The Effects of Vibration Frequency on Oxidative Stress, Digestive Enzymes and ATPases of Crimson Snapper (Lutjanus erythropterus) Fry during Transport
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Simulation of Transport Experiments
2.3. Sample Collection and Analysis
2.4. Data Analysis
3. Results
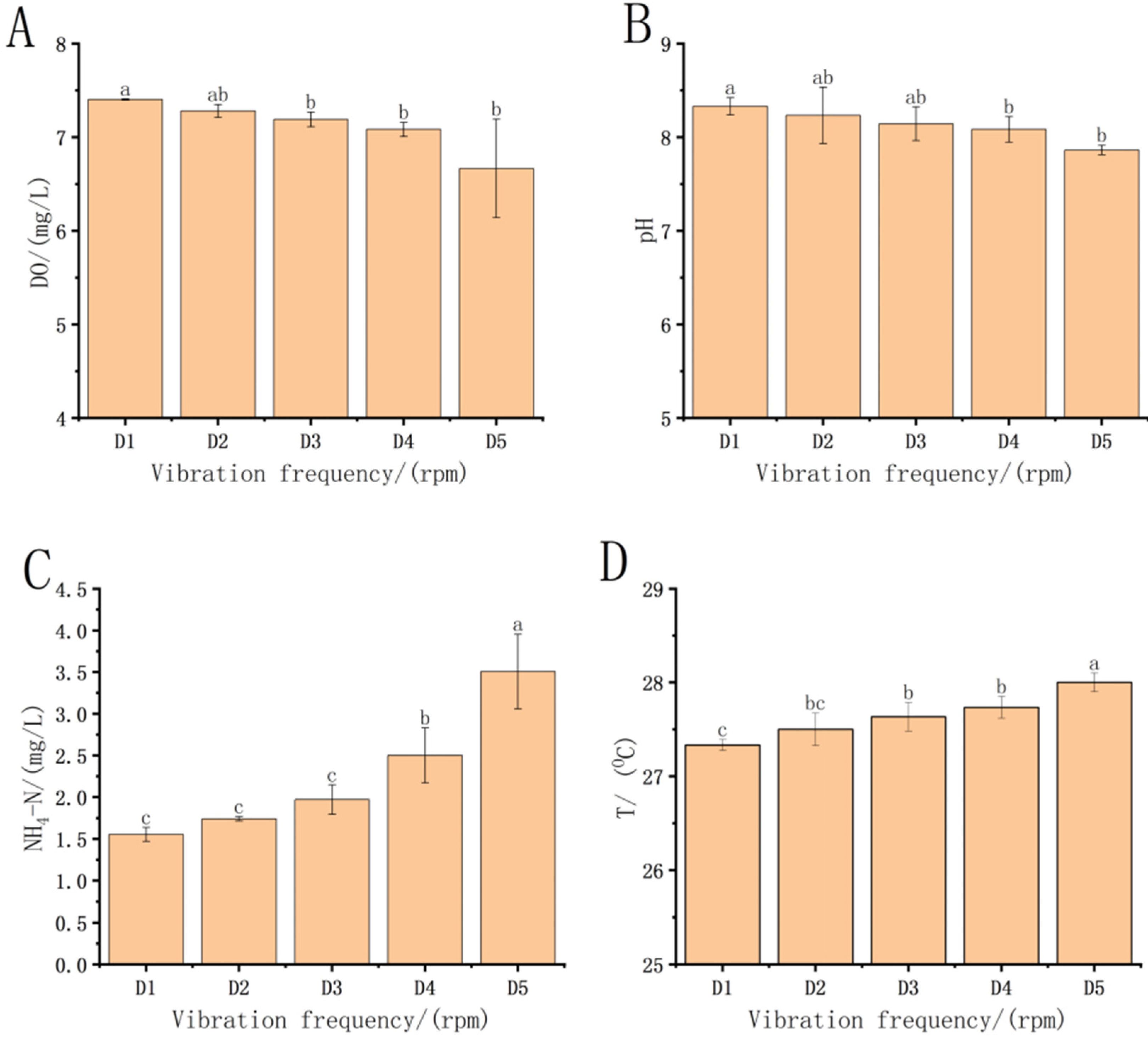
3.1. Changes in the Treatment Groups after Transportation
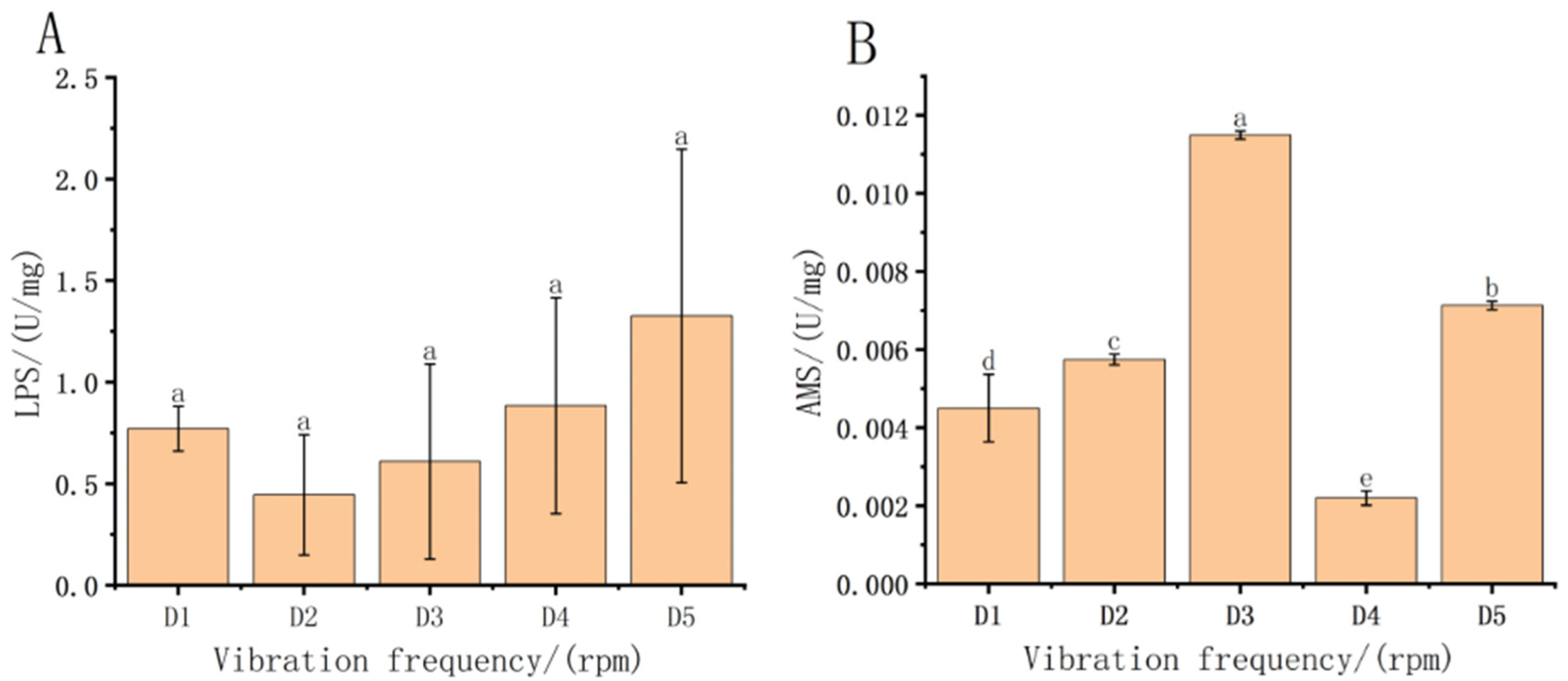
3.2. Effect of Vibration Frequency Stress on Oxidative Stress Biomarkers in Fish Fry in Each Treatment Group
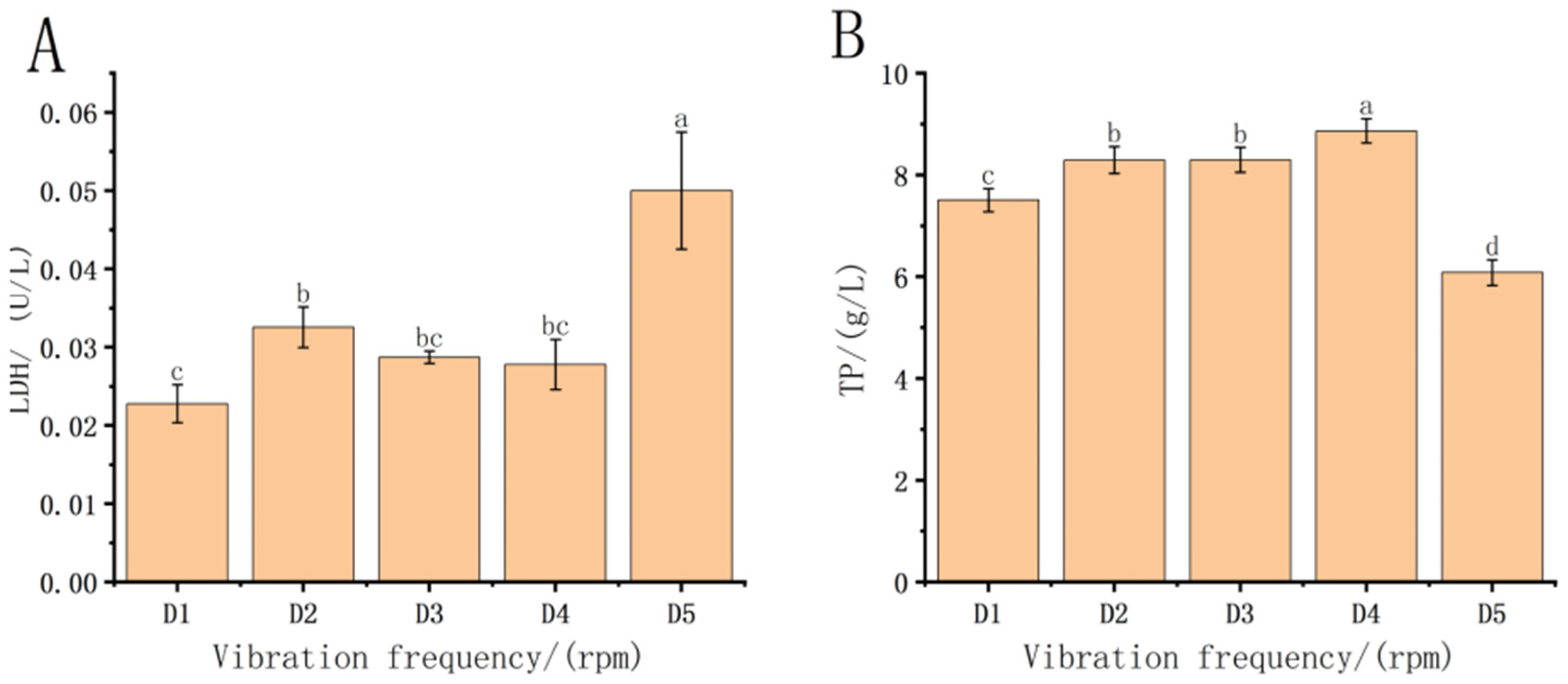
3.3. Effect of Vibration Frequency Stress on Digestive Enzymes in Fish Fry
3.4. Effect of Vibration Frequency Stress on ATPase in Fish Fry
4. Discussion
4.1. Impact of Transport on Water Quality in Each Treatment Group
4.2. Effect of Transport on Oxidative Stress Biomarkers in Each Treatment Group
4.3. Effect of Transport on Digestive Enzymes in Each Treatment Group
4.4. Effect of Post-Transport Treatment Groups on ATPase
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhao, X.; Sun, Z.; Xu, H.; Song, N.; Gao, T. Transcriptome and Co-Expression Network Analyses Reveal the Regulatory Pathways and Key Genes Associated with Temperature Adaptability in the Yellow Drum (Nibea albiflora). J. Therm. Biol. 2021, 100, 103071. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Zhang, X.-M. Effects of Environmental Stress on Plasma Levels of Glucose and Esr of Sebastes Schlegeli and Lateolabrax maculatus. J. Fish. Sci. China 2005, 12, 414–418. [Google Scholar]
- Demers, N.E.; Bayne, C.J. The Immediate Effects of Stress on Hormones and Plasma Lysozyme in Rainbow Trout. Dev. Comp. Immunol. 1997, 21, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Li, Z. Effects of Temperatures on the Physiological and Biochemical Indexs of Silver Carp and Bighead Carp. J. Northeast. Norm. Univ. (Nat. Sci. Ed.) 1996, 2, 108–122. [Google Scholar]
