average shell length was 0.913 ± 0.242 mm. At 2, 4, and 6 weeks, shell length measurements were taken from 20 randomly selected clams per replicate (three replicates per treatment) using a vernier caliper. At experimental termination (week 6), all live clams were counted to calculate the total percentage survival (final number of clams/Initial number of clams × 100).
2.6. Clam and Microalgae Fatty Acid Profile
At experimental termination, clams and microalgae were freeze-dried for 48 h and ground to a fine powder with a mortar and pestle. Microalgae were first filtered on a 2 μm GF filter and collected filtrate placed into 15 mL falcon tubes. Processed clams (50 to 70 mg, triplicate samples) and microalgae (20 mg, duplicate samples) were sent to Microbial ID Inc. (Newark, DE) for fatty acid analysis. The relative fatty acid content was determined using fatty acid methyl ester (FAME) analysis (MIDI technical note #101, Sasser). FAMEs were separated by gas chromatography (GC) using a hydrogen carrier gas and a nitrogen makeup gas. The electrical signal from the GC detector was compared to a stored database of the Sherlock pattern recognition software. Individual fatty acids from microalgae and post-set sunray clams are presented as the percentage of total fatty acids. The FA profile of microalgae is listed below (Table 2).

Table 2.
Fatty acid profile (as a percentage of total fatty acid) of microalgae species used to feed post-set sunray venus clams (Macrocallista nimbosa). ND: not detected. Data are presented as the means ± SD (n = 2).
2.7. Statistical Analysis
All data were tested for assumptions of normality and homogeneity of variance using Shapiro–Wilk and Levene’s tests. Survival data and fatty acid content, which are expressed as proportions, were arcsine square-root transformed prior to analysis. One-way analysis of variance (ANOVA) was used to evaluate differences among the dietary treatments. Tukey’s honestly significant difference test and a test of simple effects were used when significant differences were found among response variables. Differences among means were considered significant at p < 0.05. All data were analyzed using SPSS v. 27 (IBM, Armonk, NY, USA).
3. Results
3.1. Growth
Significant differences (p < 0.05) in growth were seen among treatments at 2, 4, and 6 weeks (Table 3). The final size of post-set clams fed various microalgae diets was significantly different (p = 0.002). The largest clams were those fed T4 (TL + DL + CN + TW), while the smallest clams were those fed T2 (CN + TW), i.e., the diatom-only diet. Tetra- and tri-algal species diets outperformed bi-algal species diets regardless of whether they were diatom- or flagellate-dominant. Although the average size was the smallest in clams fed T2, final size variations were also higher in T2 than in other treatments.

Table 3.
Size (mm, mean length ± SD) of post-set sunray venus clams (Macrocallista nimbosa) fed live microalgae diets. Different letters in the same column indicate significant differences among the treatments (one-way ANOVA, α = 0.05, a > b > c). DPF: days post fertilization.
There was a significant difference in daily growth rate among treatment groups (p = 0.006) (Figure 2). Sunray venus clams fed T4 (TL + DL + CN + TW) had the highest average daily growth (80.4 µm ± 5.8 µm), while the lowest daily growth rate was seen in T2 (CN + TW) (57.4 µm ± 10.0 µm). There was no significant difference (p < 0.05) among the three bi-algal dietary treatments or between the two tri-algal dietary treatments.
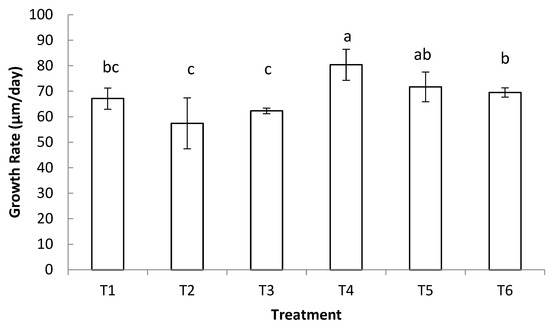
Figure 2.
Daily growth rate of post-set sunray venus clams (Macrocallista nimbosa) fed live microalgae diets. Different letters indicate significant differences among the treatments (one-way ANOVA, α = 0.05, a > b > c). Each bar represents the mean ± SD of three replicates.
3.2. Survival
The survival of post-set sunray venus clams was significantly affected by dietary treatment (p = 0.0008) (Figure 3). Clams fed T3 (TL + CN) had the highest survival (88.1% ± 6.3%), whereas the lowest survival (61.9% ± 6.7%) was seen in clams fed T5 (TL + DL + CN). There was no statistical difference in the survival of post-set clams fed T2 and T5 or T1, T4, and T6.
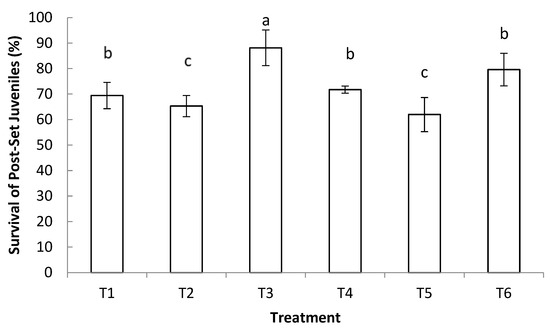
Figure 3.
