1. Introduction
The most important operating variable during Atlantic salmon aquaculture, representing around 47% of the cost of production in major salmon-producing countries, is feed [
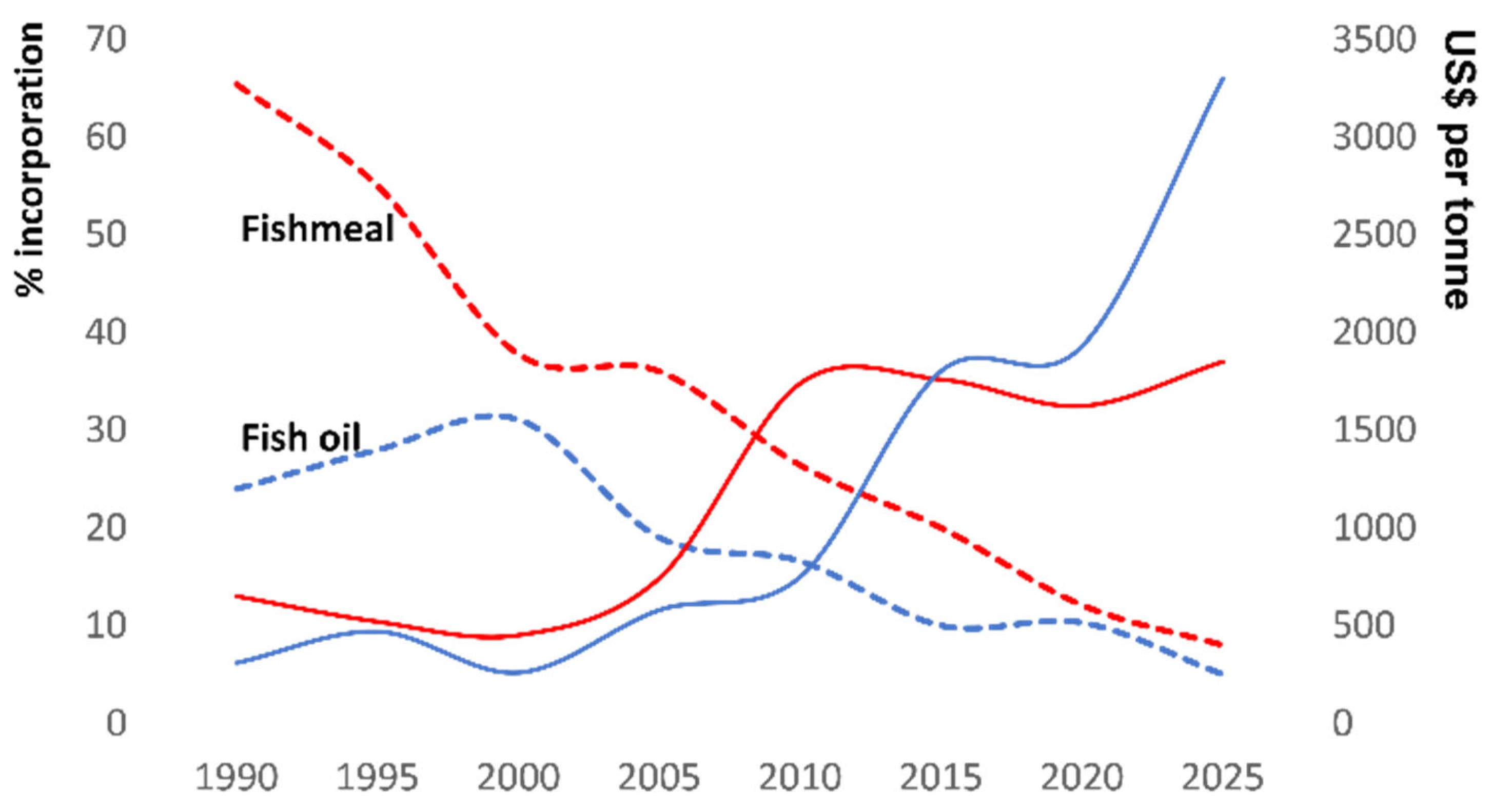
1]. Traditionally, salmon feed has relied heavily on fishmeal (FM) and fish oil (FO) as major ingredients [
2,
3]. This is because FM has high nutrient density, is decidedly digestible and palatable, and has an outstanding profile of essential amino acids, vitamins, and minerals. Because salmon and other vertebrates lack the δ12 and δ15 desaturases needed to convert oleic acid (18:1 n-9) into linoleic (C18:2 ω-6) and α-linolenic (18:3 ω-3) acid, they are unable to synthesize essential omega-3 fatty acids (EFAs) such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) de novo [
4,
5]. For these reasons, FO, an excellent source of EPA and DHA, became the gold standard provider for farmed salmon. However, over the last few decades, there has been a steady reduction in the use of FM/FO in salmon feeds (
Figure 1), and this trend is likely to continue for a variety of reasons. First, catches from industrial fisheries, which embody the key raw material for aquafeeds (i.e., forage fish), have experienced declines and fluctuations due to overfishing, natural events (e.g., El-Niño Southern Oscillation events), and climate change-induced effects [
6,
7]. Second, a greater proportion of forage fishes are now being used directly as human food [
8,
9], and third, competition for FM and marine oils has increased from the animal feeds sector, supplement, functional food, cosmetic, and pharmaceutical industries [
10,
11,
12]. These factors have combined to raise the cost of FM and FO (
Figure 1), and have spurred research into the use of alternative, more sustainable, and often cost-effective ingredients, which are now finding a more prominent place in marine carnivore feeds.
Conventionally, the replacement of FM in salmon feeds has been undertaken using both plant-derived and animal by-product ingredients [
18]. Some diets, and especially those having higher levels of plant proteins, have been associated with reduced feed intake, poorer feed conversion, and corresponding growth penalties [
19,
20,
21,
22,
23,
24]. This diminished growth has been attributed to several factors, including imbalances in dietary essential (EAA) and dispensable (DAA) amino acids, the presence of indigestible starch/fiber, anti-nutritional factors (ANFs), and decreased feed palatability [
24,
25]. Similarly, while many studies have determined that Atlantic salmon can be raised using vegetable oils, disproportionate dietary levels can change gut architecture, its microbiome and gene expression, as well as impair barrier functions and absorptive processes while reducing overall vigor and growth [