- Wang, W.B.; Li, A.-H. The Effect of Environmental Stress to Fish Immune System. J. Fish. China 2002, 26, 368–374. [Google Scholar]
- Singh Kumar, R.; Vartak, V.R.; Balange, A.K.; Ghughuskar, M.M. Water Quality Management During Transportation of Fry of Indian Major Carps, Catla catla (Hamilton), Labeo rohita (Hamilton) and Cirrhinus mrigala (Hamilton). Aquaculture 2004, 235, 297–302. [Google Scholar] [CrossRef]
- Tort, L. Stress and Immune Modulation in Fish. Dev. Comp. Immunol. 2011, 35, 1366–1375. [Google Scholar] [CrossRef]
- Urbinati, E.C.; De Abreu, J.S.; Da Silva Camargo, A.C.; Landinez Parra, M.A. Loading and Transport Stress of Juvenile Matrinxa (Brycon cephalus, Characidae) at Various Densities. Aquac. Amst. 2004, 229, 389–400. [Google Scholar] [CrossRef]
- Tang, D.Y.; Zheng, Z.; Dong, J. Study on Ammonia-Nitrogen Adsorption from Low Concentration Wastewater by Modified Zeolite and Its Desorption. Chin. J. Environ. Eng. 2011, 5, 293–296. [Google Scholar]
- Wang, Q.; Jun, M.; Jing, X. Effects of Low Temperature and Alive Transportation on Stress and Meat Quality of Sea Bass (Lateolabrax maculatus). J. Chin. Inst. Food Sci. Technol. 2022, 22, 11. [Google Scholar]
- Celi, M.; Francesco, F.; Giulia, M.; Lucrezia, G.; Maria, Q.E.; Vincenzo, M.; Salvatore, M.; Mirella, V.; Giuseppa, B. Vessel Noise Pollution as a Human Threat to Fish: Assessment of the Stress Response in Gilthead Sea Bream (Sparus aurata, Linnaeus 1758). Fish Physiol. Biochem. 2016, 42, 631641. [Google Scholar] [CrossRef] [PubMed]
- Neo, Y.Y.; Hubert, J.; Bolle, L.; Winter, H.V.; Cate, C.T.; Slabbekoorn, H. Sound Exposure Changes European Seabass Behavior in a Large Outdoor Floating Pen: Effects of Temporal Structure and a Ramp-up Procedure. Environ. Pollut. 2016, 214, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.-C.; Du, F.-K.; Nie, Z.-J.; Yin, W.-J.; Xu, P.; Gu, R.-B. Effects of 10‰ Salinity to the Plasma Osmotic Pressure, Cortisol, Glucose and Liver Glycogen in Colilia Nasus Stressed during Loading and Transportation. Acta Hydrobiol. Sin. 2015, 39, 66–72. [Google Scholar]
