Identification and Characterization of GYS and GSK3β Provides Insights into the Regulation of Glycogen Synthesis in Jinjiang Oyster Crassostrea ariakensis
Abstract
1. Introduction
2. Materials and Methods
2.1. Oyster Collection and Sampling
2.2. Glycogen Content Determination
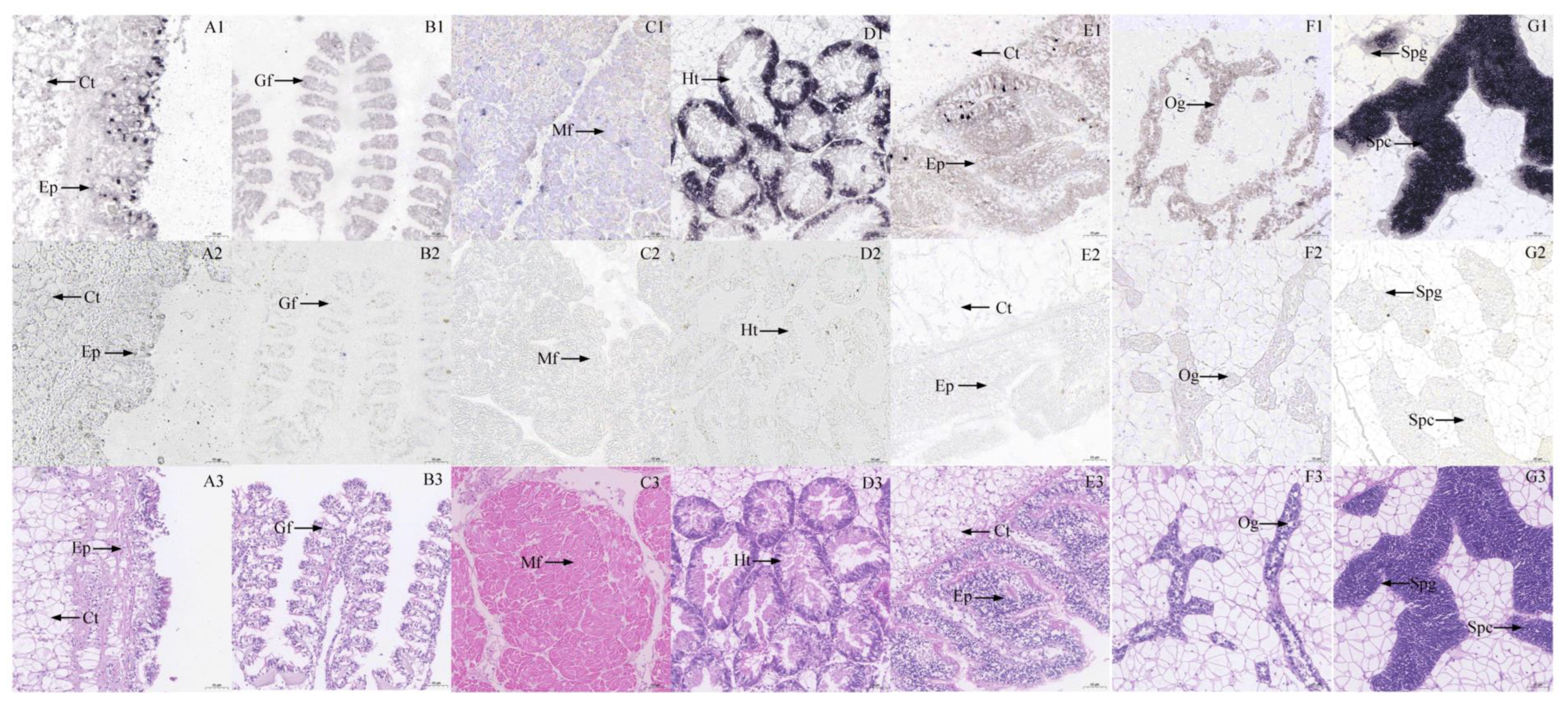
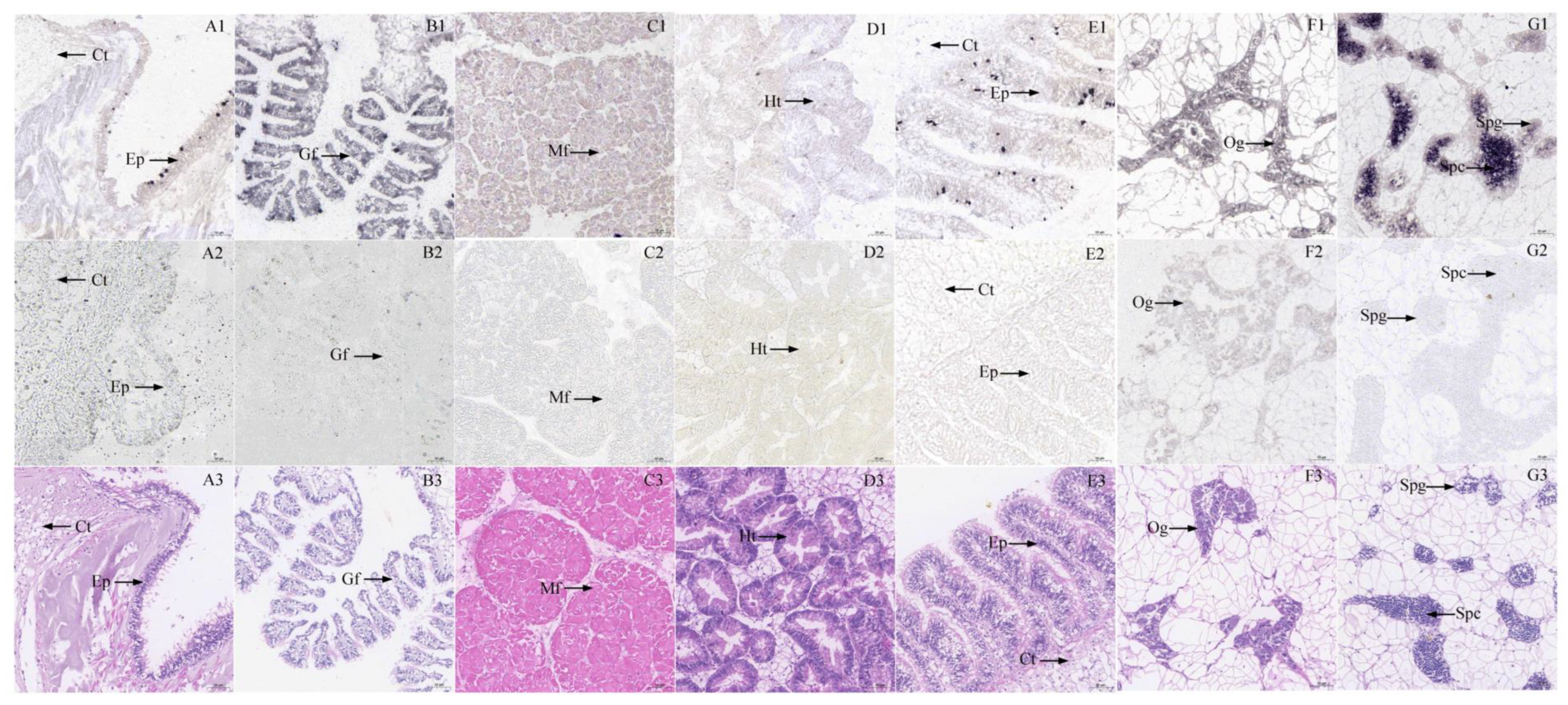
2.3. In Situ Hybridization
2.4. CDS Sequence Amplification and Analyses
| Primer Name | Purpose | Nucleotide Sequence (5′→3′) |
|---|---|---|
| C-GYS-L | CDS sequence verification | GGTTCTTGACCTGATCTAACTGCTGA |
| C-GYS-R | GCACTTGCCAACACTCCAGTATTAT | |
| C-GSK3β-L | ATCCGTCAGGTTTCACGAGC | |
| C-GSK3β-R | ATGAATAGCTTGCAACAGCTTAGGATG | |
| q-GYS-L | qRT-PCR expression analysis | TACTGTCTGCTGGGTCCCTATAACG |
| q-GYS-R | CCTCATTGCCTGAAGTGTCTGTCTG | |
| q-GSK3β-L | GTCAGCCGCCTTCTGGAATATACTC | |
| q-GSK3β-R | CAACCTGGTGCTAGGATCTCTTAGTTC | |
| β-actin-F | CTGTGCTACGTTGCCCTGGACTT | |
| β-actin-R | TGGGCACCTGAATCGCTCGTT | |
| R-GSK3β-1L | RNAi | GATCACTAATACGACTCACTATAGGGGAAATGGGTCTTTTGGGGTT |
| R-GSK3β-1R | GATCACTAATACGACTCACTATAGGGGTCTCCAGGGAAAATGGGTT | |
| R-GSK3β-2L | GATCACTAATACGACTCACTATAGGGCACAAGGTGTGTGCCATAGG | |
| R-GSK3β-2R | GATCACTAATACGACTCACTATAGGGTATATTCCAGAAGGCGGCTG | |
| R-GSK3β-3L | GATCACTAATACGACTCACTATAGGGGTCTGGCTCCGACAGAACTC | |
| R-GSK3β-3R | GATCACTAATACGACTCACTATAGGGCCAACACTTTTGCACTTCCA | |
| ISH-GYS-probe | In situ hybridization | GCGGTCGTGCCAGGGGATTCCTATGCTGCATCATCATACATCATCAT |
| ISH-GSK3β-probe | TCTCCAGTTACTGCTCCAGGATCACTTGCGTTTCATCATCATACATCATCAT | |