26,
27,
28,
29,
30]. Accordingly, while there exists an ever-growing collection of alternative proteins and oils, each has its own nutritional characteristics, technical characteristics, and constraints that may influence their suitability for producing practical salmon feed formulations.
Slower feeding and reduced feed intake are common issues observed with aquafeeds containing high levels of alternative, and usually unfamiliar ingredients. Diminished feed consumption leads to lower and slower growth, and lessens financial gain while increasing the potential for environmental degradation—and in RAS-based systems, increasing handling and solids removal combined with failing water quality. Various strategies have been employed to overcome these predicaments including, but not limited to, optimizing pellet size, shape, color, and texture, pellet resilience, and buoyancy in the water column. Fundamentally, any offered feed must, of course, be appealing to the target species to ensure optimal consumption. A frequently used tactic to attract and stimulate food consumption has been to utilize so-called feed palatants and/or attractants. A variety of studies have determined that a range of nucleotides, amino acids, and others, either alone or in combination, act as potent gustatory and olfactory stimuli that initiate arousal, searching, and consummatory behaviors in fishes [
31,
32,
33].
Antarctic krill meal (KM) has been favored as a salmon feed additive due to several factors. For example, electrophysiological evaluations of olfactory and gustatory responses are augmented when diets are supplemented with KM and extracts thereof [
34]; when added to soybean-based diets, KM has been reported to have an overall beneficial effect on growth when compared to FM-containing feed [
35]. The feed stimulating activity of KM is believed to be due to synergism between amino acids and/or nucleotides [
35,
36,
37]. Many trials, with various species of marine, diadromous, and freshwater fishes, at various points in their production cycle, have reported that KM products act as feed attractants/stimulants in non-FM-based diets and as FM replacers, providing a significant benefit [
38,
39,
40,
41,
42,
43,
44]. These studies variously describe increased growth, feed intake, conversion efficiencies, enhanced coloration, and favorable fatty acid (FA) profiles in treated fish, including salmonids [
42]. However, there are instances recorded in the literature [
45,
46,
47,
48,
49] where the addition of KM to diets, or as a FM replacer at various concentrations, had variable, no beneficial, and even negative effects, and these too include studies with salmonids [
50,
51,
52,
53].