- Liu, Z. The Main Reason and Countermeasures of Unfavorable for Transportation in Cultured Freshwater Fishes. Freshw. Fish. 2002, 32, 61–62. [Google Scholar]
- Cui, K.; Fu, Z.; Cheng, D.; Yang, Q.; Ma, Z.; Qin, J.G.; Hu, J. Development of Immune Functionality in Larval and Juvenile Crimson Snapper Lutjanus erythropterus (Bloch 1790). Aquac. Rep. 2018, 10, 1–7. [Google Scholar] [CrossRef]
- Sfakianakis, D.G.; Koumoundouros, G.; Divanach, P.; Kentouri, M. Osteological Development of the Vertebral Column and of the Fins in Pagellus erythrinus (L. 1758). Temperature Effect on the Developmental Plasticity and Morpho-Anatomical Abnormalities. Aquaculture 2004, 232, 407–424. [Google Scholar] [CrossRef]
- Liang, Q.; Afriyie, G.; Chen, Z.; Xu, Z.; Dong, Z.; Guo, Y.; Wang, Z. Analysis of Opsin Gene Family of Crimson Snapper (Lutjanus erythropterus). Gene 2022, 807, 145960. [Google Scholar] [CrossRef]
- Koumoundouros, G.; Gagliardi, F.; Divanach, P.; Boglione, C.; Cataudella, S.; Kentouri, M. Normal and Abnormal Osteological Development of Caudal Fin in Sparus aurata L. Fry. Aquaculture 1997, 149, 215–226. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Wang, Z.D.; Guo, Y.S.; Liu, L.; Yu, J.; Zhang, S.; Liu, S.J.; Liu, C.W. Morphological Characters and Transcriptome Profiles Associated with Black Skin and Red Skin in Crimson Snapper (Lutjanus erythropterus). Int. J. Mol. Sci. 2015, 16, 26991–27004. [Google Scholar] [CrossRef]
- Bo, W.U.; Jing, X.I.E. Effects of the Dissovied Oxygen Level and the Vibration on Oxidative Stress of Grouper during Water Transport. Food Mach. 2019, 35, 137–142+82. [Google Scholar]
- Derby, C.D. Cephalopod Ink: Production, Chemistry, Functions and Applications. Mar. Drugs 2014, 12, 2700–2730. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.I.E.; Wang, Q. Progress in Understanding Environmental Stress and Physiological Regulatory Mechanism in Aquatic Animals During Live Transportation. Food Sci. 2021, 42, 319–325. [Google Scholar]