| Fluorescent labeling sequences | ATGATGATGTATGATGATGT |
2.5. Relative Quantification of Gene Expression
2.6. GSK3β RNA Interference In Vivo
2.7. Statistical Analysis
3. Results
3.1. CDS Characterization of GYS and GSK3β
3.2. Histocytology Localization of GYS and GSK3β Expression in Six Tissues
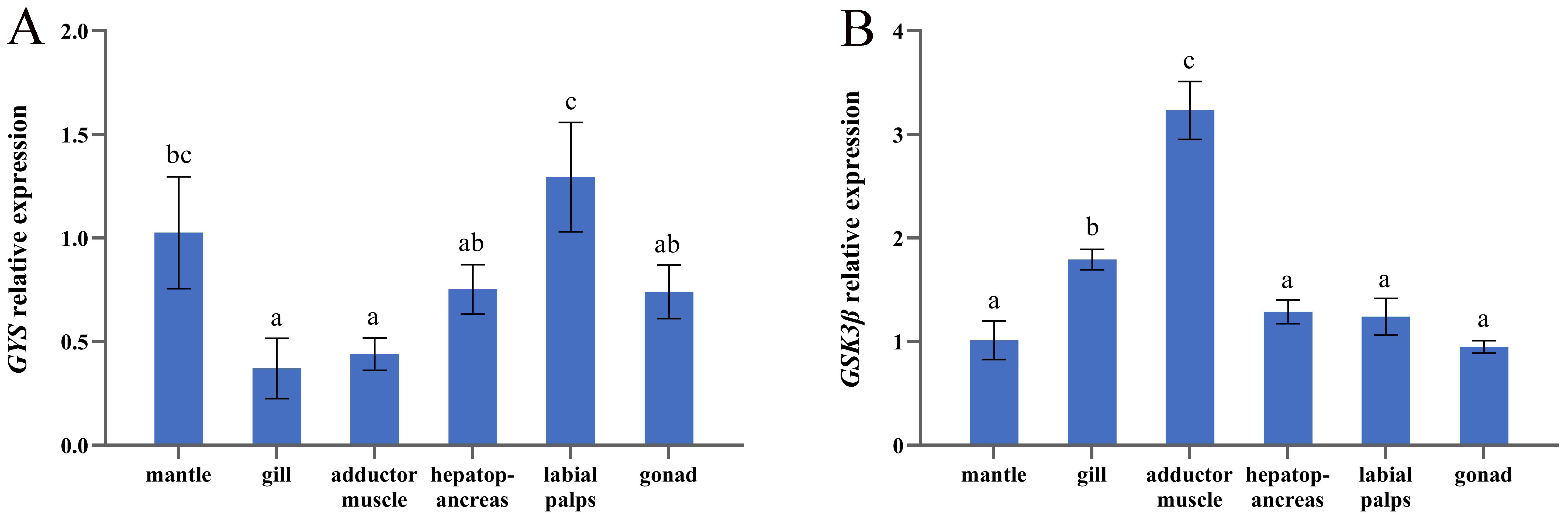
3.3. mRNA Expression of GYS and GSK3β in Six Tissues
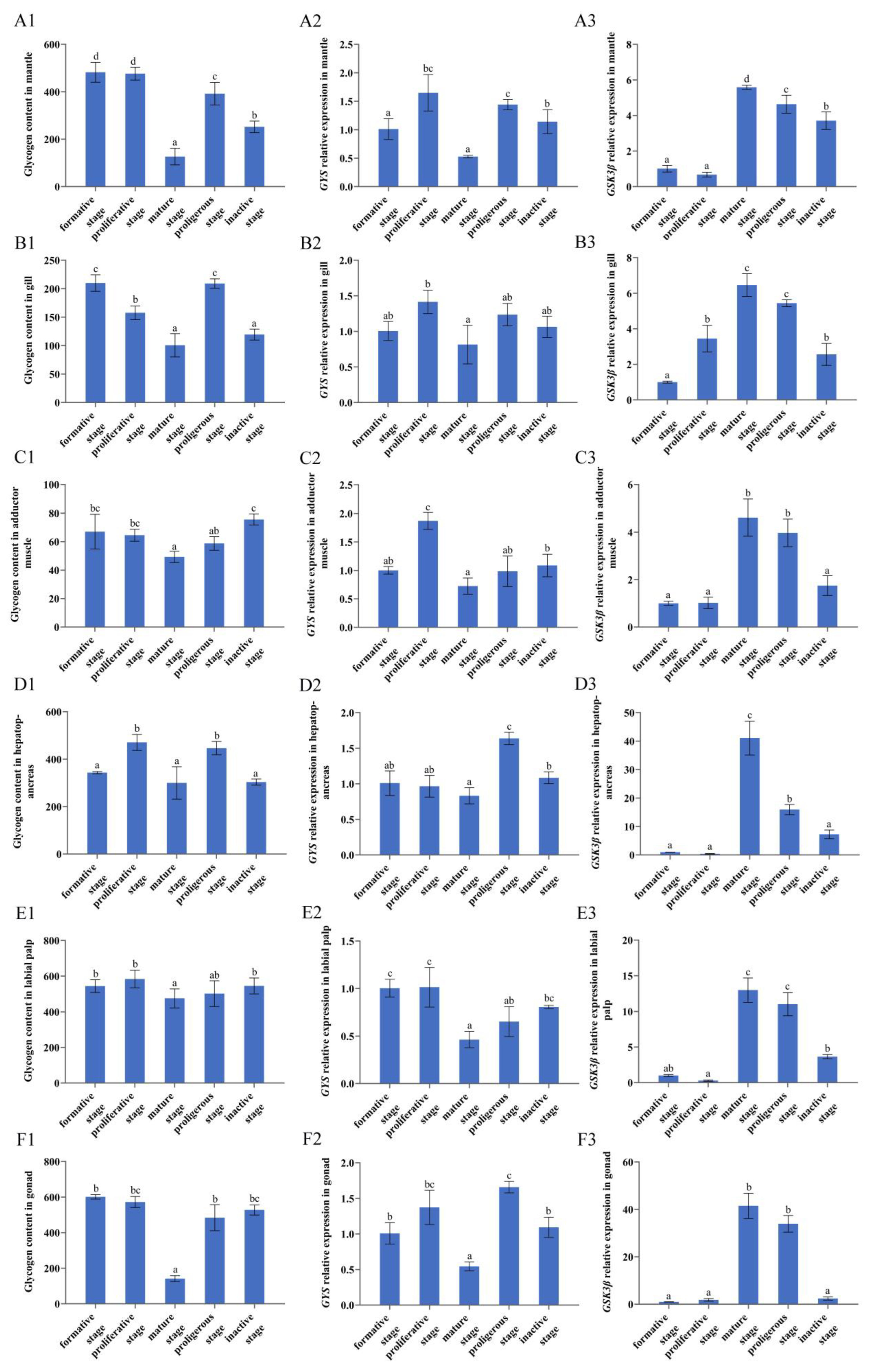
3.4. Glycogen Content in Tissues at Different Reproductive Stages
3.5. GYS and GSK3β Expression in Six Tissues at Different Reproductive Stages
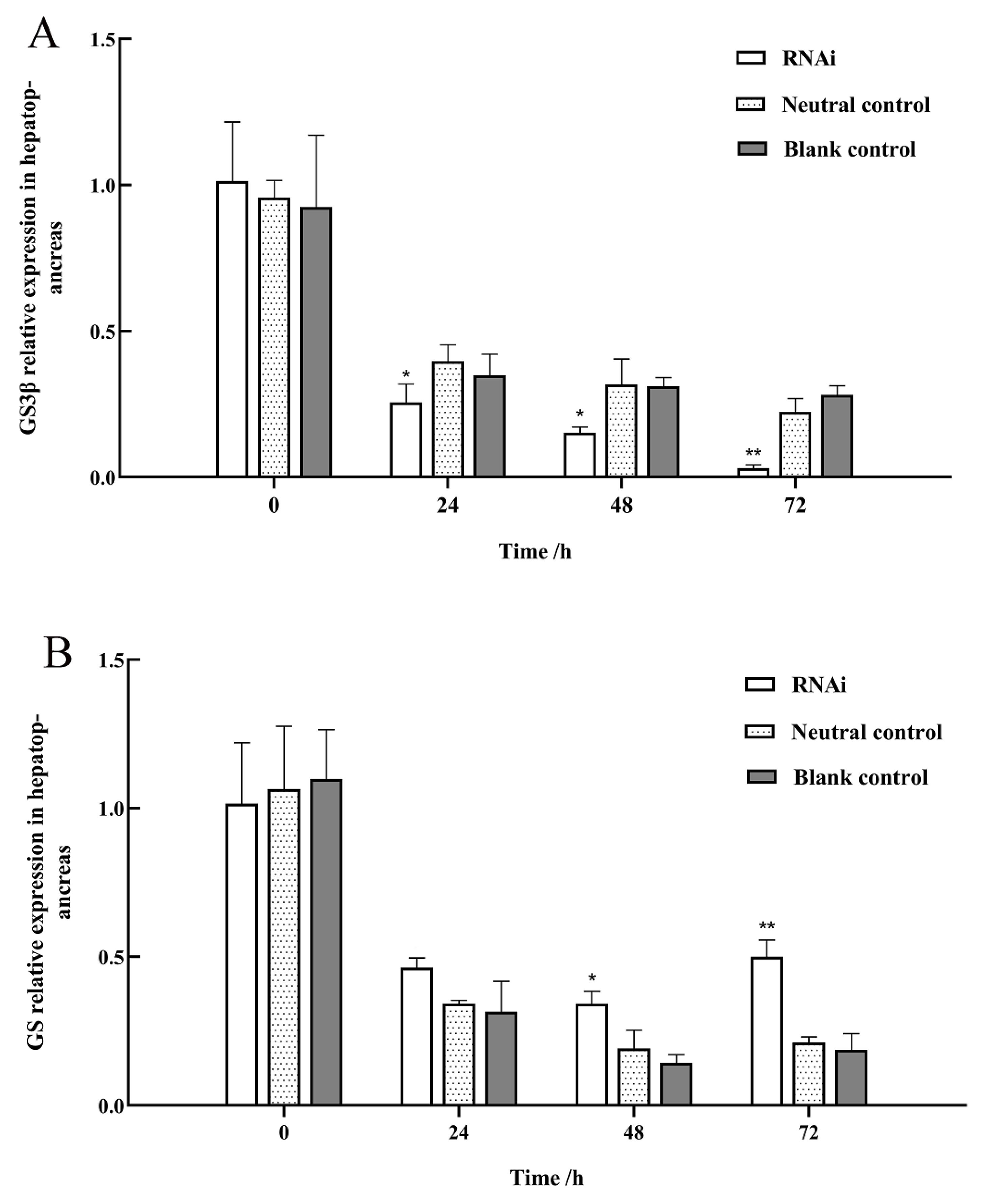
3.6. Effect of GSK3β Gene Silencing on the Relative Expression Levels of GYS
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, M.; Allen, S.K., Jr. A review of published work on Crassostrea ariakensis. J. Shellfish. Res. 2003, 22, 1–20. [Google Scholar]
- Ren, J.; Hou, Z.; Wang, H.; Sun, M.-A.; Liu, X.; Liu, B.; Guo, X. Intraspecific Variation in Mitogenomes of Five Crassostrea Species Provides Insight into Oyster Diversification and Speciation. Mar. Biotechnol. 2016, 18, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Li, Q.; Yu, H.; Kong, L. Biochemical Composition and Nutritional Value of Different Shell Color Strains of Pacific Oyster Crassostrea gigas. J. Ocean Univ. China 2018, 17, 897–904. [Google Scholar] [CrossRef]