Accordingly, there remains some doubt regarding the merit of using KM and its associated products in aquafeeds. Nevertheless, most krill caught today are utilized by the aquafeed industry [
54]. Moreover, an increasing number of reports point to sharp declines in regional Antarctic krill densities due to climate-induced changes in ocean temperature, currents, recruitment, acidification, overfishing, or a combination of these factors [
55,
56,
57,
58,
59,
60]. If krill populations experience negative consequences due to increased fishing pressures and climate change, various analyses and predictive models forecast corresponding perturbations in predator species (seabirds, seals, cetaceans) abundance [
61,
62,
63]. Hence, it is clearly relevant to verify the value of using KM in salmon diets. Here, we examine the impact of incorporating KM into FM/FO-free diets in which animal- or plant-based proteins replace that of FM, while FO is substituted by vegetable and algal oils. KM was integrated into the experimental diets at 0, 2.5, and 5% levels. The response of post-smolt Atlantic salmon to the test diets was also assessed against a FM/FO-based feed over a period of 90 days with minimum physical disturbance to the animals.
3. Results
There were no differences in weight, length, or condition factor (K) between treatment groups at the start of the trial (
Table 4), and test diets had no negative impact on the survival of experimental fish over the length of the study. A total of two mortalities (“jumpers”) were recorded over the 90-day trial, and these fish originated from different tanks and dietary treatments. Replacement of FO with vegetable and algal oils while altering dietary profiles of individual FAs did not affect total dietary saturated, monounsaturated, or polyunsaturated fatty acids (
Table 3). Higher (
p < 0.05) levels of C14:0, C15:0, C16:1 ω9, C20:1 ω9, and C22:1 ω9 were measured in the C1 feed when compared against all other diets. Unlike all other diets, the C1 feed also incorporated C18:3 ω6, C20:2 ω6, C20:3 ω3, and C20:3 ω6 (
p < 0.001). Dietary ω-3: ω-6 ratios were higher (
p < 0.05) for the C1 feed when compared against all other diets (
Table 3).
Measurements made during tank stock reduction (day 45) revealed that fish fed the P1 diet, the plant-based feed without KM, were smaller (
p < 0.02) in terms of weight and length when examined against all other groups (
Table 4).
K was higher in fish that were fed animal-based feeds supplemented with KM compared against all other diets (
p > 0.012), and by trial end, differences in weight, length, and
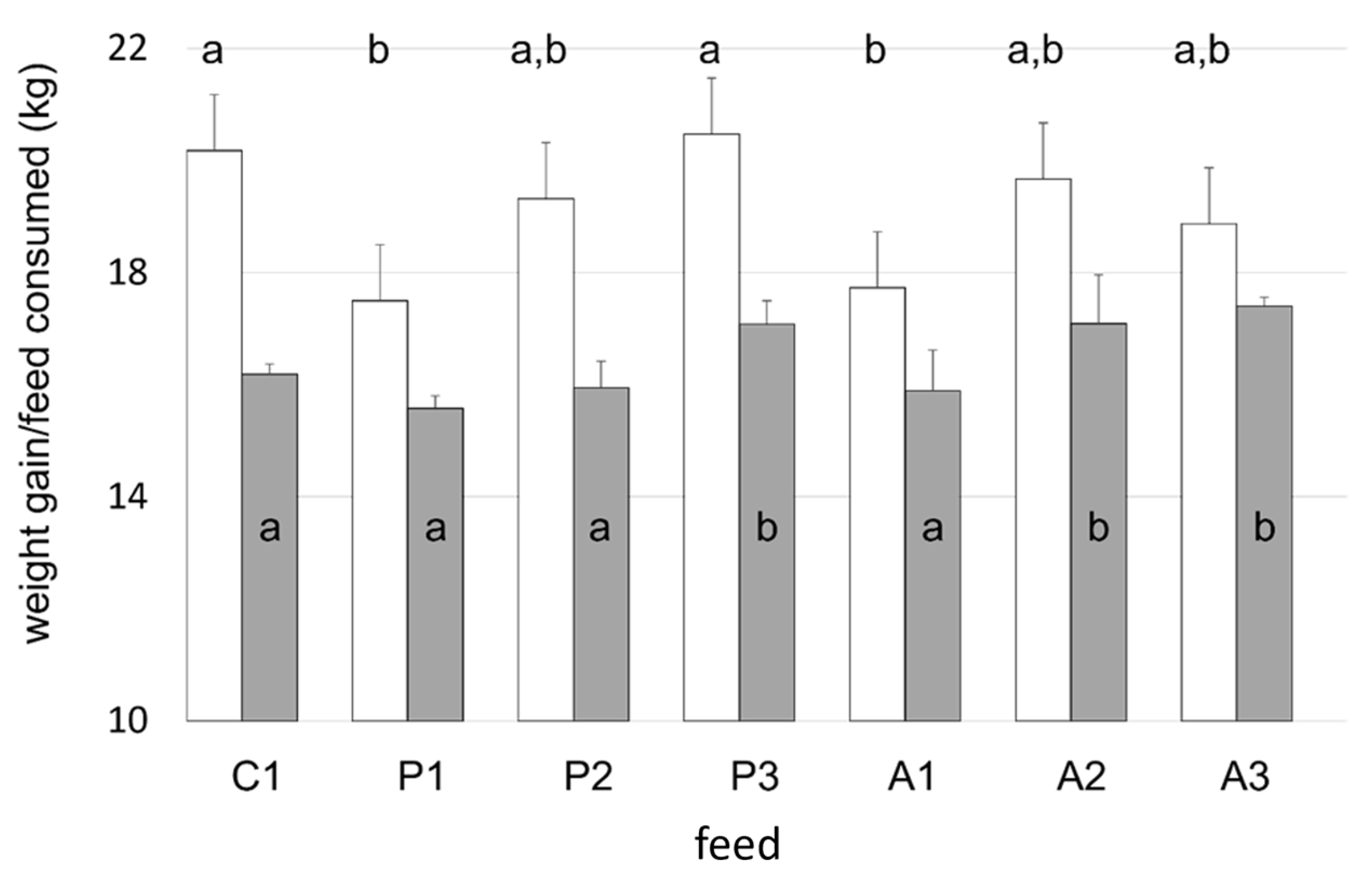
K were discerned between dietary groups. The supplementation of plant-based diets with KM resulted in a dose-dependent increase in weight gain of 6% and 10% for the 2.5% and 5% KM inclusion rates, respectively, even though dietary protein and lipid levels were equivalent to fish fed the diet devoid of krill (P1). The addition of KM to plant protein-based feeds increased total feed consumption at a 5% inclusion rate (P3) (