Survival of post-set sunray venus clams (Macrocallista nimbosa) fed various live microalgal species combinations. Different letters indicate significant differences among the treatments (one-way ANOVA, α = 0.05, a > b > c). Each bar represents the mean ± SD of three replicates.
3.3. Clam Fatty Acid Profile
The FA profiles of post-set sunrays fed the various dietary treatments are presented in Table 4. Significant differences were seen in percentages of saturated fatty acids (SFAs) (p = 0.019), monounsaturated fatty acids (MUFAs) (p < 0.0001), and polyunsaturated fatty acids (PUFAs), n-3 PUFAs (p < 0.0001) and n-6 PUFAs (p < 0.0001). Regardless of diet, sunrays had higher total PUFAs or SFAs compared to MUFAs. PUFAs were the most abundant FA group in clams fed diets T2 and T4, SFAs were the most abundant FA group in clams fed T1, and proportions were similar in clams fed T3 and T5. The dominant FA in all sunrays was 16:0, followed by either 20:5n-3 eicosapentaenoic acid (EPA) (T2) or 22:6n-3 docosahexaenoic acid (DHA) (T1, T3–T6). Other FAs found in high percentages in sunray clams included 17:0, 16:1n-7, 18:1n-9, and 20:2n-6. Clams fed the flagellate-only T1 (TL + DL) diet had the highest DHA and lowest arachidonic acid (ARA), (20:4n-6) percentages, while clams fed the diatom-only T2 (CN + TW) diet had the lowest DHA and highest ARA percentages. Clams fed diets T3 and T5 had similar DHA, EPA, and ARA percentages; likewise, clams fed diets T4 and T6 had similar percentages of the same three highly unsaturated fatty acids (HUFAs).

Table 4.
Proportions of fatty acids of post-set sunray venus clams (Macrocallista nimbosa) fed different diet combinations of live microalgae.
4. Discussion
The choice of microalgal combinations affects growth, survival, and fatty acid (FA) composition [39,40,41]. As such, bivalve nutritional research has focused on determining optimal live algal combinations [11,42,43].
This is the first known study to explore various live algae diets to enhance the production of post-set sunray clams, an alternative clam species reared in hard clam hatcheries in FL. Post-set sunray clams achieved the highest growth when fed a tetra-algal dietary combination consisting of two flagellates (T. lutea and D. lutheri) and two diatoms (C. neogracile and T. weissflogii), while survival was highest in the bi-algal T. lutea and C. neogracile treatment.
It is generally agreed that mixed microalgae bivalve diets result in increased production compared to mono-algal diets due to a more nutritionally complete dietary profile [11,39,41,44,45], yet this is not always the case. For the saltwater clam (Meretrix meretrix), a mono-species diet of I. galbana resulted in better performance than a mixed-species diet [46]. In contrast, mixed diets outperformed mono-species diets in blue mussel (Mytilus edulis) and northern quahog (hard clam) (M. mercenaria) larvae and post-set clams [35,45,47].
Bivalve hatcheries typically switch from a mono (flagellate) to a bi-algal diet (diatom and flagellate) once clams reach the post-set stage. A standard post-set hard clam diet consists of feeding equal proportions of C. neogracile and T. lutea, although proportions are not strictly adhered to and are often based on the condition of available algae. Algal species suitable for hatchery culture, such as I. galbana, D. lutheri, Tetraselmis suecica, T. pseudonana, and C. neogracile, have been shown to have enhanced nutritional quality compared to other species [33,48].
The growth and survival of sunrays was improved when fed a mixed species diet consisting of equal proportions of flagellates and diatoms, likely due to the complementary nutrition benefits offered. The reported survival seen with post-set sunray clams in this 6 week study is higher than but comparable to the mean survival of 50% seen in post-set sunray clams reared to 1 mm seed at the FAU-HBOI bivalve hatchery (Laramore, unpublished data). Higher growth and survival were reported in hard clam larvae fed a bi-algal diet over a flagellate-based diet; however, the species of diatom fed was important [35]. Increased survival in blue mussel (M. galloprovincialis) larvae was reported when diatoms were added to a flagellate-based diet in various proportions [43]. Both the proportion and the species of diatom added affected survival but not growth.
The sunray clams in the present study showed increased growth when diatoms were included in the diet; however, growth was lowest when fed a diatom-only diet. Other studies have shown similar results. Inclusion of diatoms in bivalve diets significantly promoted growth rates in oysters (Pinctada margaritifera L.), scallops (Nodipecten subnodosus), hard clams (M. mercenaria), and geoduck clams (Panopea generosa) [24,28,35,47,49], whereas diatom-only or diatom-dominant diets have been shown to result in decreased growth in oysters (Crassostrea gigas) and hard clams (M. mercenaria) [47,50]. Lower survival was likewise reported in juvenile hard clams fed a diatom-only or a diatom-dominant diet [47].