- Bartlett, J.K.; Maher, W.A.; Purss, M.B. Near infra-red spectroscopy quantitative modelling of bivalve protein, lipid and glycogen composition using single-species versus multi-species calibration and validation sets. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 193, 537–557. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, C.C.; Jurczak, M.J.; Danos, A.M.; Brady, M.J. Glycogen branches out: New perspectives on the role of glycogen metabolism in the integration of metabolic pathways. Am. J. Physiol. Metab. 2006, 291, E1–E8. [Google Scholar] [CrossRef] [PubMed]
- Ojea, J.; Pazos, A.J.; Martínez, D.; Novoa, S.; Sánchez, J.L.; Abad, M. Seasonal variation in weight and biochemical composition of the tissues of Ruditapes decussatus in relation to the gametogenic cycle. Aquaculture 2004, 238, 451–468. [Google Scholar] [CrossRef]
- Liu, S.; Li, L.; Wang, W.; Li, B.; Zhang, G. Characterization, fluctuation and tissue differences in nutrient content in the Pacific oyster (Crassostrea gigas) in Qingdao, northern China. Aquac. Res. 2020, 51, 1353–1364. [Google Scholar] [CrossRef]
- Smolders, R.; Bervoets, L.; De Coen, W.; Blust, R. Cellular energy allocation in zebra mussels exposed along a pollution gradient: Linking cellular effects to higher levels of biological organization. Environ. Pollut. 2004, 129, 99–112. [Google Scholar] [CrossRef]
- Maguire, G.B.; Gardner, N.; Nell, J.A.; Kent, G.N.; Kent, A.S. Studies on triploid oysters in Australia. 2. Growth, condition index gonad area, and glycogen content of triploid and diploid Pacific oysters, Crassostrea gigas, from oyster leases in Tasmania, Australia. Aquaculture 1995, 137, 357. [Google Scholar] [CrossRef]