- Hao, D.; Qiwei, W.; Fang, G.; Jianyi, L.; Deguo, Y.; Xihua, C.; Yan, Z. Transport Stress Catabatic Effect of Anesthetic Benzocaine on American Shad Alosa Sapidissima. J. Fish. Sci. China 2006, 13, 787–793. [Google Scholar]
- Liu, S.X.; Zhou, S.-J.; Han, M.-Y.; Wang, Y.-F.; Hong, J.-W.; Gu, Z.-F.; Ma, Z.-H. Effects of Density Stress on Water Quality, Survival Rate, Immune Enzyme Activities, and Serotonation Index of Trachinotus ovatus. Mar. Sci. 2019, 43, 70–80. [Google Scholar]
- Gomes, L.C. Edsandra Campos Chagas, Richard Philip Brinn, Rodrigo Roubach, Carlos Eduardo Coppati, and Bernardo Baldisserotto. Use of Salt During Transportation of Air Breathing Pirarucu Juveniles (Arapaima gigas) in Plastic Bags. Aquaculture 2006, 256, 521–528. [Google Scholar] [CrossRef]
- Battisti, E.K.; Rabaioli, A.; Uczay, J.; Sutili, F.J.; Lazzari, R. Effect of Stocking Density on Growth, Hematological and Biochemical Parameters and Antioxidant Status of Silver Catfish (Rhamdia quelen) Cultured in a Biofloc System. Aquaculture 2020, 524, 735213. [Google Scholar] [CrossRef]
- Hvas, M.; Oppedal, F. Physiological Responses of Farmed Atlantic Salmon and Two Cohabitant Species of Cleaner Fish to Progressive Hypoxia. Aquaculture 2019, 512, 734353. [Google Scholar] [CrossRef]
- Sun, J.L.; Zhao, L.L.; Liao, L.; Tang, X.H.; Cui, C.; Liu, Q.; He, K.; Ma, J.D.; Jin, L.; Yan, T.; et al. Interactive Effect of Thermal and Hypoxia on Largemouth Bass (Micropterus salmoides) Gill and Liver: Aggravation of Oxidative Stress, Inhibition of Immunity and Promotion of Cell Apoptosis. Fish Shellfish. Immunol. 2020, 98, 923–936. [Google Scholar] [CrossRef]
- Lu, Z.-F.; Huang, H.; Huang, X.M.; Huang, W.Z. Effects of Hypoxic Stress on Antioxidant and Energy Metabolism of Hybrid Grouper (Epinephelus fuscoguttatus♀ × Epinephelus lanceolatus♂). J. Guangdong Ocean Univ. 2022, 42, 13–19. [Google Scholar]
