Evaluating Suitability of Fishing Areas for Squid-Jigging Vessels in the Northwest Pacific Ocean Derived from AIS Data
Abstract
1. Introduction
2. Materials and Methods
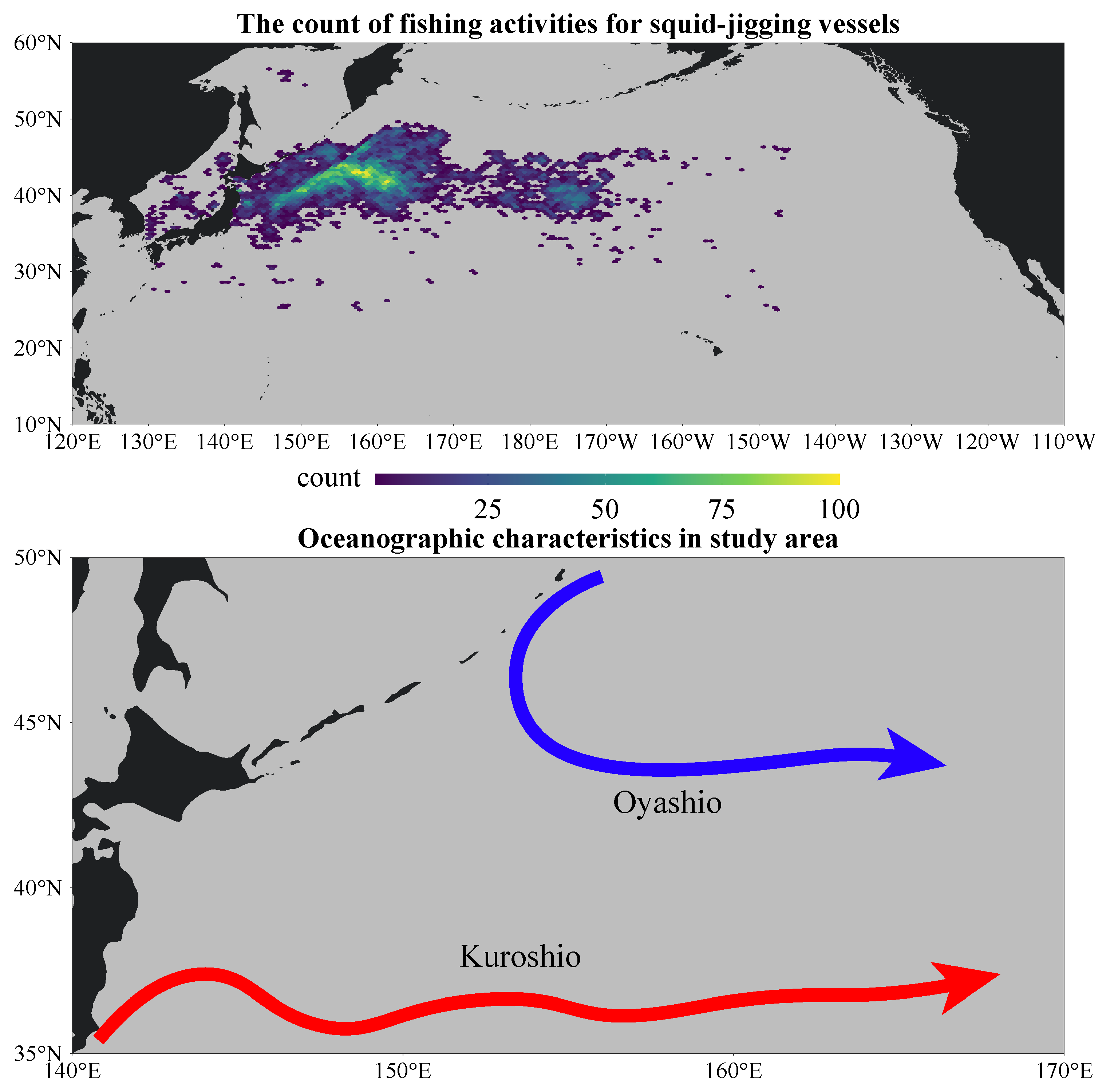
2.1. Study Area
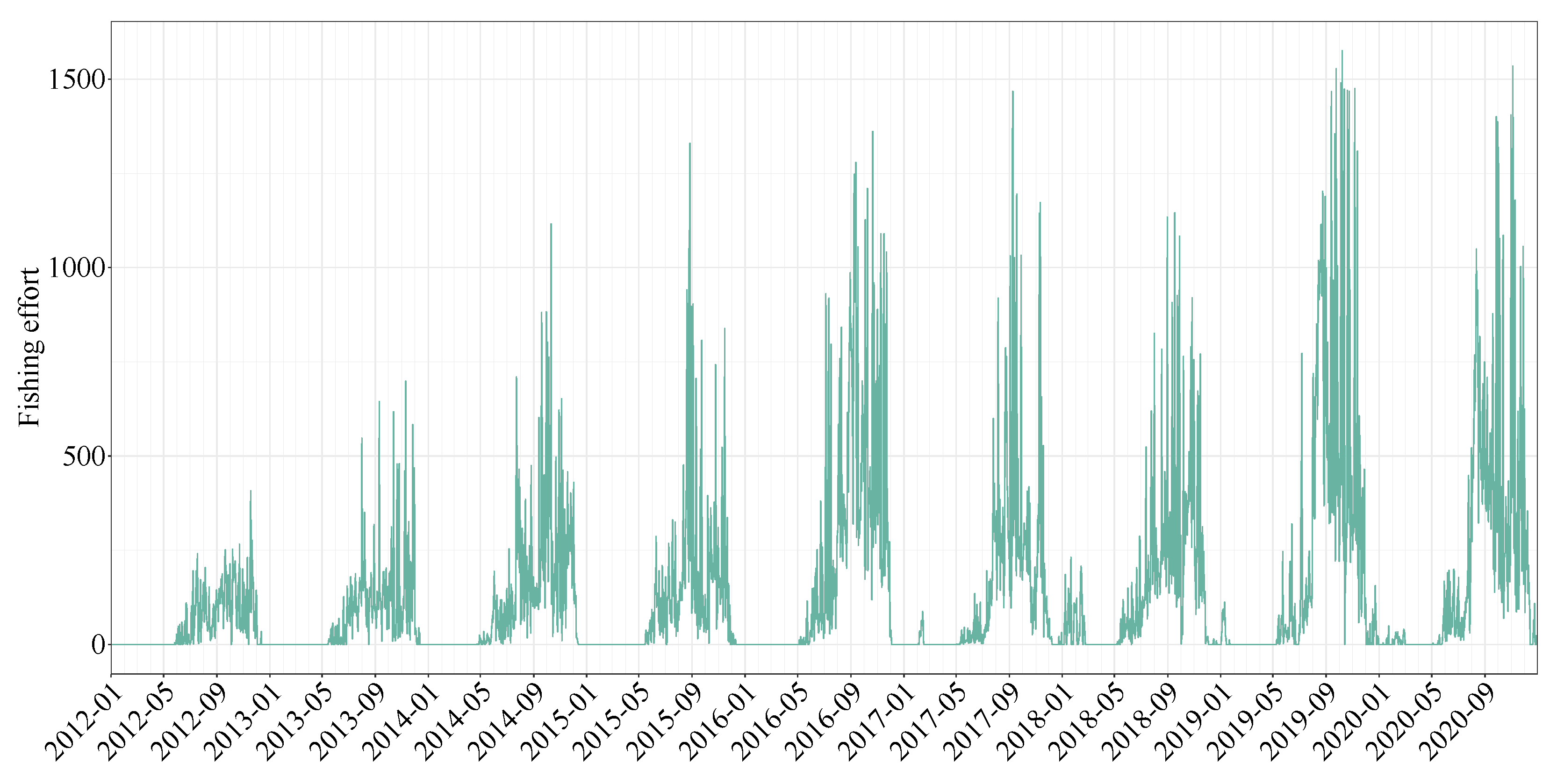
2.2. Fishing Effort Data and Fishery Data
2.3. Marine Environmental Data
2.4. BRT Model
2.5. HSI Model
2.6. Suitable Fishing Areas for Squid-Jigging Vessels
2.7. Evaluating O. bartramii Potential Habitat
3. Results
3.1. Parameters and Performance of the BRT Models
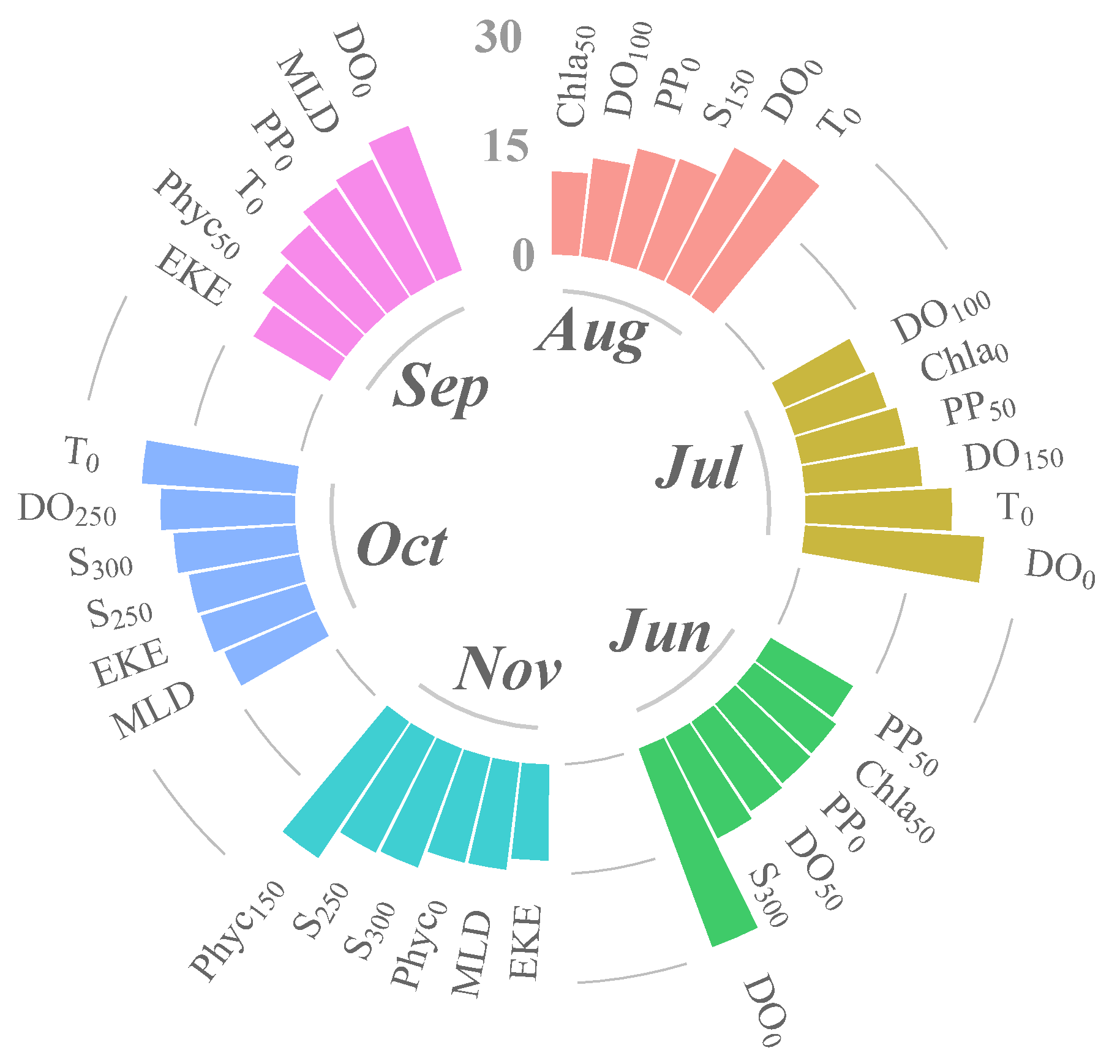
3.2. Relative Influence of Environmental Variables
3.3. Analysis of SI Curves
3.4. Analysis of Different HSI Models
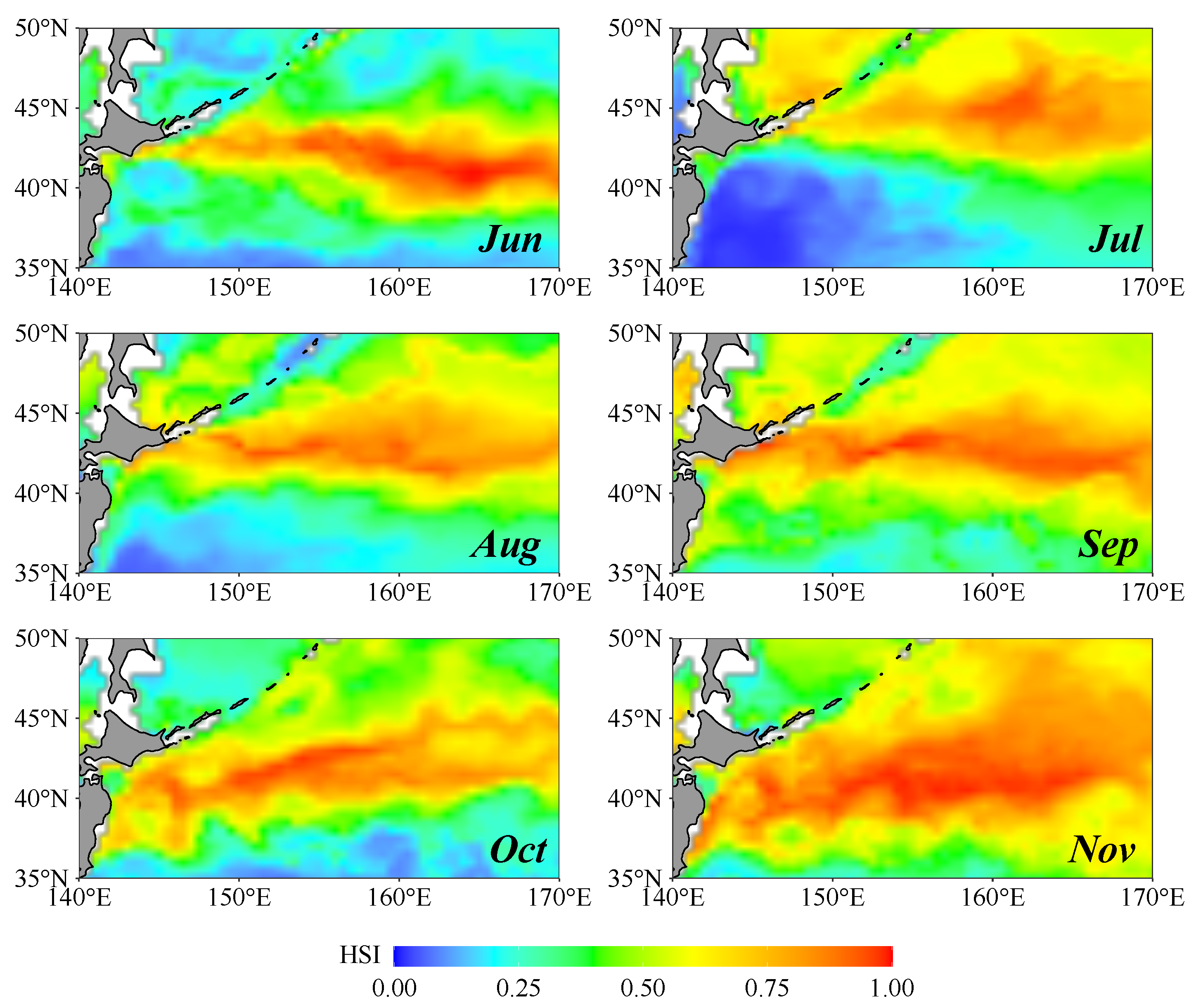
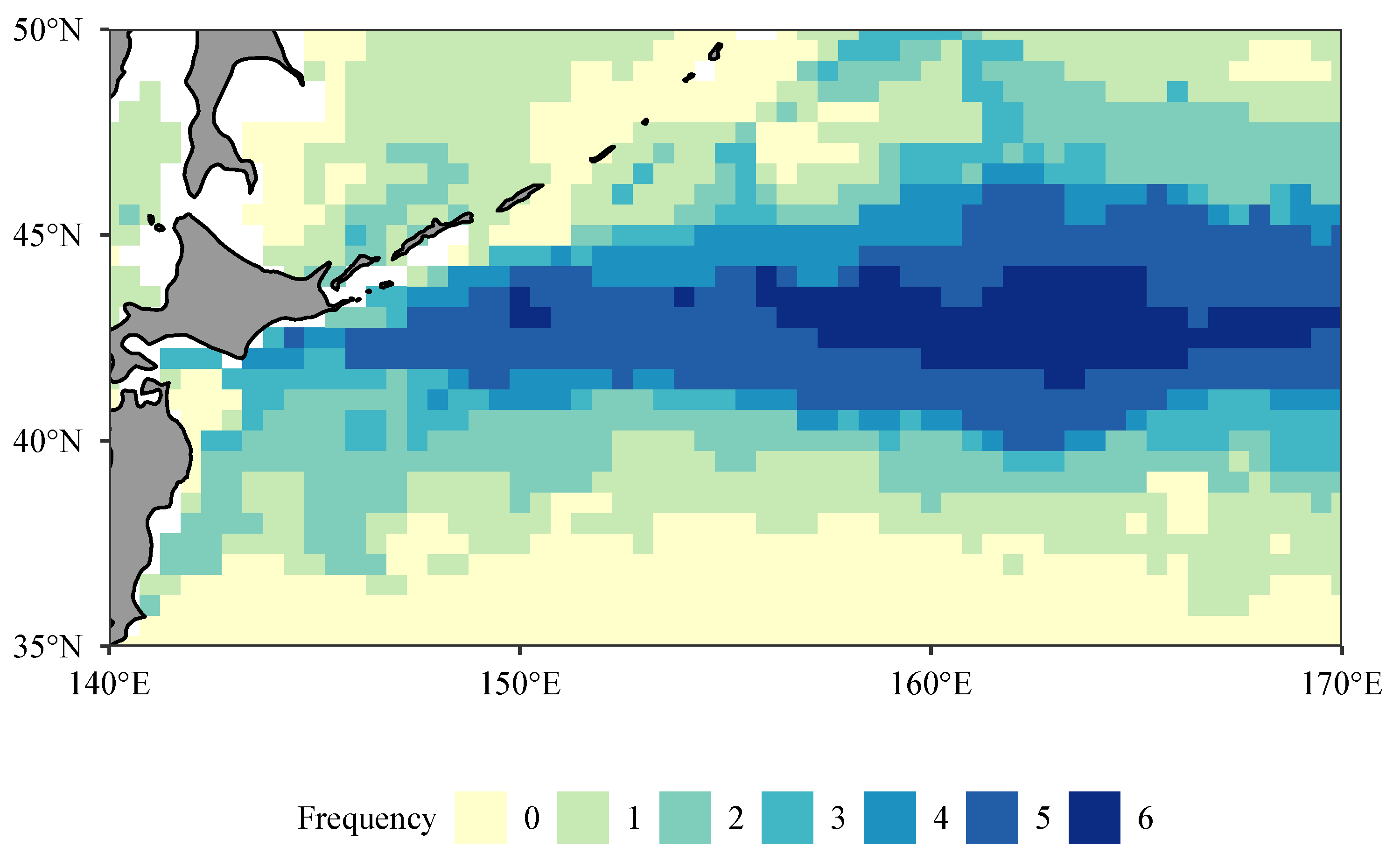
3.5. Spatial Distribution of Hotspots for Fishing
3.6. Revealing Squid Potential Habitat
4. Discussion
4.1. Model Construction
4.2. Influence of Oceanographic Conditions
4.3. Comparison for HSI Model
4.4. Analysis of the Suitable Fishing Area for Squid-Jigging Vessels
4.5. Fishing Effort in Comparison to Catch
4.6. Perspectives for Fisheries Management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cimino, M.A.; Anderson, M.; Schramek, T.; Merrifield, S.; Terrill, E.J. Towards a Fishing Pressure Prediction System for a Western Pacific EEZ. Sci. Rep. 2019, 5, 461. [Google Scholar] [CrossRef] [PubMed]
- Natale, F.; Gibin, M.; Alessandrini, A.; Vespe, M.; Paulrud, A. Mapping Fishing Effort through AIS Data. PLoS ONE 2015, 10, e0130746. [Google Scholar] [CrossRef] [PubMed]
- Damien, L.G.; Cyril, R.; Françoise, G.; David, B. Defining high-resolution dredge fishing grounds with Automatic Identification System (AIS) data. Aquat. Living Resour. 2017, 30, 39. [Google Scholar] [CrossRef]