Polyunsaturated fatty acids (PUFAs) play important roles in bivalve growth and development [31,51]. DHA has been shown to be integral in maintaining the membrane structure and functional integrity of tissues, and EPA has been shown to play a vital role in growth [52,53]; more importantly, as bivalves have limited ability to synthesize these FAs, they must be provided through their diet [25,54,55]. For this reason, nutritional research in bivalves has focused on n-3 PUFAs, although feed studies have shown that ARA (20:4 n-6) plays a key role in fish larval development, as well as immune response stress response in fish and oysters [56,57,58,59,60].
Differing profiles for diatoms and flagellates have been reported in the literature [61,62]. The most common algal combination fed to post-set clams consists of a mixture of Isochrysis sp. and Chaetoceros sp. as flagellates and diatoms, which complement each other nutritionally [33]. However, variability is sometimes seen within these categories, which is why a mixed-species diet is recommended. The FA profile of microalgae fed was primarily reflected in the FA profile of the clam tissue, as flagellates and diatoms also generally differ with respect to nutritional profile. Of the four microalgae fed in the present study T. lutea and D. lutheri were high in DHA. Although C. neogracile contained high levels of EPA compared to T. lutea, concentrations in D. lutheri and T. weissflogii were higher. The greater amounts of DHA present in the two flagellate species are reflected in the clam tissues, in that the most DHA was found in the flagellate-only diet, and the least amount of DHA was found in the diatom-only diet. This was not the case with EPA. Highest levels of DHA were found in the bi-algal diatom diet, with the lowest levels in the bi-algal flagellate diet. In both instances, diets containing a mix of diatoms and flagellates had moderate and similar amounts of both EPA and DHA.
The best-performing diet in terms of both growth and survival for sunrays in the present study was the tetra-algal diet containing equal proportions of diatoms and flagellates. Sunrays fed the two tri-algal diets showed a differential response in terms of production even though clam FA profiles were similar, with clams fed the tri-algal diatom-dominant diet exhibiting increased survival, and those fed the tri-algal flagellate-dominant diet exhibiting increased growth. Sunrays fed the bi-algal diatom diet performed poorly in terms of both growth and survival, and they differed greatly in terms of FA profile compared to clams in other treatments, having significantly higher concentrations of EPA, and significantly lower concentrations of DHA. Clams fed the bi-flagellate diet performed better in terms of survival than in growth, suggesting perhaps that DHA is more important for survival and EPA is more important for growth. Survival of mussel larvae was enhanced by increasing levels of DHA, even when EPA levels were similar [43]. High levels of EPA, found in C. neogracile, were found to be correlated with inconsistent mussel performance, while moderate EPA levels found in the bi-algal T. lutea and C. neogracile diet resulted in high performance [50].
Arachidonic acid (ARA) plays a role in the formation of eicosanoids, and it is crucial to various physiological and pathological processes in marine bivalves [59,60]. ARA was shown to be important in maturation and immune response of the mangrove oyster (Crassostrea corteziensis) but had no effect on the growth [60].
The ARA concentration was low (<1%) in all four microalgae species fed. However, higher levels of ARA were seen in both species of diatoms, which is reflected in that the bi-algal diatom diet had the highest and the bi-algal flagellate diet had the lowest ARA levels. In contrast, post-set clam tissues had high (2.8–6%) ARA concentrations. This has likewise been reported in the common cockle (Cerastoderma edule) and the northern hard clam [23,47].
Some species (i.e., M. mactroides) are thought to be able to convert linolenic acid (18:2 n-6) into ARA [63]; however, whether this occurs in venerid clams is unclear. T. lutea contained the highest levels of linolenic acid of the microalgae fed in the present study, and, although clams fed the bi-algal flagellate diet had the highest amount of linolenic acid in their tissues, they had the lowest levels of ARA. Neither a high nor a low ARA concentration enhanced growth or survival. The sunrays fed the bi-algal diatom treatment had a significantly larger proportion of ARA, while sunrays fed the bi-algal flagellate treatment had a significantly lower proportion of ARA in their tissues.
Long-chain PUFA ratios may be more important than absolute levels [64,65]. An unbalanced EPA/ARA ratio is thought to lead to harmful effects, as EPA can inhibit the formation of eicosanoids from 20:4n-6 [66]. Development of sea urchin larvae (Paracentrotus lividus) was enhanced when the dietary EPA/DHA ratio was lower, and the EPA/ARA ratio was higher [65,67]. Clams fed the poorest-performing diet in this study, the bi-algal diatom diet, had high EPA/DHA and EPA/ARA ratios, while the converse was seen in all other treatments. Clam tissue FA profiles reflected microalgae species. This was evident in the EPA/DHA, as DHA concentrations were higher in both flagellate species despite there being differences in EPA concentrations. Conversely, this was not seen with the EPA/ARA ratio, likely due to the ARA microalgae concentrations being low in all species, while EPA levels were quite variable, leading to an extremely high EPA/ARA ratio of 89 in D. lutheri, compared to 3.5 for T. lutea. Only one other known study detailed the FA content of sunrays [68]. Although not a dietary study, the post-set sunrays were fed a T. lutea and C. neogracile bi-algal diet. The clam total PUFA n-3 and n-6 values were lower in that study than in the present study; however, EPA/DHA and EPA/ARA ratios were similar.