- Samain, J.F. Review and perspectives of physiological mechanisms underlying genetically-based resistance of the Pacific oyster Crassostrea gigas to summer mortality. Aquat. Living Resour. 2011, 24, 227–236. [Google Scholar] [CrossRef]
- Cao, C.; Wang, W.-X. Copper-induced metabolic variation of oysters overwhelmed by salinity effects. Chemosphere 2017, 174, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Berthelin, C.; Kellner, K.; Mathieu, M. Storage metabolism in the Pacific oyster (Crassostrea gigas) in relation to summer mortalities and reproductive cycle (West Coast of France). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2000, 125, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Wood, E.J. Textbook of Biochemistry with Clinical Correlations, 2nd ed.; Devlin, T.M., Ed.; John Wiley & Sons: New York, NY, USA, 1987; Volume 15, p. 47. [Google Scholar] [CrossRef]
- Stalmans, W.; Bollen, M.; Mvumbi, L. Control of glycogen synthesis in health and disease. Diabetes/Metab. Res. Rev. 1987, 3, 127–161. [Google Scholar] [CrossRef] [PubMed]
- Frame, S.; Cohen, P. GSK3 takes centre stage more than 20 years after its discovery. Biochem. J. 2001, 359, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zuo, B.; Xu, D.; Ren, Z.; Zhang, H.; Li, X.; Lei, M.; Xiong, Y. Alternative Splicing of the Porcine Glycogen Synthase Kinase 3β (GSK-3β) Gene with Differential Expression Patterns and Regulatory Functions. PLoS ONE 2012, 7, e40250. [Google Scholar] [CrossRef]