- Wang, Y.; Li, J.; Li, J.T.; He, Y.Y.; Chang, Z.Q.; Liu, D.Y. Effects of pH Stress on Antioxidant System Enzyme Activities and Gene Expression of Fenneropenaeus Chinensis. J. Fish. Sci. China 2011, 18, 556–564. [Google Scholar] [CrossRef]
- Allen, L.J.; Kinney, E.C. Proceedings of the Bio-Engineering Symposium for Fish Culture. FCS Pub. 1981, 317, 266–274. [Google Scholar]
- Cao, S.; Zhao, D.; Huang, R.; Xiao, Y.; Xu, W.; Liu, X.; Gui, Y.; Li, S.; Xu, J.; Tang, J.; et al. The Influence of Acute Ammonia Stress on Intestinal Oxidative Stress, Histology, Digestive Enzymatic Activities and Pept1 Activity of Grass Carp (Ctenopharyngodon idella). Aquac. Rep. 2021, 20, 100722. [Google Scholar] [CrossRef]
- Goss, G.G.; Wood, C.M. The Effects of Acid and Acid/Aluminum Exposure on Circulating Plasma Cortisol Levels and Other Blood Parameters in the Rainbow Trout, Salmo gairdneri. J. Fish Biol. 2010, 32, 63–76. [Google Scholar] [CrossRef]
- Zhang, K.F. Antioxidant Defense System in Animals. Chin. J. Zool. 2007, 2, 153–160. [Google Scholar] [CrossRef]
- Paital, B.; Chainy, G.B. Seasonal Variability of Antioxidant Biomarkers in Mud Crabs (Scylla serrata). Ecotoxicol. Environ. Saf. 2013, 87, 33–41. [Google Scholar] [CrossRef]
- Gupta, I.; Parihar, A.; Malhotra, P.; Singh, G.B.; Lüdtke, R.; Safayhi, H.; Ammon, H.P. Effects of Boswellia Serrata Gum Resin in Patients with Ulcerative Colitis. Eur. J. Med. Res. 1997, 2, 37–43. [Google Scholar]
- Liu, W.; Ji, D.; Shan, L.; Zheng, C.; Chen, S.; Li, S.; Yan, M.; Wu, J.; Xie, Q.; Zhou, Z. Effects of Ozone on the Histological Structure and the Antioxidant Systems in Gills of Juvenile Oplegnathus fasciatus. J. Fish. China 2011, 35, 1384–1391. [Google Scholar]
- Hu, J.; Wu, K.; Ye, L.; Wang, Y. Effect of Acute Salinity Stress on Catalase of Juvinile Amphiprion clarkii. South China Fish. Sci. 2015, 11, 73–78. [Google Scholar]
- Martínez-Álvarez, R.M.; Morales, A.E.; Sanz, A. Antioxidant Defenses in Fish: Biotic and Abiotic Factors. Rev. Fish Biol. Fish. 2005, 15, 75–88. [Google Scholar] [CrossRef]