Nutritional components other than FA, such as lipids, proteins, carbohydrates, minerals, and vitamins, present in the diets might also have affected sunray post-set production. High carbohydrate levels were shown to increase growth in juvenile oysters (Ostrea edulis) and scallops (Pecten maximus) [69,70], while higher protein levels increased growth in juvenile mussels (Mytilus trossulus) [71].
Due to the amount of clam tissue required for the determination of proximate and mineral analysis and the size and number of clams available at experimental termination, this analysis was unfortunately not conducted. Proximate and mineral analysis of algae was not performed for a similar reason. However, the literature concerning clam nutritional profiles other than FAs indicates that clams are high in protein, low in fat, and high in minerals. Proximate analysis of assorted clam species such the striped venus clam, Chamelea gallina, Anadora semillis, the blood ark, Anadora ovalis, the ponderous ark, Noetia ponderosa, and the hard clam Mercenaria mercenaria reported protein levels of 7–16 g/100 g wet weight and lipid levels of 0.8–1.5 g/100 g wet weight dependent on species and season [72,73,74]. These studies showed clams to be a good source of minerals such as sodium, calcium, magnesium, phosphorus, iron, and zinc, which also varied with season. As seasonal variation is often associated with a change in the availability of various phytoplankton species, it is likely that clams fed different combinations of algae would also show differences in some of these nutritional components.
Microalgae species used in the present study were previously analyzed for these nutritional components, and the values are reported in the literature. The microalga D. lutheri has been shown to contain high levels of protein, carbohydrates, and lipids compared to the other three species of microalgae used in this study [12]. Both diatom species were found to have similarly higher protein and similarly lower carbohydrate and lipid concentrations compared to T lutea [12]. The enhanced performance in growth seen with the tetra-algal species diet that included D. lutheri over that of the bi-species T. lutea and C. neogracile diet may have been due to increased concentrations of protein, carbohydrates, and/or lipids, as well as differing FA profiles. Survival, however, was not enhanced in diets containing D. lutheri, suggesting that these nutritional components may have less of an effect on survival compared to growth.
5. Conclusions
A typical hard clam hatchery diet consists of feeding a microalgae diet consisting of T. lutea and C. neogracile. Few studies have been conducted concerning an optimal hard clam diet, and none have been conducted with sunrays. A recent study aimed at determining whether other mixed algae dietary combinations would increase hard clam production over the standard hard clam hatchery diet concluded that a tetra-algal species combination of T. lutea, D. lutheri, C. neogracile, and C. nana enhanced both growth and survival. While sunrays fed a tetra-gal species combination of T. lutea, D. lutheri, C. neogracile and T. weissflogii had higher growth, those fed the standard hard clam bi-algal diet showed the highest survival. This poses a conundrum for clam hatcheries considering sunray culture as the cost of production of multiple microalgae species is likely to be higher. Individual hatchery operators will need to conduct a cost–benefit analysis to determine whether any economic benefits are to be gained by producing multiple algal species to increase growth of post-set sunrays, as no survival benefit was achieved.
Author Contributions
Conceptualization, E.P., S.L. and L.S.; Methodology, E.P.; Data curation, E.P., S.L. and J.B.; Writing—original Draft Preparation, E.P. and S.L.; Writing—Review and Editing, S.L., L.S., J.B. and P.S.W.; Supervision, S.L., J.B. and P.S.W.; Funding Acquisition, S.L., L.S. and J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Florida Sea Grant project R/LR-A-56.
Institutional Review Board Statement
Invertebrate culture studies are not subject to institutional review.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available upon request from the corresponding author.
Acknowledgments
The authors specially thank Md Hasson for support in the lab and Richard Baptiste for assistance in sunray venus clam spawning and larval rearing. This is FAU-HBOI contribution #2315.
Conflicts of Interest
The authors declare no conflict of interest.
References
- USDA. Florida Aquaculture Industry Overview. United States Department of Agriculture. 2020. Available online: https://www.fdacs.gov/content/download/91723/file/FDACS-P-02145-2020FLAquacultureIndustryOverview.pdf (accessed on 30 October 2021).
- Gustafson, R.G.; Creswell, R.L.; Jacobsen, T.R.; Vaughan, D.E. Larval biology and mariculture of the angelwing clam, Cyrtopleura costata. Aquaculture 1991, 95, 257–279. [Google Scholar] [CrossRef]
- Blake, N.J.; Adams, C.; Degner, R.; Sweat, D. Aquaculture and Marketing of the Florida Bay Scallop in Crystal River, Florida; Technical Paper; Gainesville, F.L., Ed.; Florida Sea Grant College Program: Gainesville, FL, USA, 2000; Volume 106. [Google Scholar]
- Power, A.J.; Sturmer, L.; Lucas, C.; Walker, R.L.; Manley, J. Gametogenic cycle of the ponderous ark, Noetia ponderosa (Say, 1822), from Cedar Kay, Florida. J. Shellfish Res. 2005, 24, 69–73. [Google Scholar]
- Sturmer, L.N.; Nunez, J.; Creswell, R.; Baker, S.M. The Potential of Blood ark and Ponderous Ark Aquaculture in Florida, Florida Sea Grant Technical Paper, TP-169; Florida Sea Grant College Program: Gainesville, FL, USA, 2009; 81p.