- Cohen, P.I.; Frame, S. The renaissance of GSK3. Nat. Rev. Mol. Cell Biol. 2001, 2, 769–776. [Google Scholar] [CrossRef]
- Rommel, C.; Bodine, S.; Clarke, B.A.; Rossman, R.; Nunez, L.; Stitt, T.N.; Yancopoulos, G.D.; Glass, D.J. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat. Cell Biol. 2001, 3, 1009–1013. [Google Scholar] [CrossRef]
- Bacca, H.; Huvet, A.; Fabioux, C.; Daniel, J.-Y.; Delaporte, M.; Pouvreau, S.; Van Wormhoudt, A.; Moal, J. Molecular cloning and seasonal expression of oyster glycogen phosphorylase and glycogen synthase genes. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2005, 140, 635–646. [Google Scholar] [CrossRef]
- Li, B.; Meng, J.; Li, L.; Liu, S.; Wang, T.; Zhang, G. Identification and Functional Characterization of the Glycogen Synthesis Related Gene Glycogenin in Pacific Oysters (Crassostrea gigas). J. Agric. Food Chem. 2017, 65, 7764–7773. [Google Scholar] [CrossRef]
- Liu, S.; Li, L.; Meng, J.; Song, K.; Huang, B.; Wang, W.; Zhang, G. Association and Functional Analyses Revealed That PPP1R3B Plays an Important Role in the Regulation of Glycogen Content in the Pacific Oyster Crassostrea gigas. Front. Genet. 2019, 10, 106. [Google Scholar] [CrossRef]
- Zeng, Z.; Ni, J.; Ke, C. Expression of glycogen synthase (GYS) and glycogen synthase kinase 3β (GSK3β) of the Fujian oyster, Crassostrea angulata, in relation to glycogen content in gonad development. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2013, 166, 203–214. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Li, X.; Li, J.; Zhou, Y.; Xiang, Z.; Ma, H.; Noor, Z.; Mo, R.; Zhang, Y.; Yu, Z. Seasonal variations in biochemical composition and nutritional quality of Crassostrea hongkongensis, in relation to the gametogenic cycle. Food Chem. 2021, 356, 129736. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Chen, X.; Yu, M.; Ren, J.; Hu, J.; Shao, C.; Zhou, L.; Sun, X.; Yu, T.; Zheng, Y.; et al. Chromosome-level genome and population genomic analysis provide insights into the evolution and environmental adaptation of Jinjiang oyster Crassostrea ariakensis. Mol. Ecol. Resour. 2022, 22, 1529–1544. [Google Scholar] [CrossRef] [PubMed]
- Chen, X. Establish a Method for Determination of Oyster Glycogen Content and Study on the Characteristics of Jinjiang Oyster Glycogen Changes; Shanghai Ocean University: Shanghai, China, 2021. [Google Scholar] [CrossRef]
- Xu, R.; Li, Q.; Yu, H.; Kong, L. Oocyte maturation and origin of the germline as revealed by the expression of Nanos-like in the Pacific oyster Crassostrea gigas. Gene 2018, 663, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, B.; Liu, Z.; Chen, X.; Yu, T.; Wang, Z.; Sun, X.; Zhou, L.; Zheng, Y. Establishment of near-infrared model for glycogen content in freeze-dried tissues of Jinjiang oyster Crassostrea ariakensis. Prog. Fish. Sci. 2023, 44, 210–218. [Google Scholar] [CrossRef]