- Ferrà, C.; Tassetti, A.N.; Grati, F.; Pellini, G.; Polidori, P.; Scarcella, G.; Fabi, G. Mapping change in bottom trawling activity in the Mediterranean Sea through AIS data. Mar. Policy 2018, 94, 275–281. [Google Scholar] [CrossRef]
- Kroodsma, D.A.; Mayorga, J.; Hochberg, T.; Miller, N.A.; Boerder, K.; Ferretti, F.; Wilson, A.; Bergman, B.; White, T.D.; Block, B.A.; et al. Tracking the global footprint of fisheries. Science 2018, 359, 904–908. [Google Scholar] [CrossRef]
- Le Tixerant, M.; Le Guyader, D.; Gourmelon, F.; Queffelec, B. How can Automatic Identification System (AIS) data be used for maritime spatial planning? Ocean. Coast. Manag. 2018, 166, 18–30. [Google Scholar] [CrossRef]
- Russo, E.; Monti, M.A.; Mangano, M.C.; Raffaetà, A.; Sarà, G.; Silvestri, C.; Pranovi, F. Temporal and spatial patterns of trawl fishing activities in the Adriatic Sea (Central Mediterranean Sea, GSA17). Ocean. Coast. Manag. 2020, 192, 105231. [Google Scholar] [CrossRef]
- White, T.D.; Ferretti, F.; Kroodsma, D.A.; Hazen, E.L.; Carlisle, A.B.; Scales, K.L.; Bograd, S.J.; Block, B.A. Predicted hotspots of overlap between highly migratory fishes and industrial fishing fleets in the northeast Pacific. Sci. Adv. 2019, 5, eaau3761. [Google Scholar] [CrossRef]
- Selig, E.; Nakayama, S.; Wabnitz, C.; Österblom, H.; Spijkers, J.; Miller, N.; Bebbington, J.; Sparks, J. Revealing global risks of labor abuse and illegal, unreported, and unregulated fishing. Nat. Commun. 2022, 13, 1612. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, H.; Fan, W.; Shi, H.; Fei, Y.; Yuan, S. Behaviour Impact Analysis of Tuna Purse Seiners in the Western and Central Pacific Based on the BRT and GAM Models. Front. Mar. Sci. 2022, 9, 881036. [Google Scholar] [CrossRef]
- Fei, Y.; Yang, S.; Fan, W.; Shi, H.; Zhang, H.; Yuan, S. Relationship between the Spatial and Temporal Distribution of Squid-Jigging Vessels Operations and Marine Environment in the North Pacific Ocean. J. Mar. Sci. Eng. 2022, 10, 550. [Google Scholar] [CrossRef]