- Scarpa, J.; Sturmer, L.N.; Nuñez, J.; Creswell, R.L. Evaluation of the sunray venus clam Macrocallista nimbosa for aquaculture in Florida. J. Shellfish Res. 2008, 27, 1051. [Google Scholar]
- Scarpa, J.; Sturmer, L.N.; Adams, C.M.; Creswell, R.L.; Nuñez, J. Sunray Venus Clam: A New Species to Diversify the Florida Aquaculture Hard Clam Industry; Florida Sea Grant Final Technical Report, Project R/LR-A-44; Florida Sea Grant College Program: Gainesville, FL, USA, 2009; p. 23. [Google Scholar]
- Abbott, R.T. American Seashells: The Marine Mollusca of the Atlantic and Pacific Coasts of North America, 2nd ed.; Van Nostrand Reinhold: New York, NY, USA, 1974. [Google Scholar]
- Sturmer, L.N.; Scarpa, J.; Laramore, S.E.; Creswell, L. Evaluation of the sunray venus clam Macrocallista nimbosa under field nursery and growout culture conditions in Florida. J. Shellfish Res. 2009, 28, 734. [Google Scholar]
- Loosanoff, V.L.; Davis, H.C. Rearing of bivalve mollusks. Adv. Mar. Biol. 1963, 1, 1–136. [Google Scholar]
- Epifanio, C. Growth in bivalve molluscs: Nutritional effects of two or more species of algae in diets fed to the American oyster Crassostrea virginica (Gmelin) and the hard clam Mercenaria mercenaria (L.). Aquaculture 1979, 18, 1–12. [Google Scholar] [CrossRef]
- Brown, M.R.; Jeffery, S.W.; Volkman, J.K.; Dunstan, G.A. Nutritional properties of microalgae for mariculture. Aquaculture 1997, 151, 315–331. [Google Scholar] [CrossRef]
- Whetstone, J.M.; Sturmer, L.N.; Oesterling, M.J. Biology and Culture of the Hard Clam (Mercenaria mercenaria); SRAC Publication No. 433; Southern Regional Aquaculture Center: Stoneville, MS, USA, 2005. [Google Scholar]
- Marshall, R.; McKinley, S.; Pearce, C.M. Effects of nutrition on larval growth and survival in bivalves. Rev. Aquac. 2010, 2, 33–55. [Google Scholar] [CrossRef]
- Bendif, E.M.; Probert, I.; Schroeder, D.C.; de Vargas, C. On the description of Tisochrysis lutea gen. nov. sp. nov. and Isochrysis nuda sp. nov. in the Isochrysidales, and the transfer of Dicrateria to the Prymnesiales (Haptophyta). J. App. Phycol. 2013, 25, 1763–1776. [Google Scholar] [CrossRef]
- Brown, M.R.; Blackburn, S.I. Live microalgae as feeds in aquaculture hatcheries. In Advances in Aquaculture Hatchery Technology; Allan, G., Burnell, G., Eds.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 117–156, 157e–158e. [Google Scholar]
- Wikfors, G.H.; Ferris, G.E.; Smith, B.C. The relationship between gross biochemical composition of cultured algal foods and growth of the hard clam, Mercenaria mercenaria (L.). Aquaculture 1992, 108, 135–154. [Google Scholar] [CrossRef]
- Gallagher, S.M.; Mann, R.; Sasaki, G.C. Lipid as an index of growth and viability in three species of bivalve larvae. Aquaculture 1986, 56, 81–103. [Google Scholar] [CrossRef]
- Whyte, J.N.C.; Bourne, N.; Ginther, N.G. Biochemical changes during embryogenesis in the rock scallop Crassadoma gigantea. Mar. Biol. 1990, 106, 239–244. [Google Scholar] [CrossRef]
- Pernet, F. Effect of varying dietary levels of ω6 polyunsaturated fatty acids during the early ontogeny of the sea scallop, Placopecten magellanicus. J. Exp. Mar. Biol. Ecol. 2005, 327, 115–133. [Google Scholar] [CrossRef]
- Fernandez-Reiriz, M.J.; Perez-Camacho, A.; Peteiro, L.G.; Labarta, U. Growth and kinetics of lipids and fatty acids of the clam Venerupis pullastra during larval development and postlarvae. Aquac. Nutr. 2011, 17, 13–23. [Google Scholar] [CrossRef]
- Matias, D.; Joaquim, S.; Ramos, M.; Sobral, P.; Leitao, A. Biochemical compounds’ dynamics during larval development of the carpet-shell clam Ruditapes decussatus (Linnaeus, 1758): Effects of mono-specific diets and starvation. Helgol. Mar. Res. 2011, 65, 369–379. [Google Scholar] [CrossRef]
- Reis Batista, I.; Kamermans, P.; Verdegem, M.C.J.; Smaal, A.C. Growth and fatty acid composition of juvenile Cerastoderma edule (L.) fed live microalgae diets with different fatty acid profiles. Aquac. Nutr. 2014, 20, 132–142. [Google Scholar] [CrossRef]
- Liu, W.; Pearce, C.M.; McKinley, R.S.; Forster, I.P. Nutritional value of selected species of microalgae for larvae and early post-set juveniles of the Pacific geoduck clam, Panopea generosa. Aquaculture 2016, 452, 326–341. [Google Scholar] [CrossRef]
- Soudant, P.; Marty, Y.; Moal, J.; Masski, H.; Samain, J.F. Fatty acid composition of polar lipid classes during larval development of scallop Pecten maximus (L.) Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1998, 121, 279–288. [Google Scholar] [CrossRef]
- Helm, M.M.; Bourne, N.; Lovatelli, A. Hatchery Culture of Bivalves, a Practical Manual; FAO Fisheries Technical Paper 471; Food and Agriculture Organization of the United Nations: Rome, Italy, 2004; Available online: https://www.fao.org/3/y5720e/y5720e00.htm (accessed on 30 October 2021).