- Swift, M.L.; Thomas, T.P.; Humphrey, C.L. Characteristics of glycogen synthase activity in the oyster, Crassostrea virginica Gmelin. Comp. Biochem. Physiol. Part B Comp. Biochem. 1988, 90, 361–365. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, S.; He, J.; Yao, H.; Lin, Z.; Dong, Y. Glycogen content fluctuation and association analysis with polymorphism of gys gene of Sinonovacula constricta in the Zhejiang population. J. Fish. China 2021, 45, 415–423. [Google Scholar] [CrossRef]
- Sastry, A.N.; Blake, N.J. Regulation of gonad development in the bay scallop, Aequipecten irradians Lamarck. Biol. Bull. 1971, 140, 274–283. [Google Scholar] [CrossRef]
- Vassalo, M.T. Lipid storage and transfer in the scallop Chlamys Hericia Gould. Comp. Biochem. Physiol. 1973, 44, 1169–1175. [Google Scholar]
- Hamano, K.; Awaji, M.; Usuki, H. cDNA structure of an insulin-related peptide in the Pacific oyster and seasonal changes in the gene expression. J. Endocrinol. 2005, 187, 55–67. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Song, K.; Meng, J.; Li, L.; Zhang, G. Integrated application of transcriptomics and metabolomics provides insights into glycogen content regulation in the Pacific oyster Crassostrea gigas. BMC Genom. 2017, 18, 713. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Zhong, T.; Guo, J.; Li, L.; Zhang, H.; Wang, L. Transcriptional regulation of pig GYS1 gene by glycogen synthase kinase 3β (GSK3β). Mol. Cell. Biochem. 2017, 424, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Gabbott, P.A.; Whittle, M.A. Glycogen synthetase in the sea mussel Mytilus edulis L.—II. Seasonal changes in glycogen content and glycogen synthetase activity in the mantle tissue. Comp. Biochem. Physiol. Part B Comp. Biochem. 1986, 83, 197–207. [Google Scholar] [CrossRef]