- Arkhipkin, A.I.; Rodhouse, P.G.K.; Pierce, G.J.; Sauer, W.; Sakai, M.; Allcock, L.; Arguelles, J.; Bower, J.R.; Castillo, G.; Ceriola, L.; et al. World Squid Fisheries. Rev. Fish. Sci. Aquac. 2015, 23, 92–252. [Google Scholar] [CrossRef]
- Dong, E.; Shi, S.; He, J.; Tang, F. On state of squid jigging fishery and professional upgrading transformation of squid fishing vessels in the North Pacific—Take China Aquatic Products Zhoushan Marine Fisheries Co., LTD as an example. Fish. Inf. Strategy 2020, 35, 208–215. [Google Scholar] [CrossRef]
- Watanabe, H.; Kubodera, T.; Ichii, T.; Kawahara, S. Feeding habits of neon flying squid Ommastrephes bartramii in the transitional region of the central North Pacific. Mar. Ecol. Prog. Ser. 2004, 266, 173–184. [Google Scholar] [CrossRef]
- Nishikawa, H.; Toyoda, T.; Masuda, S.; Ishikawa, Y.; Sasaki, Y.; Igarashi, H.; Sakai, M.; Seito, M.; Awaji, T. Wind-induced stock variation of the neon flying squid (Ommastrephes bartramii) winter-spring cohort in the subtropical North Pacific Ocean. Fish. Oceanogr. 2015, 24, 229–241. [Google Scholar] [CrossRef]
- Anderson, C.I.; Rodhouse, P.G. Life cycles, oceanography and variability: Ommastrephid squid in variable oceanographic environments. Fish. Res. 2001, 54, 133–143. [Google Scholar] [CrossRef]
- Brander, K. Impacts of climate change on fisheries. J. Mar. Syst. 2010, 79, 389–402. [Google Scholar] [CrossRef]
- Brown, S.K.; Buja, K.R.; Jury, S.H.; Monaco, M.E.; Banner, A. Habitat Suitability Index Models for Eight Fish and Invertebrate Species in Casco and Sheepscot Bays, Maine. N. Am. J. Fish. Manag. 2000, 20, 408–435. [Google Scholar] [CrossRef]
