Effect of Feed Texture and Dimensions, on Feed Waste Type and Feeding Efficiency in Juvenile Sagmariasus verreauxi
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Systems
2.2. Experimental Feeds
- (1)
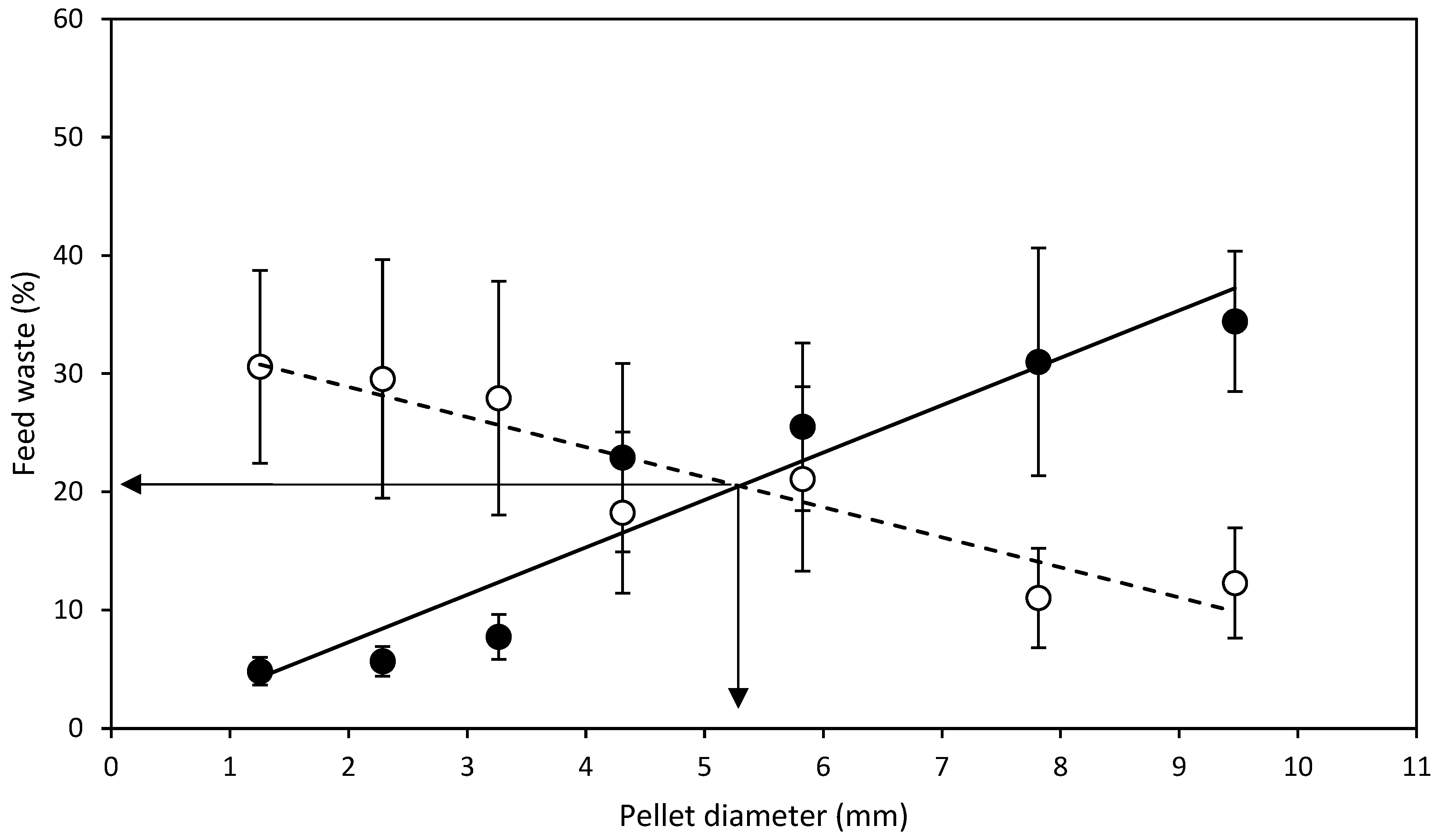
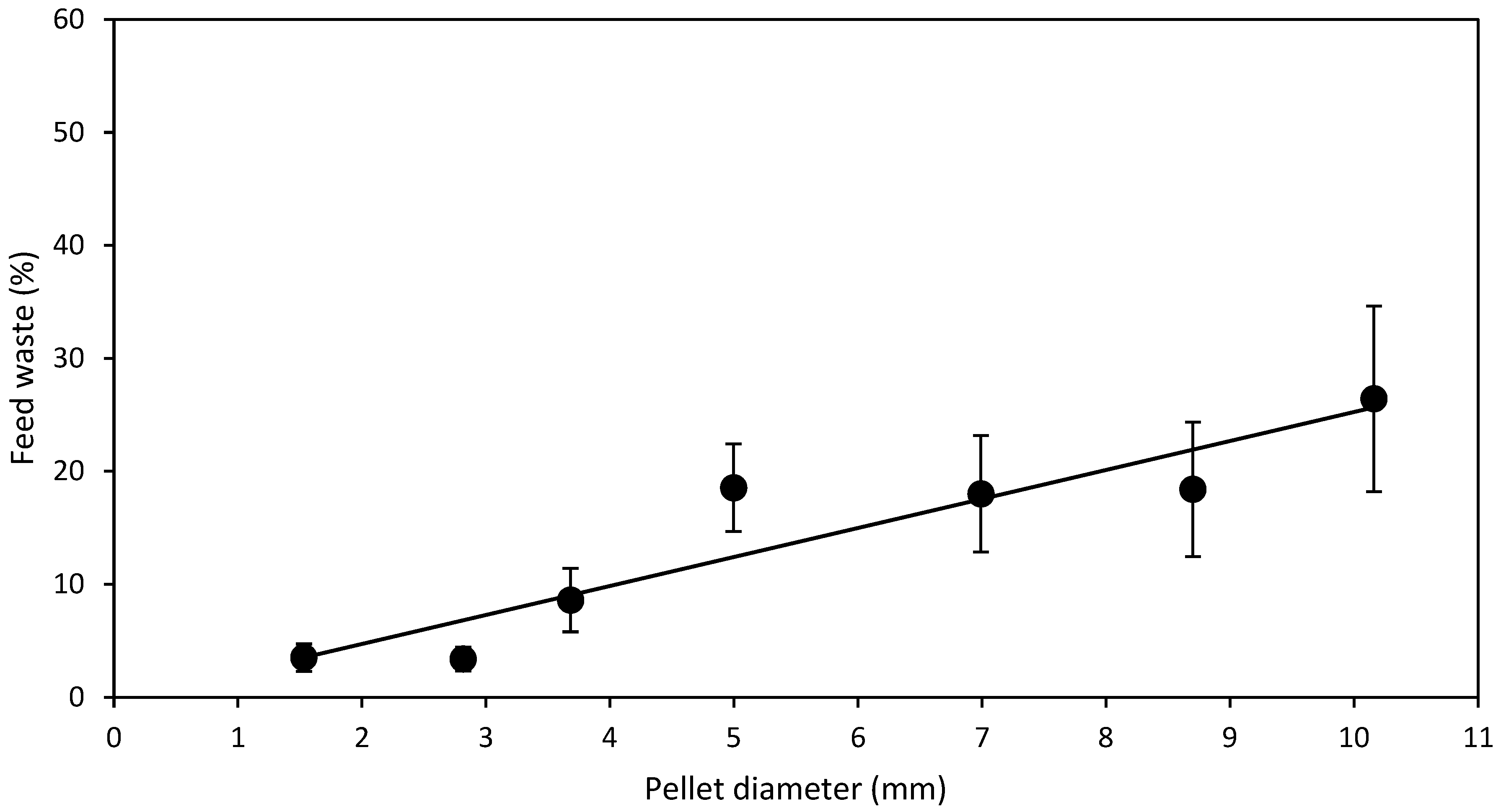
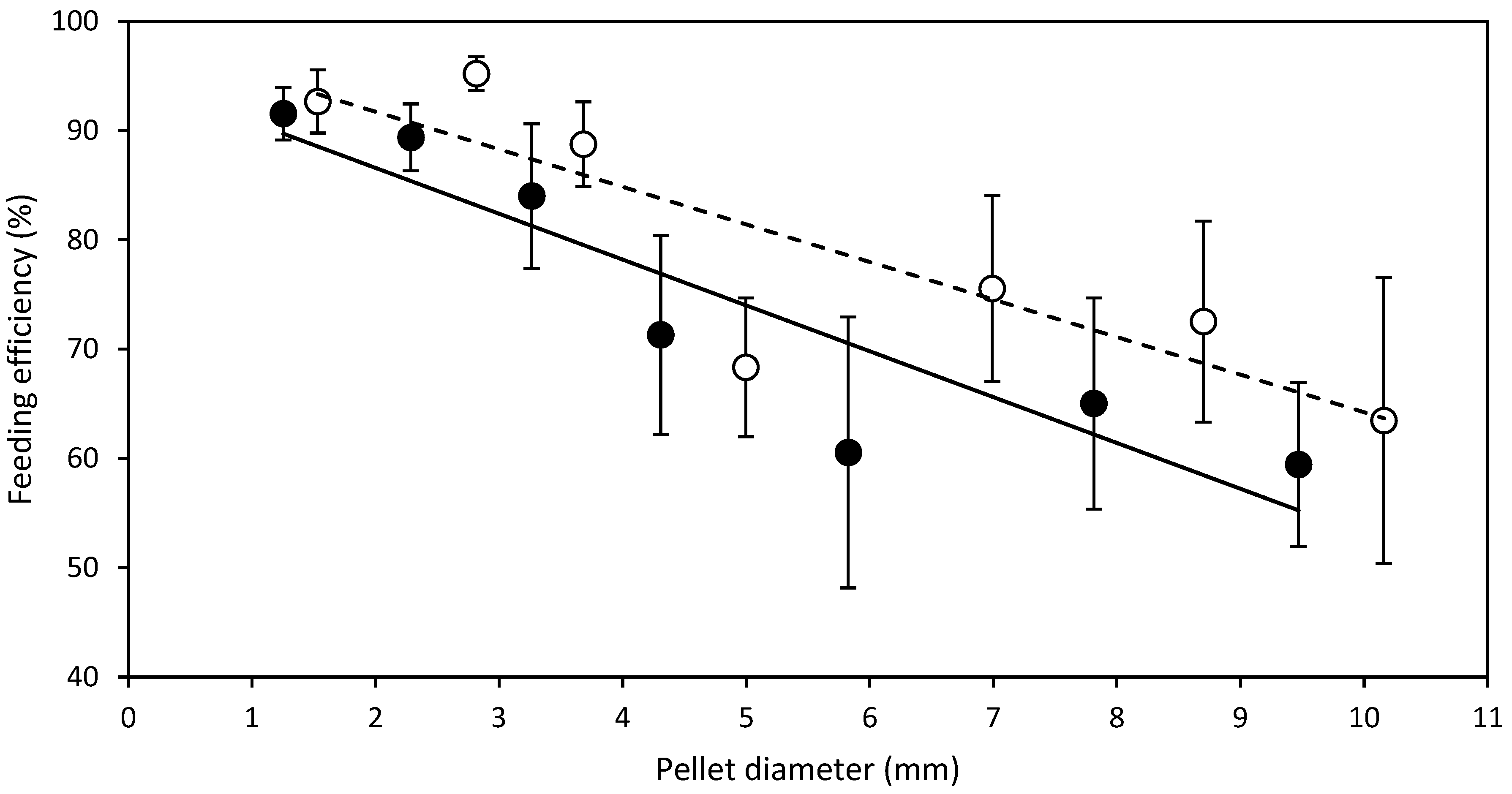
- D expt.—SMP feed strands of diameters, 1.5, 2.8, 3.7, 5.0, 7.0, 8.7, 10.2 mm were manufactured and cut to a standard length of 20 mm regardless of the diameter to produce SMP. Half of the manufactured SMP feed strands were dried from 6 to 24 h depending on the diameter, in a Steridium DS500 dryer, at 45 °C to a moisture content of 9.9 ± 0.6% to produce HDP. After drying of SMP to HDP, there was a reduction in the aforementioned SMP pellet diameters to 1.3, 2.3, 3.3, 4.3, 5.8, 7.8 and 9.5 mm HDP, respectively. Therefore, in total, 14 different feeds were produced from one batch, 7 SMP and 7 HDP.