- Suquet, M.; de Kermoysan, G.; Araya, R.G.; Queau, I.; Lebrun, L.; Le Souchu, P.; Mingant, C. Anesthesia in Pacific oyster, Crassostrea gigas. Aquat. Living Resour. 2009, 22, 29–34. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, Z.; Chen, X.; Zhou, L.; Sun, X.; Yu, T.; Wang, X.; Zheng, Y.; Wu, B. Identification and Characterization of GYS and GSK3β Provides Insights into the Regulation of Glycogen Synthesis in Jinjiang Oyster Crassostrea ariakensis. Fishes 2023, 8, 65. https://doi.org/10.3390/fishes8020065
Wang Y, Liu Z, Chen X, Zhou L, Sun X, Yu T, Wang X, Zheng Y, Wu B. Identification and Characterization of GYS and GSK3β Provides Insights into the Regulation of Glycogen Synthesis in Jinjiang Oyster Crassostrea ariakensis. Fishes. 2023; 8(2):65. https://doi.org/10.3390/fishes8020065
Chicago/Turabian StyleWang, Yan, Zhihong Liu, Xi Chen, Liqing Zhou, Xiujun Sun, Tao Yu, Xiaomei Wang, Yanxin Zheng, and Biao Wu. 2023. "Identification and Characterization of GYS and GSK3β Provides Insights into the Regulation of Glycogen Synthesis in Jinjiang Oyster Crassostrea ariakensis" Fishes 8, no. 2: 65. https://doi.org/10.3390/fishes8020065
APA StyleWang, Y., Liu, Z., Chen, X., Zhou, L., Sun, X., Yu, T., Wang, X., Zheng, Y., & Wu, B. (2023). Identification and Characterization of GYS and GSK3β Provides Insights into the Regulation of Glycogen Synthesis in Jinjiang Oyster Crassostrea ariakensis. Fishes, 8(2), 65. https://doi.org/10.3390/fishes8020065