- Yu, W.; Yang, Y.; Chen, H.; Zhou, Q.; Zhang, Y.; Huang, X.; Huang, Z.; Li, T.; Zhou, C.; Ma, Z.; et al. Effects of Dietary Chitosan on the Growth, Health Status and Disease Resistance of Golden Pompano (Trachinotus ovatus). Carbohydr. Polym. 2023, 300, 120237. [Google Scholar] [CrossRef]
- Cao, X.; Gao, M.; Yang, X. Effects of Acute Hypoxia Stress on the Activities of Antioxidant Enzymes and Phosphatase in Seriola Aureovittata. J. Anhui Agric. Sci. 2022, 50, 88–91. [Google Scholar]
- Mao, R.X.; Liu, F.J.; Zhang, X.F.; Zhang, Y.; Cao, D.C.; Lu, C.Y.; Liang, L.Q.; Sun, X.W. Studies on Quantitative Trait Loci Related to Activity of Lactate Dehydrogenase in Common Carp (Cyprinus carpio). Yi Chuan Hered. 2009, 31, 407–411. [Google Scholar] [CrossRef] [PubMed]
- Valarmathi, S.; Azariah, J. Effect of Copper Chloride on the Enzyme Activities of the Crab Sesarma quadratum (Fabricius). Turk. J. Zool. 2003, 27, 253–256. [Google Scholar]
- Cheng, C.H.; Ye, C.X.; Guo, Z.X.; Wang, A.L. Immune and Physiological Responses of Pufferfish (Takifugu obscurus) under Cold Stress. Fish Shellfish. Immunol. 2017, 64, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.H.; Fang, Z.Q.; Zheng, W.B.; Wu, Y.Y.; Ma, G.Z. Experiment with Effect of Cadmium on Activity of Superoxide Dismutase in Gill and Liver Tissue of Crucian. J. Saf. Environ. 2003, 3, 13–16. [Google Scholar]
- Yang, H.; Wang, H.; Liu, J.-H.; Liang, G.-D. Effect of High Temperature on the Growth and Enzyme Activities of Superoxide Dismutase and Lactate Dehydrogenase of Gift Nile Tilapia Juveniles (Oreochromis niloticus). J. Guangdong Ocean Univ. 2014, 34, 15–20. [Google Scholar]
- Zhang, C.; Zhang, Y.; Gao, Q.; Peng, S.; Shi, Z. Effect of Low Salinity Stress on Antioxidant Function in Liver of Juvenile Nibea albiflora. South China Fish. Sci. 2015, 11, 59–64. [Google Scholar]
- Yu, Z.; Guo, W.C.; Yang, Z.G.; Zhang, K. Advances in the Study of Hemotological Indices of Fish. J. Shanghai Fish. Univ. 2001, 10, 163–165. [Google Scholar]