- Chang, Y.J.; Sun, C.L.; Chen, Y.; Yeh, S.Z.; DiNardo, G.; Su, N.J. Modelling the impacts of environmental variation on the habitat suitability of swordfish, Xiphias gladius, in the equatorial Atlantic Ocean. ICES J. Mar. Sci. 2013, 70, 1000–1012. [Google Scholar] [CrossRef]
- Tian, S.; Chen, X.; Chen, Y.; Xu, L.; Dai, X. Evaluating habitat suitability indices derived from CPUE and fishing effort data for Ommatrephes bratramii in the northwestern Pacific Ocean. Fish. Res. 2009, 95, 181–188. [Google Scholar] [CrossRef]
- de Souza, E.N.; Boerder, K.; Matwin, S.; Worm, B. Improving Fishing Pattern Detection from Satellite AIS Using Data Mining and Machine Learning. PLoS ONE 2016, 11, e0158248. [Google Scholar] [CrossRef]
- Gong, C.; Chen, X.; Gao, F.; Tian, S. Effect of spatial and temporal scales on habitat suitability modeling: A case study of Ommastrephes bartramii in the Northwest Pacific Ocean. J. Ocean Univ. China 2014, 13, 1043–1053. [Google Scholar] [CrossRef]
- Feng, Y.; Cui, L.; Chen, X.; Chen, L.J.; Yang, Q. Impacts of changing spatial scales on CPUE-factor relationships of Ommastrephes bartramii in the northwest Pacific. Fish. Oceanogr. 2019, 28, 143–158. [Google Scholar] [CrossRef]
- Ichii, T.; Mahapatra, K.; Sakai, M.; Inagake, D.; Okada, Y. Differing body size between the autumn and the winter-spring cohorts of neon flying squid (Ommastrephes bartramii) related to the oceanographic regime in the North Pacific: A hypothesis. Fish. Oceanogr. 2004, 13, 295–309. [Google Scholar] [CrossRef]
- Nishikawa, H.; Igarashi, H.; Ishikawa, Y.; Sakai, M.; Kato, Y.; Ebina, M.; Usui, N.; Kamachi, M.; Awaji, T. Impact of paralarvae and juveniles feeding environment on the neon flying squid (Ommastrephes bartramii) winter–spring cohort stock. Fish. Oceanogr. 2014, 23, 289–303. [Google Scholar] [CrossRef]