p < 0.001) with a corresponding increase in tank biomass (
Figure 2) such that overall biomass gain for P3 fish was identical to that measured in control tanks. Krill additions to salmon maintained on diets comprising mainly animal protein expressed differences in weight gain (
Figure 2;
Table 4), although not in a dose-dependent manner. Fish fed diets containing animal proteins (A1–A3) were shorter than control salmon, and this was reflected by differences in
K (
Table 4). As the duration of trial
K increased, irrespective of feed type presented (
p < 0.05), and by termination, all Atlantic salmon fed krill meal had the highest
K, ranging from 1.37 to 1.39 and above (
Table 4), which is considered a well-proportioned fish for the weight–length range [
64].
The average daily group feed intake throughout the trial varied from 189.8 ± 2.7 to 212.3 ± 2.2 g per day. The greatest by the A3 group and the lowest feed consumption was exhibited by the P1 group, with the former being higher than that expressed by all other treatment groups. The lowest cumulative feed intake was recorded for the P1 group, but this did not differ from that of fish receiving the C1, P2, and A1 diets. The addition of KM increased feed ingestion by fish fed the animal protein-based diets (A2 and A3) and the plant-based feed at the 5% inclusion level (P3;
Figure 2). The lower weight recorded for the P1-fed fish at day 45 was accompanied by a reduction (
p < 0.004) in their SGRs relative to all other groups (
Table 5). As anticipated, SGRs decreased (
p < 0.05, Student’s
t-test) between day 45 and 90 for all groups, reflecting the increasing fish weight. The only difference in SGR attained at trial end was observed when comparing the plant protein-based diets void of krill (P1). Mimicking the results recorded for weight gain and SGR, FCRs of salmon fed the P1 diet were higher (
p < 0.007) than all other groups (range 0.78 ± 0.06 to 0.85 ± 0.07) at the trial’s mid-point (day 45;
Table 5). By trial end, the highest and most comparable biological FCRs were observed for P1 and A3 fish (
p < 0.04). The FM/FO-based control diet (C1) returned a biological FCR that was identical to that recorded at the mid-point, whereas all other diets tested expressed higher values, except for the P1 feed, which exhibited an improvement (
Table 5). Examination of daily food intake, expressed as a percentage of body weight,
viz. SFR, revealed that the highest intake was established by fish fed the P3, A2, and A3 diets, and this differed (
p < 0.03) to that of the lowest intake illustrated by salmon fed the control (C1) diet (
Table 5). Calculation of the thermal growth coefficient (TGC) revealed that the P3 treatment resulted in the highest value (2.66 ± 0.04), differing (
p < 0.02) only to the result for fish fed the P1 feed (2.39 ± 0.09). All other treatment groups had similar values for the TGC ranging from 2.51 to 2.62.