- Zhang, H.; Qi, N.; Zhang, Y.; Fan, W.; Liu, H.; Long, L.; Guan, C. Effects of Temperature on Growth, Hematology, and Immune Responses of Subadult Chinese Sturgeon (Acipenser sinensis Gray 1835) under Different Ammonia Nitrogen Conditions in Recirculating Aquaculture System. J. Appl. Ichthyol. 2019, 35, 313–322. [Google Scholar] [CrossRef]
- Sun, X.L.; Xing, K.-Z.; Chen, C.-X.; Wang, Q.-K.; Yu, X.-Q.; Hu, J.-C. The Effects of Acute Temperature Stress on Blood Parameters in Half-Smooth Tongue-Sole (Cynoglossus semilaevis). Fish. Sci. 2010, 29, 387–392. [Google Scholar]
- Zhou, S.J.; Hu, J.; Yu, G.; Yang, Q.-B.; Yang, R.; Liu, Y.-J.; Ma, Z.-H. Effects of Photoperiod on Digestive Enzyme Activity in Larval and Juvenile Barramundi Lates Calcarifer (Bloch). Mar. Sci. 2018, 42, 63–69. [Google Scholar]
- Thongprajukaew, K.; Kovitvadhi, S.; Kovitvadhi, U.; Preprame, P. Effects of Feeding Frequency on Growth Performance and Digestive Enzyme Activity of Sex-Reversed Nile Tilapia, Oreochromis niloticus (Linnaeus, 1758). Agric. Nat. Resour. 2017, 51, 292–298. [Google Scholar] [CrossRef]
- Chen, J.; Xi, S.; Qin, C.; Guo, Y.; Pan, W.; Shao, G. Effects of Light Intensity on Growth and Digestive Enzyme Activities of Sea Urchin (Anthocidaris crassispina) Larvae. Prog. Fish. Sci. 2021, 42, 125–131. [Google Scholar]
- Liang, M.J.; Ma, Y.M.; Zhang, H.X.; Wang, S.K.; Lin, S.G. Comparison between Digestive Enzyme Activities of Sparus Macrocephalus in Summer and Winter and Study on the Effects of Reactive Temperature and Ph on These Activities. Acta Oceanol. Sin. 2006, 28, 167–171. [Google Scholar]
- Alam, M.; Frankel, T.L. Gill Atpase Activities of Silver Perch, Bidyanus bidyanus (Mitchell), and Golden Perch, Macquaria ambigua (Richardson): Effects of Environmental Salt and Ammonia. Aquaculture 2006, 251, 118–133. [Google Scholar] [CrossRef]
- Evans, D.H.; Piermarini, P.M.; Choe, K.P. The Multifunctional Fish Gill: Dominant Site of Gas Exchange, Osmoregulation, Acid-Base Regulation, and Excretion of Nitrogenous Waste. Physiol. Rev. 2005, 85, 97–177. [Google Scholar] [CrossRef] [PubMed]