- Pronker, A.E.; Nevejan, N.M.; Peene, F.; Geijsen, P.; Sorgeloos, P. Hatchery broodstock conditioning of the blue mussel Mytilus edulis (Linnaeus 1758). Part I. Impact of different micro-algae mixtures on broodstock performance. Aquac. Int. 2008, 16, 297–307. [Google Scholar] [CrossRef]
- Martínez-Fernández, E.; Southgate, P.C. Use of tropical microalgae as food for larvae of the black-lip pearl oyster Pinctada margaritifera. Aquaculture 2007, 263, 220–226. [Google Scholar] [CrossRef]
- Mourente, G.; Lubián, L.M.; Odriozola, J.M. Total fatty acid composition as a taxonomic index of some marine microalgae used as food in marine aquaculture. Hydrobiologia 1990, 203, 147–154. [Google Scholar] [CrossRef]
- Renaud, S.M.; Parry, D.L.; Thinh, L. Microalgae for use in tropical aquaculture I: Gross chemical and fatty acid composition of twelve species of microalgae from the Northern Territory, Australia. J. App. Phycol. 1994, 6, 337–345. [Google Scholar] [CrossRef]
- Patil, V.; Källqvist, T.; Olsen, E. Fatty acid composition of 12 microalgae for possible use in aquaculture feed. Aquac. Int. 2007, 15, 1–9. [Google Scholar] [CrossRef]
- Myers, J.A.; Boisvert, R.N. The economics of producing algae and bivalve seed in hatcheries. Aquaculture 1990, 86, 163–179. [Google Scholar] [CrossRef]
- Benemann, J. Microalgae aquaculture feeds. J. App. Phycol. 1992, 4, 233–245. [Google Scholar] [CrossRef]
- Coutteau, P.; Sorgeloos, P. The use of algal substitutes and the requirement for live algae in the hatchery and nursery of bivalve molluscs: An international survey. J. Shellfish Res. 1992, 11, 467–476. [Google Scholar]
- Hassan, M.M.; Parks, V.; Laramore, S. Optimizing microalgae diets for hard clam, Mercenaria mercenaria, larvae culture. Aquac. Rep. 2021, 20, 100716. [Google Scholar] [CrossRef]
- Guillard, R.R.L.; Ryther, J.H. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt and Detonula confervacea. Can. J. Microbiol. 1962, 8, 229–239. [Google Scholar] [CrossRef]
- Mamat, N.Z.; Alfaro, A.C. Evaluation of microalgal and formulated diets for the culture of the New Zealand pipi clam Paphiesaustralis. Int. Aquat. Res. 2014, 6, 57–69. [Google Scholar] [CrossRef]
- Epifanio, C. Comparison of yeast and algal diets for bivalve molluscs. Aquaculture 1979, 16, 187–192. [Google Scholar] [CrossRef]
- Milke, L.M.; Bricelj, V.M.; Parrish, C.C. Growth of postlarval sea scallops, Placopecten magellanicus, on microalgal diets, with emphasis on the nutritional role of lipids and fatty acids. Aquaculture 2004, 234, 293–317. [Google Scholar] [CrossRef]
- Ronquillo, J.D.; Fraser, J.; McConkey, A.J. Effect of mixed microalgal diets on growth and polyunsaturated fatty acid profile of European oyster (Ostrea edulis) juveniles. Aquaculture 2012, 360, 64–68. [Google Scholar] [CrossRef]
- Ren, Y.; Liu, W.; Pearce, C.M.; Forster, I.; McKinley, R.S. Effects of selected mixed-algal diets on growth and survival of early postset juveniles of the Pacific geoduck clam, Panopea generosa (Gould, 1850). Aquac. Nutr. 2015, 21, 152–161. [Google Scholar] [CrossRef]
- Helm, M.M. Mixed algal feeding of Ostrea edulis larvae with Isochrysis galbana and Tetraselmis suecica. J. Mar. Biol. Assoc. UK 1977, 57, 1019–1029. [Google Scholar] [CrossRef]
- Pettersen, A.K.; Turchini, G.M.; Jahangard, S.; Ingram, B.A.; Sherman, C.D.H. Effects of different dietary microalgae on survival, growth, settlement and fatty acid composition of blue mussel (Mytilus galloprovincialis) larvae. Aquaculture 2010, 309, 115–124. [Google Scholar] [CrossRef]
- Wong, W.; Levinton, J. Culture of the blue mussel Mytilus edulis (Linnaeus, 1758) fed both phytoplankton and zooplankton: A microcosm experiment. Aquac. Res. 2004, 35, 965–969. [Google Scholar] [CrossRef]