- (2)
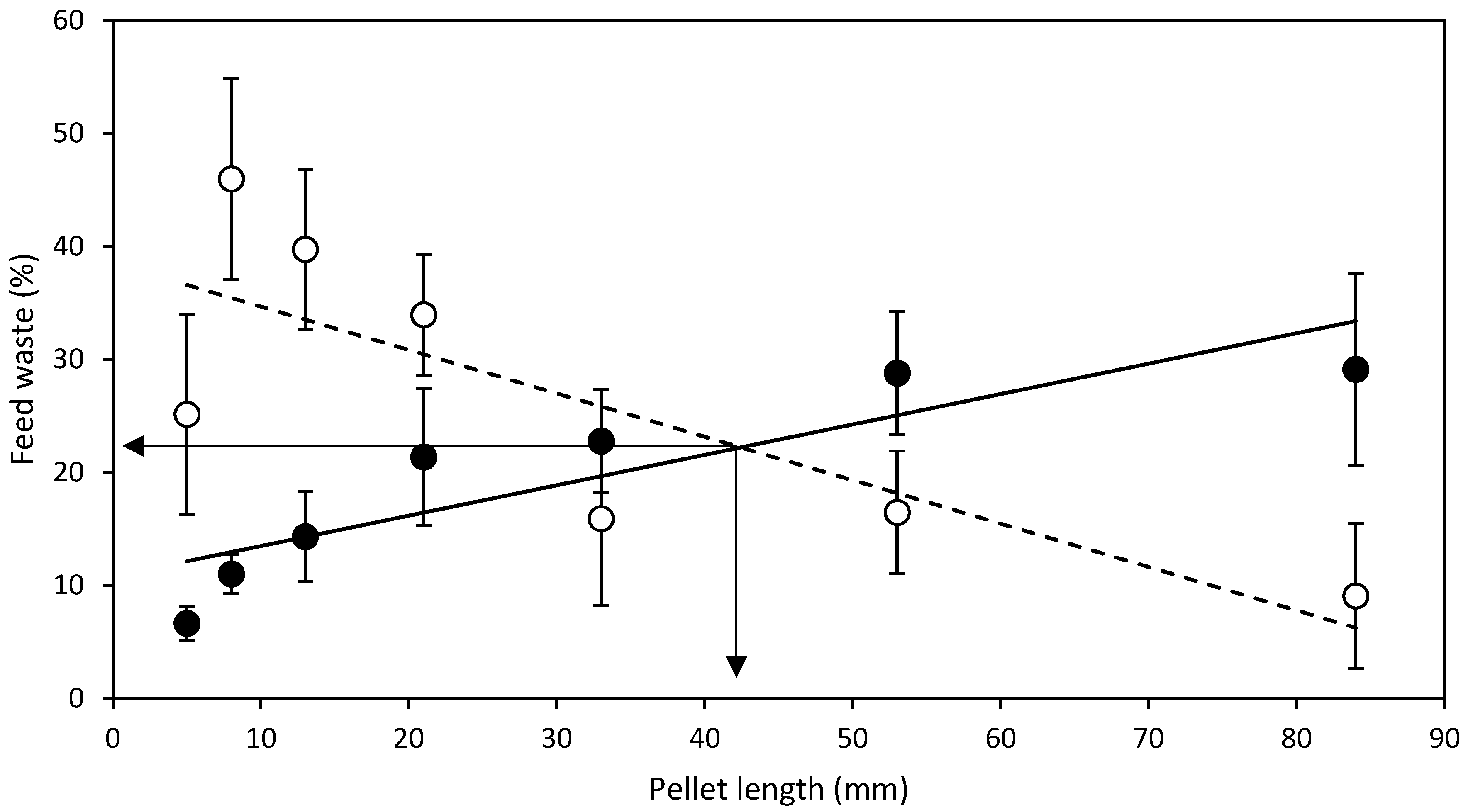
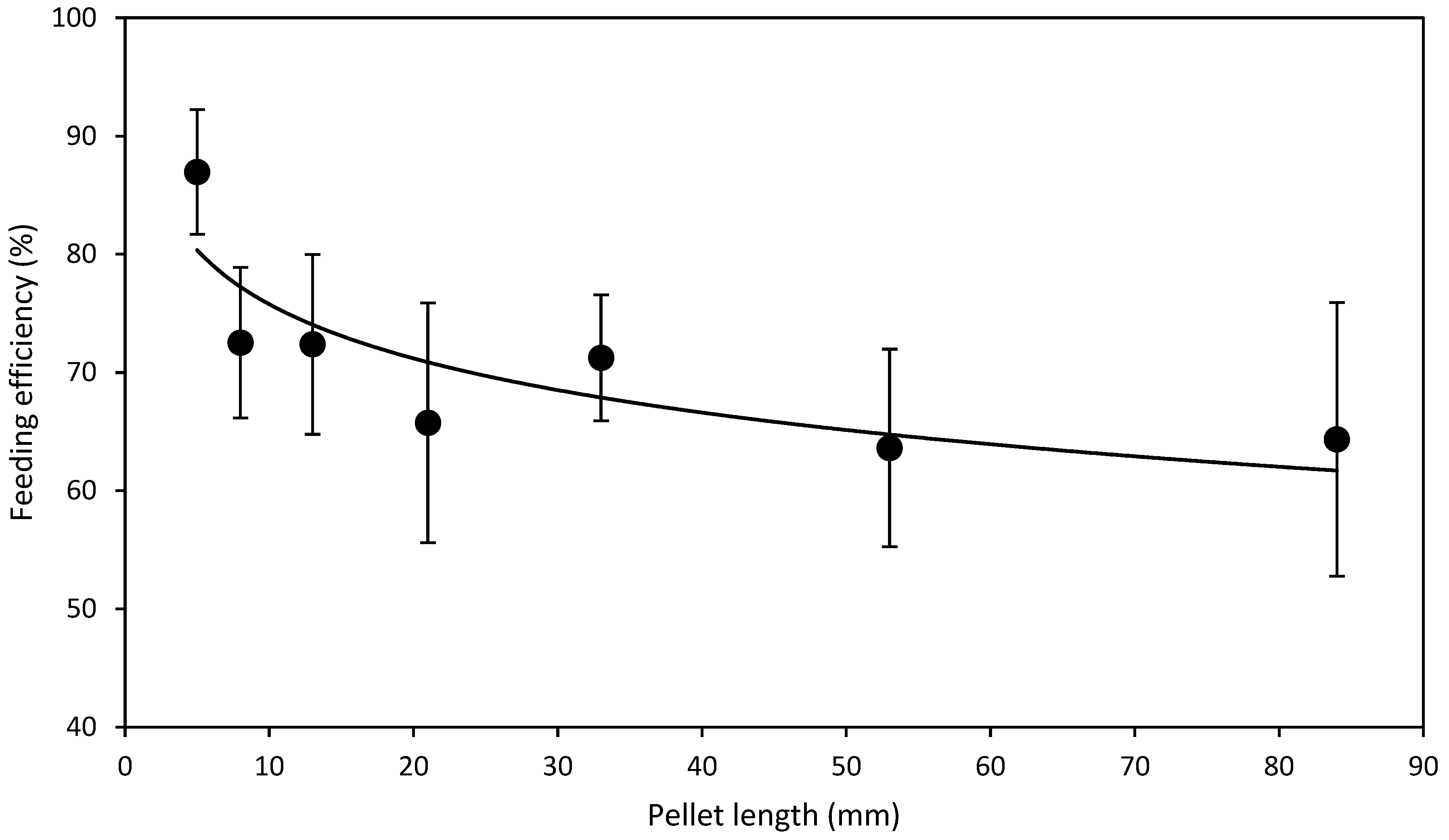
- L expt.—To test the effect of pellet length, one batch of HDP standard diameter of 4.3 mm were made and cut to lengths 5, 8, 13, 21, 33, 53 and 84 mm. A standard 4.3 mm diameter HDP was selected as it represented the first equal or lower (≤) available point where levels of FRW and NFRW were closest in HDP diameter tested in D expt.