- Wang, J.; Yu, W.; Chen, X.; Lin, L.; Chen, Y. Detection of potential fishing zones for neon flying squid based on remote-sensing data in the Northwest Pacific Ocean using an artificial neural network. Int. J. Remote Sens. 2015, 36, 3317–3330. [Google Scholar] [CrossRef]
- Wang, J.; Cheng, Y.; Lu, H.; Chen, X.; Lin, L.; Zhang, J. Water Temperature at Different Depths Affects the Distribution of Neon Flying Squid (Ommastrephes bartramii) in the Northwest Pacific Ocean. Front. Mar. Sci. 2022, 8, 741620. [Google Scholar] [CrossRef]
- Yu, W.; Chen, X.; Yi, Q.; Chen, Y. Spatio-temporal distributions and habitat hotspots of the winter-spring cohort of neon flying squid Ommastrephes Bartramii in relation to oceanographic conditions in the Northwest Pacific Ocean. Fish. Res. 2016, 175, 103–115. [Google Scholar] [CrossRef]
- Yu, W.; Chen, X.; Yi, Q.; Chen, Y. Influence of oceanic climate variability on stock level of western winter-spring cohort of Ommastrephes bartramii in the Northwest Pacific Ocean. Int. J. Remote Sens. 2016, 37, 3974–3994. [Google Scholar] [CrossRef]
- Chen, P.; Chen, X.; Yu, W.; Lin, D. Interannual Abundance Fluctuations of Two Oceanic Squids in the Pacific Ocean Can Be Evaluated through Their Habitat Temperature Variabilities. Front. Mar. Sci. 2021, 8, 770224. [Google Scholar] [CrossRef]
- Murata, M.; Nakamura, Y.N. Seasonal migration and diel vertical migration of the neon flying squid, Ommastrephes bartramii in the North Pacific. In Proceedings of the International Symposium on Large Pelagic Squids; Okutani, T., Ed.; Japan Marine Fishery Resources Research Center: Yokohama, Japan, 1998; pp. 13–30. [Google Scholar]
- Scharffenberg, M.G.; Stammer, D. Seasonal variations of the large-scale geostrophic flow field and eddy kinetic energy inferred from the TOPEX/Poseidon and Jason-1 tandem mission data. J. Geophys. Res. Oceans 2010, 115. [Google Scholar] [CrossRef]