- Galley, T.H.; Batista, F.M.; Braithwaite, R.; King, J.; Beaumont, A.R. Optimisation of larval culture of the mussel Mytilus edulis (L.). Aquac. Int. 2010, 18, 315–325. [Google Scholar] [CrossRef]
- Tang, B.; Liu, B.; Wang, G.; Zhang, T.; Xiang, J. Effects of various algal diets and starvation on larval growth and survival of Meretrix meretrix. Aquaculture 2006, 254, 526–533. [Google Scholar] [CrossRef]
- Hassan, M.M.; Perri, E.; Parks, V.; Laramore, S. Growth, survival, and fatty acid profile of hard clam, Mercenaria mercenaria, juveniles fed live microalgae diets. Aquac. Fish Fisheries 2022, in press. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Raja, R.; Ravi Kumar, R.; Ganesan, V.; Anbazhagan, C. Microalgae: A sustainable feed source for aquaculture. World J. Microbiol. Biotechnol. 2011, 27, 1737–1746. [Google Scholar] [CrossRef]
- Saucedo, P.E.; González-Jiménez, A.; Acosta-Salmón, H.; Mazón-Suástegui, J.M.; Ronsón-Paulín, J.A. Nutritional value of microalgae-based diets for lions-paw scallop (Nodipecten subnodosus) juveniles reared at different temperatures. Aquaculture 2013, 392–395, 113–119. [Google Scholar] [CrossRef]
- Rico-Villa, B.; Le Coz, J.R.; Mingant, C.; Robert, R. Influence of phytoplankton diet mixtures on microalgae consumption, larval development and settlement of the Pacific oyster Crassostrea gigas (Thunberg). Aquaculture 2006, 256, 377–388. [Google Scholar] [CrossRef]
- Kapranova, L.L.; Nekhoroshev, M.V.; Malakhova, L.V.; Ryabushko, V.I.; Kapranov, S.V.; Kuznetsova, T.V. Fatty acid composition of gonads and gametes in the Black Sea bivalve mollusk Mytilus galloprovincialis Lam. at different stages of sexual maturation. J. Evol. Biochem. Physiol. 2019, 55, 448–455. [Google Scholar] [CrossRef]
- Marty, Y.; Delaunay, F.; Moal, J.; Samain, J.F. Changes in the fatty acid composition of Pecten maximus (L.) during larval development. J. Exp. Mar. Biol. Ecol. 1992, 163, 221–234. [Google Scholar] [CrossRef]
- Gagné, R.; Tremblay, R.; Pernet, F.; Miner, P.; Samain, J.F.; Olivier, F. Lipid requirements of the scallop Pecten maximus (L.) during larval and post-larval development in relation to addition of Rhodomonas salina in diet. Aquaculture 2010, 309, 212–221. [Google Scholar] [CrossRef]
- Soudant, P.; Chu, F.L.E.; Samain, J.F. Lipid requirements in some economically important marine bivalves. J. Shellfish Res. 2000, 19, 605–612. [Google Scholar]
- Geng, S.; Zhou, C.; Chen, W.; Yu, S.; Huang, W.; Huan, T.; Xu, J.; Yan, S. Fatty acid and sterol composition reveal food selectivity of juvenile ark shell Tegillarca granosa Linnaeus after feeding with mixed microalgae. Aquaculture 2016, 455, 109–117. [Google Scholar] [CrossRef]
- Emata, A.C.; Ogata, H.Y.; Garibay, E.S.; Furuita, H. Advanced broodstock diets for the mangrove red snapper and a potential importance of arachidonic acid in eggs and fry. Fish Physiol. Biochem. 2003, 28, 489–491. [Google Scholar] [CrossRef]
- Carrier, J.K., III; Watanabe, W.O.; Harel, M.; Rezek, T.C.; Seaton, P.J.; Shafer, T.H. Effects of dietary arachidonic acid on larval performance, fatty acid profiles, stress resistance and expression of Na+/K+ ATPase mRNA in black sea bass Centropristis striata. Aquaculture 2011, 319, 111–121. [Google Scholar] [CrossRef]
- Torrecillas, S.; Betancor, M.B.; Caballero, M.J.; Rivero, F.; Robaina, L.; Izquierdo, M.; Montero, D. Supplementation of arachidonic acid rich oil in European sea bass juveniles (Dicentrarchus labrax) diets: Effects on growth performance, tissue fatty acid profile and lipid metabolism. Fish Physiol. Biochem. 2018, 44, 283–300. [Google Scholar] [CrossRef]
- Delaporte, M.; Soudant, P.; Moal, J.; Giudicelli, E.; Lambert, C.; Seguineau, C.; Samain, J.F. Impact of 20,4n-6 supplementation of the fatty acid composition and hemocyte parameters of the Pacific oyster Crassostrea gigas. Lipids 2006, 6, 567–576. [Google Scholar] [CrossRef]