At trial termination, the examination of twelve randomly taken fish from each treatment (n = 3 per tank; 12 per treatment) revealed no between-group differences in weight, length, visceral mass, or weight of the intestine (
Table 6;
Figure 3). Fillet yields too, were identical. Evaluation of fillet proximate composition revealed no differences between treatments for lipid, moisture, or ash contents (
Table 6). However, protein levels were affected, with salmon fed the plant-based diets expressing higher levels (
p < 0.047). Irrespective of experimental diet fed, whole-body lipid levels were the same proportionately for all groups (
Table 7). However, fish fed the control (C1) FM/FO-based diet expressed higher (
p < 0.05) myristic (C14:0) and palmitoleic (C16:1 ω9) acid levels, as well as elevated docosapentaenoic acid levels (C22:5 ω3;
Table 6). The most plentiful of the monounsaturated fatty acids, probably reflecting the use of canola oil in the feeds, was oleic acid (C18:1 ω9).
4. Discussion
Atlantic salmon fed on a primarily plant-based diet grew ~220% throughout the current trial without mortality. Moreover, values for their SFR, PPV, PER, morphological characteristics, and composition did not vary to those of control fish fed a FM/FO-based diet. Their growth rates, however, lagged behind all other treatment groups from day 45 onwards. These observations are thus generally in agreement with many other studies on Atlantic salmon where FM has been replaced with various plant proteins [
20,
21,
22,
23,
65,
66]. For example, in a comparable study, using fish of similar size and fed various plant concentrate-based feeds together with canola oil, the DHA-EPA rich marine algae
Nannochloropsis oceanica and low levels of FO, a 123–157% increase in body weight was observed after 84-days; however, growth of the FM/FO control group was greater (
p < 0.001) [
67]. In the present study, feed intake and TGCs were similar to that of other experiments with plant-based feeds, whereas FCRs were higher but still well within previously recorded ranges for Atlantic salmon. As is the case with FO alternatives (
op. cit.), replacement of FM with blends of plant proteins, including those that incorporate high levels of soy protein concentrate, have been reported to cause dysbiosis and influence the intestinal transcriptome of Atlantic salmon [
68]. Indeed, it is well established that when fed diets containing legumes, salmon can develop enteropathy, especially in the distal intestine [
27,
69]. This condition is characterized by changes in the dimensions of intestinal folds, reductions in enterocytic absorptive vacuoles, and infiltration of various inflammatory cells into the
lamina propria. Plant proteins may also influence the activities of digestive enzymes [
70,
71]. These dysfunctions, together with those that impact the expression of genes involved in enteritis, among a packet of others [
72], could potentially cause changes to gut permeability and barrier function—effects that could partially explain the increased FCR and reduced fish performance noted here.