- Elith, J.; Leathwick, J.R.; Hastie, T. A working guide to boosted regression trees. J. Anim. Ecol. 2008, 77, 802–813. [Google Scholar] [CrossRef] [PubMed]
- Manel, S.; Williams, H.C.; Ormerod, S. Evaluating presence-absence models in ecology: The need to account for prevalence. J. Appl. Ecol. 2001, 38, 921–931. [Google Scholar] [CrossRef]
- Yu, W.; Chen, X.; Zhang, Y.; Yi, Q. Habitat suitability modelling revealing environmental-driven abundance variability and geographical distribution shift of winter–spring cohort of neon flying squid Ommastrephes bartramii in the northwest Pacific Ocean. ICES J. Mar. Sci. 2019, 76, 1722–1735. [Google Scholar] [CrossRef]
- Yu, W.; Chen, X. Habitat suitability response to sea-level height changes: Implications for Ommastrephid squid conservation and management. Aquac. Fish. 2021, 6, 309–320. [Google Scholar] [CrossRef]
- Alabia, I.D.; Saitoh, S.I.; Igarashi, H.; Ishikawa, Y.; Usui, N.; Kamachi, M.; Awaji, T.; Seito, M. Ensemble squid habitat model using three-dimensional ocean data. ICES J. Mar. Sci. 2016, 73, 1863–1874. [Google Scholar] [CrossRef]
- Brodie, S.; Jacox, M.G.; Bograd, S.J.; Welch, H.; Dewar, H.; Scales, K.L.; Maxwell, S.M.; Briscoe, D.M.; Edwards, C.A.; Crowder, L.B.; et al. Integrating Dynamic Subsurface Habitat Metrics Into Species Distribution Models. Front. Mar. Sci. 2018, 5, 219. [Google Scholar] [CrossRef]
- Melo-Merino, S.M.; Reyes-Bonilla, H.; Lira-Noriega, A. Ecological niche models and species distribution models in marine environments: A literature review and spatial analysis of evidence. Ecol. Model. 2020, 415, 108837. [Google Scholar] [CrossRef]
- Sakurai, Y.; Kiyofuji, H.; Saitoh, S.I.; Yamamoto, J.; Goto, T.; Mori, K.; Kinoshita, T. Stock fluctuations of the Japanese common squid, Todarodes pacificus, related to recent climate changes. Fish. Sci. 2002, 68, 226–229. [Google Scholar] [CrossRef][Green Version]