2.3. Feeding and Feed Waste Collection
2.4. Data Analysis
3. Results
3.1. Diameter Experiment
3.2. Length Experiment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheppard, J.K.; Bruce, M.P.; Jeffs, A.G. Optimal feed pellet size for culturing juvenile spiny lobster Jasus edwardsii (Hutton, 1875) in New Zealand. Aquac. Res. 2002, 33, 913–916. [Google Scholar] [CrossRef]
- Zoutendyk, P. Feeding, defaecation and absorption efficiency in the Cape rock lobster Jasus lalandii. S. Afr. J. Mar. Sci. 1988, 6, 59–65. [Google Scholar] [CrossRef]
- Lau, C.J. Feeding Behavior of the Hawaiian Slipper Lobster, Scyllarides Squammosus, with a Review of Decapod Crustacean Feeding Tactics on Molluscan Prey. Bull. Mar. Sci. 1987, 41, 378–391. [Google Scholar]
- Cox, S.L.; Jeffs, A.G.; Davis, M. Developmental changes in the mouthparts of juvenile Caribbean spiny lobster, Panulirus argus: Implications for aquaculture. Aquaculture 2008, 283, 168–174. [Google Scholar] [CrossRef]
- Watling, L. The natural history of Crustacea, Functional morphology and diversity. In Feeding and Digestive System; Oxford University Press: Oxford, UK, 2013; Volume 1, pp. 237–260. [Google Scholar]
- Francis, D.S.; Salmon, M.L.; Kenway, M.J.; Hall, M.R. Palinurid lobster aquaculture: Nutritional progress and considerations for successful larval rearing. Rev. Aquac. 2014, 6, 180–203. [Google Scholar] [CrossRef]
- Smith, D.M.; Irvin, S.J.; Mann, D. Optimising the physical form and dimensions of feed pellets for tropical spiny lobsters. In Proceedings of the Spiny Lobster Aquaculture Symposium, Nha Trang, Vietnam, 9–10 December 2008; Australian Centre for International Agricultural Research: Canberra, Australia, 2008. [Google Scholar]
- Juanes, F.; Hartwick, E.B. Prey Size Selection in Dungeness Crabs: The Effect of Claw Damage. Ecology 1990, 71, 744–758. [Google Scholar] [CrossRef]
- Fitzgibbon, Q.P.; Simon, C.J.; Smith, G.G.; Carter, C.G.; Battaglene, S.C. Temperature dependent growth, feeding, nutritional condition and aerobic metabolism of juvenile spiny lobster, Sagmariasus verreauxi. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2017, 207, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Shu-Chien, A.C.; Han, W.Y.; Carter, C.G.; Fitzgibbon, Q.P.; Simon, C.J.; Kuah, M.K.; Battaglene, S.C.; Codabaccus, B.M.; Ventura, T. Effect of dietary lipid source on expression of lipid metabolism genes and tissue lipid profile in juvenile spiny lobster Sagmariasus verreauxi. Aquaculture 2017, 479, 342–351. [Google Scholar] [CrossRef]
- Montgomery, S.; Craig, J. Distribution and abundance of recruits of the eastern rock lobster (Jasus verreauxi) along the coast of New South Wales, Australia. N. Z. J. Mar. Freshw. Res. 2005, 39, 619–628. [Google Scholar] [CrossRef]
- Department of Primary Industries, NSW, Australia. 2023. Available online: https://www.dpi.nsw.gov.au/__data/assets/pdf_file/0010/1473913/Eastern-Rock-Lobster-Report-and-Determination-2023-24-fishing-period.pdf (accessed on 1 November 2023).