In contrast to the plant-based diets, salmon presented the KM-free animal protein-based feeds performed as well as the control group. However, similar observations to those presented here have been made previously for salmon and other fish that were fed diets high in poultry meal [
73,
74,
75], although some contradictory studies have been presented [
76]. The inconsistency in accounts may reflect the use of different quality raw materials. For example, due to the variety of generally unsegregated material used for poultry meals, together with differences in processing and equipment, their protein content and nutritional quality varies—lacking certain EAAs, being high in ash, and expressing variable digestibility [
77,
78]. However, the poultry meal diets employed in the present study, which included other sources of dietary protein, expressed protein, ash, and EAA profiles not dissimilar to those of FM feeds. This may explain why the addition of KM to animal protein-based diets had no positive influence on Atlantic salmon weight growth. However, KM did increase feed consumption, and when incorporated at 5%, a significant increase in FCR occurred compared to the FM/FO control. The latter observations thereby imply that no advantage was gained with the addition of up to 5% KM in animal protein-based feeds. Similar observations have been made previously, especially with higher levels of dietary KM [
79,
80], but the noted lack of advantage generally contrasts to those of others. Thus, increased feed intake and superior growth in various species of salmonids fed FM-based diets with up to 80% KM inclusion have been commonly reported, but beyond this level, depressed growth and lower FCRs have been reported [
43]. The inconsistencies encountered with KM substitution of FM and alternative proteins in salmon diets have several potential explanations. For example, KMs are known to express seasonal, annual, and geographical variations in body composition [
57,
81] and hence nutritional quality, fluctuating in lipid and protein content, profile, and digestibility. Second, it has been established [
79] that incorporation of whole krill into meals reduces salmon growth relative to the use of partially deshelled krill meal, which was believed due to the increased presence of chitin. Whole krill-meal-based diets have a ~2.5-fold greater level of chitin than partially deshelled krill meal. The differences in chitin presence have been considered responsible for negatively impacting trypsin activities, lipase activities, and bile acid production, leading to reduced lipid and amino acid digestibility [
52,
79,
82]. Partially supporting these conclusions, the in vitro studies of [
83] also illustrated reduced amino acid digestibility with increasing dietary whole krill meal. Dietary KM has also been associated with nephrosis in Atlantic salmon, with the severity of the pathology increasing with dose [
79,
84].
In contrast to the animal protein-based feeds, benefits were observed for KM supplementation in the diets containing plant proteins. Increasing inclusion of KM resulted in improved weight gain, with fish achieving growth parity to animals fed the FM/FO-based feed. Others have reported similar outcomes, with various species presented with substantially reduced dietary FM, or its complete substitution with plant proteins [
39,
40,
49,
85,
86,
87,
88,
89]. No nutritional benefit was apparent for the plant-based KM test diets since they contained similar protein levels to the FM-FO control feeds, but differed in individual EAA content and lipid content. Since the plant-5% KM diet (P3) returned identical PERs, PPVs, 90-day bFCRs, TGC, and 90-day SGRs to those of the control, the benefits garnered by the former group can be best explained by increased feed intake due to enhanced palatability. However, because there were no differences in whole-body composition or visceral indices between the two groups, protein-sparing effects [
90,
91] cannot be discounted. The possibility also exists that the KM itself contributed nutritional benefit by providing additional nutrients to the feed, including astaxanthin and nucleotides, both of which are known to influence salmon health and growth.
In today’s volatile FO market, dietary lipids rich in omega-3 long chain polyunsaturated fatty acids (LC-PUFAs) are becoming scarcer, and depending on the FO substitution level, the presence of EPA and DHA in commercial salmon feeds can vary greatly [
92]. LC-PUFAs are engaged in a wide variety of cellular functions [
93,
94], and their requirements for laboratory-reared post-smolt Atlantic salmon range from 5 to 8% of the total pool of fatty acids (TFAs) [
95], or 10–15 g kg
−1 of the diet [
96,
97]. However, this requirement may be higher (>10% TFAs) when fish are maintained under more demanding conditions [
29] as might be experienced, for example, under net pen operations. Based on fish growth performance, the diets used in the present trial evidently attained or exceeded recommended requirements for LC-PUFAs. These observations ratify earlier experiments that determined no effect of replacing FO with plant oils on Atlantic salmon growth [
98,
99]. An important consequence of FO replacement has been the disruption of EPA:DHA ratios, with some suggesting that this may even result in essential FA deficiency in farmed salmon [