- Xue, Y.; Guan, L.; Tanaka, K.; Li, Z.; Chen, Y.; Ren, Y. Evaluating effects of rescaling and weighting data on habitat suitability modeling. Fish. Res. 2017, 188, 84–94. [Google Scholar] [CrossRef]
- Alabia, I.D.; Saitoh, S.I.; Mugo, R.; Igarashi, H.; Ishikawa, Y.; Usui, N.; Kamachi, M.; Awaji, T.; Seito, M. Seasonal potential fishing ground prediction of neon flying squid (Ommastrephes bartramii) in the western and central North Pacific. Fish. Oceanogr. 2015, 24, 190–203. [Google Scholar] [CrossRef]
- Bower, J.R.; Ichii, T. The red flying squid (Ommastrephes bartramii): A review of recent research and the fishery in Japan. Fish. Res. 2005, 76, 39–55. [Google Scholar] [CrossRef]
- Young, R.E.; Hirota, J. Description of Ommastrephes bartramii (Cephalopoda: Ommastrephidae) Paralarvae with Evidence for Spawning in Hawaiian Waters. Pac. Sci. 1990, 44, 71–80. [Google Scholar]
- Ichii, T.; Mahapatra, K.; Sakai, M.; Okada, Y. Life history of the neon flying squid: Effect of the oceanographic regime in the North Pacific Ocean. Mar. Ecol. Prog. Ser. 2009, 378, 1–11. [Google Scholar] [CrossRef]
- Yu, W.; Yi, Q.; Chen, X.; Chen, Y. Modelling the effects of climate variability on habitat suitability of jumbo flying squid, Dosidicus gigas, in the Southeast Pacific Ocean off Peru. ICES J. Mar. Sci. 2015, 73, 239–249. [Google Scholar] [CrossRef]
- Igarashi, H.; Saitoh, S.I.; Ishikawa, Y.; Kamachi, M.; Usui, N.; Sakai, M.; Imamura, Y. Identifying potential habitat distribution of the neon flying squid (Ommastrephes bartramii) off the eastern coast of Japan in winter. Fish. Oceanogr. 2018, 27, 16–27. [Google Scholar] [CrossRef]
- Bordalo-Machado, P. Fishing Effort Analysis and Its Potential to Evaluate Stock Size. Rev. Fish. Sci. 2006, 14, 369–393. [Google Scholar] [CrossRef]