- Fitzgibbon, Q.P.; Battaglene, S.C. Effect of photoperiod on the culture of early-stage phyllosoma and metamorphosis of spiny lobster (Sagmariasus verreauxi). Aquaculture 2012, 368, 48. [Google Scholar] [CrossRef]
- Simon, C.J.; James, P.J. The effect of different holding systems and diets on the performance of spiny lobster juveniles, Jasus edwardsii (Hutton, 1875). Aquaculture 2007, 266, 166–178. [Google Scholar] [CrossRef]
- Crear, B.; Hart, P.; Thomas, C. The effect of photoperiod on growth, survival, colour and activity of juvenile southern rock lobster, Jasus edwardsii. Aquac. Res. 2003, 34, 439–444. [Google Scholar] [CrossRef]
- Chittleborough, R. Environmental factors affecting growth and survival of juvenile western rock lobsters Panulirus longipes (Milne-Edwards). Mar. Freshw. Res. 1975, 26, 177–196. [Google Scholar] [CrossRef]
- Landman, M.J.; Codabaccus, B.M.; Fitzgibbon, Q.P.; Smith, G.G.; Carter, C.G. Fresh or formulated: A preliminary evaluation of fresh blue mussel (Mytilus galloprovincialis) and formulated experimental feeds with inclusion of fresh blue mussel on the growth performance of hatchery-reared juvenile slipper lobster (Thenus australiensis). Aquaculture 2021, 531, 735924. [Google Scholar]
- Simon, C.J. Feed intake and its relation to foregut capacity in juvenile spiny lobster, Jasus edwardsii. N. Z. J. Mar. Freshw. Res. 2009, 43, 195–203. [Google Scholar] [CrossRef][Green Version]
- Cunniff, P.; Washington, D. Official Methods of Analysis of AOAC International, 5th ed.; AOAC International: Rockville, MD, USA, 1999. [Google Scholar]
- Daning Tuzan, A. Factors Affecting Growth Disparity in Spiny Lobster Aquaculture: The Effect of Physiology, Behaviour and Feeding. Ph.D. Dissertation, University of Tasmania, Tasmania, Australia, 2018. [Google Scholar]
- McGaw, I.J.; Penney, C.M. Effect of meal type on specific dynamic action in the green shore crab, Carcinus maenas. J. Comp. Physiol. B 2014, 184, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Tolomei, A.; Crear, B.; Johnston, D. Diet immersion time: Effects on growth, survival and feeding behaviour of juvenile southern rock lobster, Jasus edwardsii. Aquaculture 2003, 219, 303–316. [Google Scholar] [CrossRef]
- Derby, C.D.; Steullet, P.; Horner, A.J.; Cate, H.S. The sensory basis of feeding behaviour in the Caribbean spiny lobster, Panulirus argus. Mar. Freshw. Res. 2001, 52, 1339–1350. [Google Scholar] [CrossRef]
- Kropielnicka-Kruk, K.; Trotter, A.J.; Fitzgibbon, Q.P.; Smith, G.G.; Carter, C.G. The effect of conspecific interaction on survival, growth and feeding behaviour of early juvenile tropical spiny lobster Panulirus ornatus. Aquaculture 2019, 510, 234–247. [Google Scholar] [CrossRef]
- Kropielnicka-Kruk, K.; Fitzgibbon, Q.P.; Codabaccus, B.M.; Trotter, A.J.; Giosio, D.R.; Carter, C.G.; Smith, G.G. The effect of feed frequency on growth, survival and behaviour of juvenile spiny lobster (Panulirus ornatus). Animals 2022, 12, 2241. [Google Scholar] [CrossRef] [PubMed]
| Diameter (mm) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Texture | 1.3 | 2.3 | 3.3 | 4.3 | 5.8 | 7.8 | 9.5 | Test | a | b | R2 | df | F/Chi-Sq | p |