- Luo, S.Y.; Xu, D.-D.; Lou, B.; Chen, R.-Y.; Zhan, W.; Mao, G.-M. Zhejiang Ocean University. Effects of Low Temperature Stress on Activities of Antioxidant Enzymes, Na+/-K+-Atp Enzyme and Hsp70 Content of Nibea albiflora. Mar. Sci. Bull. 2017, 36, 189–194. [Google Scholar]
- Fan, C.Y.; Ou, Y.-J.; Li, J.-E.; Yu, N.; Su, H.; Wang, G. Effects of Acute Salinity Stress on Na+-K+-Atp and Osmotic Pressure of Juvenile Trachinotus ovatus. J. Oceanogr. Taiwan Strait 2012, 31, 218–224. [Google Scholar]
- Hua, W. Effects of Ammonia Stress on the Gill Na/K-Atpase, Microstructure and Some Serum Physiological-Biochemical Indices of Juvenile Black Carp (Mylopharyngodon piceus). J. Fish. China 2012, 36, 538–545. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Guo, Y.; Zhao, X.; Zhou, S.; Ma, Z.; Yu, G.; Qin, C.; Wang, X. The Effects of Vibration Frequency on Oxidative Stress, Digestive Enzymes and ATPases of Crimson Snapper (Lutjanus erythropterus) Fry during Transport. Fishes 2023, 8, 603. https://doi.org/10.3390/fishes8120603
Li J, Guo Y, Zhao X, Zhou S, Ma Z, Yu G, Qin C, Wang X. The Effects of Vibration Frequency on Oxidative Stress, Digestive Enzymes and ATPases of Crimson Snapper (Lutjanus erythropterus) Fry during Transport. Fishes. 2023; 8(12):603. https://doi.org/10.3390/fishes8120603
Chicago/Turabian StyleLi, Jiayang, Yu Guo, Xinye Zhao, Shengjie Zhou, Zhenhua Ma, Gang Yu, Chuanxin Qin, and Xingqiang Wang. 2023. "The Effects of Vibration Frequency on Oxidative Stress, Digestive Enzymes and ATPases of Crimson Snapper (Lutjanus erythropterus) Fry during Transport" Fishes 8, no. 12: 603. https://doi.org/10.3390/fishes8120603
APA StyleLi, J., Guo, Y., Zhao, X., Zhou, S., Ma, Z., Yu, G., Qin, C., & Wang, X. (2023). The Effects of Vibration Frequency on Oxidative Stress, Digestive Enzymes and ATPases of Crimson Snapper (Lutjanus erythropterus) Fry during Transport. Fishes, 8(12), 603. https://doi.org/10.3390/fishes8120603