97]. Changes in EPA:DHA ratios, as noted in the present trial, can influence the expression of genes involved in the regulation of FA biosynthesis and metabolism in the mid- and hindgut and liver, as well as influencing immune function [
30,
95,
100,
101]. When vegetable oil replaces a large percentage of dietary FO, significant reductions in fillet 20:5ω3 (EPA) and 22:6ω3 (DHA) may also occur [
102,
103]. However, while the current study registered reduced fillet 20:5ω3, levels of 22:6ω3 remained unchanged, and this likely reflected higher dietary α-linolenic acid (18:3 ω-3), probably derived from canola oil, which salmon can bioconvert into 20:5ω3 and 22:6ω3 [
103]. The lack of effect of the non-FO diets on DHA presence apparently reflects the dietary incorporation of algal oil as an integral component. The production of heterotrophic microalgae—such as certain
Schizochytrium sp. strains—using fermentation technologies has provided the means to produce large quantities of biomass and processed oil yielding DHA and EPA. These yields are safe to use at 5% body weight [
104], and previous studies with Atlantic salmon have demonstrated the utility of
Schizochytrium biomass as a FO replacer, especially for DHA [
105,
106,
107,
108,
109]. While early studies noted the paucity of EPA in
Schizochytrium biomass, extracted oils from novel strains express EPA levels that support growth equivalent to fish fed conventional diets [
110,
111,
112]. Indeed, the same algal oil as used in the present study is now incorporated into commercial salmon diets. Nevertheless, differences were uncovered in ω3: ω6 ratios between the control and experimental diets used herein. Disturbances in omega-3 FA profiles, which provide known health benefits to consumers [
75], may reduce buyer incentive during purchasing decisions. Because of the wide-ranging health benefits of omega-3 fatty acids [
113], international, national, and non-governmental organizations suggest that the daily intake of EPA + DHA should be between 200–500 mg [
114,
115,
116]. Accordingly, a 75 g (2.8 oz.) serving of Atlantic salmon from the present trial, irrespective of dietary treatment, would exceed recommended daily intake levels (~590–745 mg).
Aquafeeds must be formulated to meet the biological requirements of the animal while being eaten in sufficient amounts to ensure survival and growth. Feed intake is reliant on various chemical, physical, and nutritional attributes that can each be modified by the ingredient selection and the processing technology used during pellet manufacture. While the present study employed a blend of seven alternative proteins and KM as a putative palatant, protein fractions from a wider variety of sources will undoubtedly become increasingly important as supply chains fluctuate and popular seafood species must be fed on cost-effective blended feeds. It is inevitable that new plant-based protein blends will be identified that more resemble the nutritional qualities of FM while reducing the potential for nutrient deficiencies [
24,
25,
117]. As pressure increases for the inclusion of alternative proteins to replace FM, the incorporation of rendered products too will inevitably expand [
118,
119]. For example, global production of chickens is estimated to be 33 billion individuals, equivalent to 102 million tons for 2023 [
120]. Raw materials left over from slaughterhouses and processing facilities represents about 30% of liveweight [
121], or around 30 million tons, which simply cannot be ignored as a potentially valuable aquafeed ingredient [
76,
119,
122]. Extensive incorporation of alternative animal proteins may lead to growth penalties, higher FCRs, and changes in body composition in salmonids [
73,
123,
124,
125,
126]. However, these ingredients appear to express a nutritional profile that more closely mimics that of FM, and lack the ANFs associated with plant products. This may provide an explanation for the growth equivalence observed for the animal protein-based diets and that of the FM/FO control group. Inexorably, these novel formulations will require the inclusion of natural feeding stimulants [
127]; as established herein, for plant-based diets, KM clearly offers potential. More extended feed trials are nonetheless warranted, since some reports indicate that the benefits of KM on feed intake may only be transitory [
43]. Moreover, partially deshelled KM can significantly decrease the stability of feed pellets in the water column when compared to traditional pellets [
84], which may be a function of the higher levels of soluble proteins in KM compared to FM [
52]. Certainly, many issues involving the use of KM as a feed palatant remain unresolved.
Industry Application
As in the case of FM/FO, it would be prudent for feed formulators to utilize several more reliable (consistent quality and cost effective) products rather than limiting themselves to a single unpredictable wild-caught ingredient. Thus, the F3 Future of Fish Feed (
F3challenge.Org (accessed on 12 January 2023)) launched the F3 Krill Replacement Challenge, utilizing a close variation of the P3 feed formula and experimental protocols described herein to identify potential krill replacements for aquafeeds. The challenge will wrap up in 2024, and results of this trial will be made available at that time, with the hope that the industry may adopt some of the tested market-ready krill replacements.