| AFI (% DW) | HDP | 64.6 ± 8.8 | 64.8 ± 10.8 | 64.3 ± 11.3 | 58.9 ± 9.9 | 53.4 ± 12.6 | 58.0 ± 10 | 53.3 ± 8.2 | ANOVA | 6,49 | 0.290 | 0.939 | |||
| Regression | −1.5 | 66.8 | 0.021 | 1,54 | 1.179 | 0.282 | |||||||||
| Leaching rate (% DW * 6 h−1) | HDP | 8.0 ± 0.2 a | 6.9 ± 0.8 ab | 8.6 ± 0.9 a | 8.7 ± 0.3 a | 7.3 ± 0.4 ab | 8.6 ± 0.0 a | 5.2 ± 0.1 b | ANOVA | 6,49 | 6.273 | 0.002 * | |||
| Regression | −0.2 | 8.6 | 0.149 | 1,19 | 3.321 | 0.084 | |||||||||
| 1.5 | 2.8 | 3.7 | 5.0 | 7.0 | 8.7 | 10.2 | |||||||||
| AFI (% DW) | SMP | 67.1 ± 11.1 | 76.3 ± 6.2 | 73.4 ± 6.6 | 49.4 ± 10.7 | 66.5 ± 9.6 | 59.0 ± 11.0 | 58.4 ± 13.1 | ANOVA | 6,49 | 0.687 | 0.661 | |||
| Regression | −1.5 | 72.9 | 0.026 | 1,54 | 1.465 | 0.231 | |||||||||
| NFRW (% DW) | SMP | 29.4 ± 10.5 | 20.3 ± 5.6 | 18.0 ± 5.0 | 32.0 ± 8.6 | 15.5 ± 5.0 | 22.6 ± 7.0 | 15.2 ± 6.3 | Regression | −1.0 | 27.5 | 0.023 | 1,54 | 1.275 | 0.264 |
| Leaching rate (% DW * 6 h−1) | SMP | 12.3 ± 0.2 a | 10.0 ± 0.3 ac | 9.1 ± 0.6 abc | 8.2 ± 0.2 abc | 6.1 ± 0.4 b | 7.6 ± 0.3 bc | 6.7 ± 1.6 bc | K-W | 6,20 | 14.615 | 0.023 * | |||
| Regression | −0.6 | 11.7 | 0.601 | 1,19 | 28.611 | 0.000 * | |||||||||
| Length (mm) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Parameter | Texture | 5 | 8 | 13 | 21 | 33 | 53 | 84 | Test | a | b | R2 | df | F/Chi-Sq | p |
| FI (% DW) | HDP | 71.4 ± 7.8 | 53.1 ± 8.1 | 56.2 ± 6.7 | 55.9 ± 7.0 | 68.7 ± 6.8 | 64.5 ± 6.5 | 70.1 ± 9.0 | Anova | 6,49 | 1.044 | 0.409 | |||
| Regression | 0.1 | 58.9 | 0.026 | 1,54 | 1.427 | 0.238 | |||||||||
| Leaching rate (% DW * 6 h−1) | HDP | 10.0 ± 0.3 a | 8.9 ± 0.4 ac | 7.7 ± 0.4 bcd | 7.7 ± 0.3 bcd | 8.1 ± 0.4 abc | 6.2 ± 0.5 bd | 6.1 ± 0.4 d | Anova | 6,49 | 12.365 | 0.000 * | |||
| Regression | −0.04 | 9.1 | 0.605 | 1,19 | 29.109 | 0.000 * | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kropielnicka-Kruk, K.; Fitzgibbon, Q.P.; Codabaccus, M.B.; Trotter, A.J.; Carter, C.G.; Smith, G.G. Effect of Feed Texture and Dimensions, on Feed Waste Type and Feeding Efficiency in Juvenile Sagmariasus verreauxi. Fishes 2023, 8, 553. https://doi.org/10.3390/fishes8110553
Kropielnicka-Kruk K, Fitzgibbon QP, Codabaccus MB, Trotter AJ, Carter CG, Smith GG. Effect of Feed Texture and Dimensions, on Feed Waste Type and Feeding Efficiency in Juvenile Sagmariasus verreauxi. Fishes. 2023; 8(11):553. https://doi.org/10.3390/fishes8110553
Chicago/Turabian StyleKropielnicka-Kruk, Katarzyna, Quinn P. Fitzgibbon, Mohamed B. Codabaccus, Andrew J. Trotter, Chris G. Carter, and Gregory G. Smith. 2023. "Effect of Feed Texture and Dimensions, on Feed Waste Type and Feeding Efficiency in Juvenile Sagmariasus verreauxi" Fishes 8, no. 11: 553. https://doi.org/10.3390/fishes8110553
APA StyleKropielnicka-Kruk, K., Fitzgibbon, Q. P., Codabaccus, M. B., Trotter, A. J., Carter, C. G., & Smith, G. G. (2023). Effect of Feed Texture and Dimensions, on Feed Waste Type and Feeding Efficiency in Juvenile Sagmariasus verreauxi. Fishes, 8(11), 553. https://doi.org/10.3390/fishes8110553