- Hurtado, M.A.; Reza, M.; Ibarra, A.M.; Wille, M.; Sorgeloos, P.; Soudant, P.; Palacios, E. Arachidonic acid (20,4 n-6) effect on reproduction, immunology, and prostaglandin E2 levels in Crassostrea corteziensis (Hertlein, 1951). Aquaculture 2009, 294, 300–305. [Google Scholar] [CrossRef]
- Volkman, J.; Jeffery, S.; Nichols, P.; Rogers, G.; Garland, C. Fatty-Acid and Lipid-Composition of 10 Species of Microalgae Used in Mariculture. J. Exp. Mar. Biol. Ecol. 1989, 128, 219–240. [Google Scholar] [CrossRef]
- Yang, Y.; Du, L.; Hosokawa, M.; Miyashita, K. Total lipids content, lipid class and fatty acid composition of ten species of microalgae. J. Oleo Sci. 2020, 69, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- DeMoreno, J.E.A.; Moreno, V.J.; Brenner, R.R. Lipid metabolism of the yellow clam, Mesodesma mactroides: 2-polyunsaturated fatty acid metabolism. Lipids 1976, 11, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Schiopu, D.; George, S.B.; Castell, J. Ingestion rates and dietary lipids affect growth and fatty acid composition of Dendraster excentricus larvae. J. Exp. Mar. Biol. Ecol. 2006, 328, 47–75. [Google Scholar] [CrossRef]
- Liu, H.; Kelly, M.S.; Cook, E.J.; Black, K.; Orr, H.; Zhu, J.X.; Dong, S.L. The effect of diet type on growth and fatty-acid composition of sea urchin larvae, I. Paracentrotus lividus (Lamarck, 1816) (Echinodermata). Aquaculture 2007, 264, 247–262. [Google Scholar] [CrossRef]
- Sargent, J.R.; McEvoy, L.A.; Bell, J.G. Requirements, presentation and sources of polyunsaturated fatty acids in marine fish larval feeds. Aquaculture 1997, 155, 117–127. [Google Scholar] [CrossRef]
- Carboni, S.; Vignier, J.; Chiantore, M.; Tocher, D.R.; Migaud, H. Effects of dietary microalgae on growth, survival and fatty acid composition of sea urchin Paracentrotus lividus throughout larval development. Aquaculture 2012, 324, 250–258. [Google Scholar] [CrossRef]
- Laramore, S.; Sturmer, L.; Baptiste, R.; Yang, H.; Sinacore, C.; Urban-Gedmadke, E. Fatty acid composition of adult and larval Sunray Venus clams Macrocallista nimbosa: Environmental and gametogenic impacts. J. Shellfish Res. 2017, 36, 403–416. [Google Scholar] [CrossRef]
- Enright, C.T.; Newkirk, G.F.; Craigie, J.S.; Castell, J.D. Evaluation of phytoplankton as diets for juvenile Ostrea edulis L. J. Exp. Mar. Biol. Ecol. 1986, 96, 1–13. [Google Scholar] [CrossRef]
- Reitan, K.I. Digestion of lipids and carbohydrates from microalgae (Chaetoceros muelleri Lemmermann and Isochrysis aff. galbana clone T-ISO) in juvenile scallops (Pecten maximus L.). Aquac. Res. 2011, 42, 1530–1538. [Google Scholar] [CrossRef]
- Kreeger, D.A.; Langdon, C.J. Effect of dietary protein content on growth of juvenile mussels, Mytilus trossulus (Gould 1850). Biol. Bull. 1993, 185, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Orban, E.; Di Lena, G.; Nevigato, T.; Casini, I.; Caproni, R.; Santaroni, G.; Giulini, G. Nutritional and commercial quality of the striped venus clam, Chamelea gallina, from the Adriatic sea. Food Chem. 2006, 101, 1063–1070. [Google Scholar] [CrossRef]
- Kim-Kabari, D.B.; Hart, A.D.; Nyeche, P.T. Nutritional composition of selected shellfish consumed in Rivers State, Nigeria. Amer. J. Food. Nutri. 2017, 5, 142–146. [Google Scholar]
- Sturmer, L.N.; Morgan, K.L.; Degner, R.L. Nutritional Composition and Marketable Shelf-Life of Blood Ark Clams and Ponderous Ark Clams; EDIS document FE568; The Department of Food and Resource Economics, Florida Cooperative Extension Service, Institute of Food and Agricultural Sciences, University of Florida: Gainesville, FL, USA, 2005; p. 6. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).