- Chen, X.; Tian, S.; Chen, Y.; Liu, B. A modeling approach to identify optimal habitat and suitable fishing grounds for neon flying squid (Ommastrephes bartramii) in the Northwest Pacific Ocean. Fish. Bull. Natl. Ocean. Atmos. Adm. 2010, 108, 1–14. [Google Scholar]
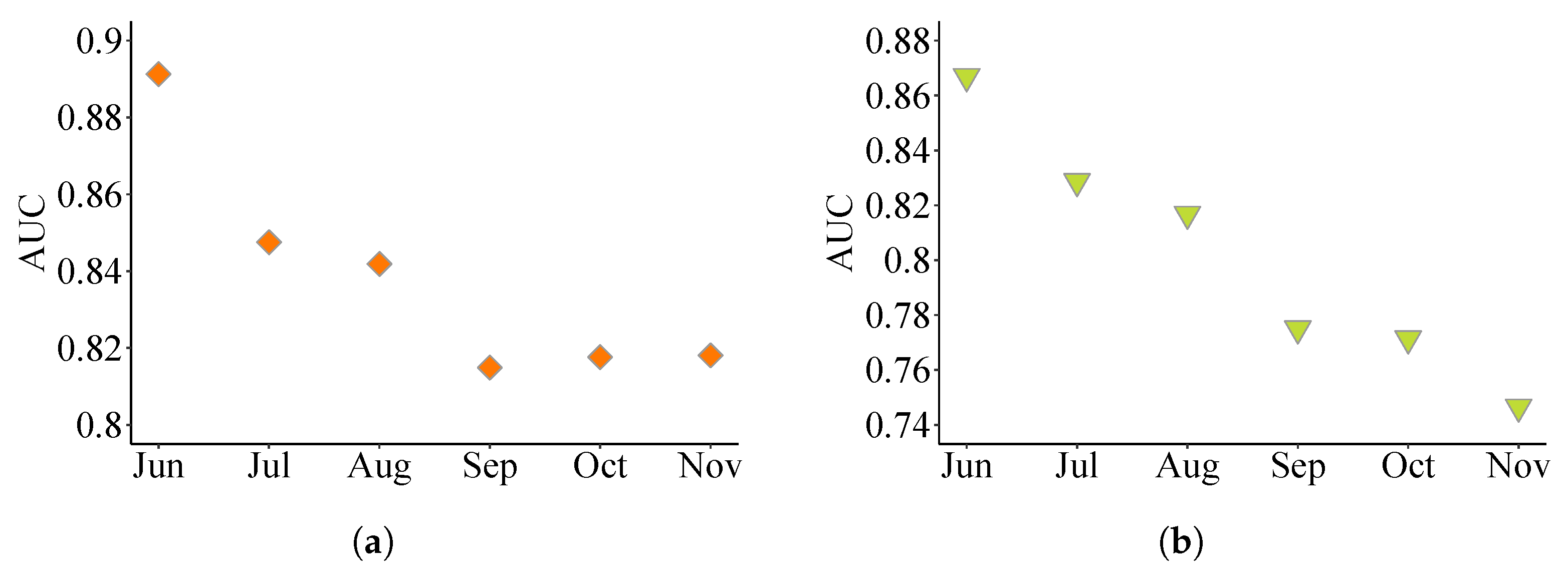


| Variables | Abbreviation | Units |
|---|---|---|
| Water temperature (dm) | ||
| Chlorophyll-a (dm) | ||
| Salinity (dm) | (dimensionless) | |
| Primary production (dm) | ||
| Dissolved oxygen (dm) | ||
| Phytoplankton concentration (dm) | ||
| Sea surface high | ||
| Mixed layer depth | ||
| Eddy kinetic energy |
| Habitat Class | Effort | Catch | ||
|---|---|---|---|---|
| Yield (h) | Frequency (%) | Yield (t) | Frequency (%) | |
| Poor | 866.6 | 5.7 | 194.9 | 10.8 |
| Normal | 11,116.5 | 37.9 | 604.5 | 31.1 |
| Suitable | 34,355.5 | 56.4 | 5716.4 | 58.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fei, Y.; Yang, S.; Huang, M.; Wu, X.; Yang, Z.; Zhao, J.; Tang, F.; Fan, W.; Yuan, S. Evaluating Suitability of Fishing Areas for Squid-Jigging Vessels in the Northwest Pacific Ocean Derived from AIS Data. Fishes 2023, 8, 530. https://doi.org/10.3390/fishes8100530
Fei Y, Yang S, Huang M, Wu X, Yang Z, Zhao J, Tang F, Fan W, Yuan S. Evaluating Suitability of Fishing Areas for Squid-Jigging Vessels in the Northwest Pacific Ocean Derived from AIS Data. Fishes. 2023; 8(10):530. https://doi.org/10.3390/fishes8100530
Chicago/Turabian StyleFei, Yingjie, Shenglong Yang, Mengya Huang, Xiaomei Wu, Zhenzhen Yang, Jiangyue Zhao, Fenghua Tang, Wei Fan, and Sanling Yuan. 2023. "Evaluating Suitability of Fishing Areas for Squid-Jigging Vessels in the Northwest Pacific Ocean Derived from AIS Data" Fishes 8, no. 10: 530. https://doi.org/10.3390/fishes8100530
APA StyleFei, Y., Yang, S., Huang, M., Wu, X., Yang, Z., Zhao, J., Tang, F., Fan, W., & Yuan, S. (2023). Evaluating Suitability of Fishing Areas for Squid-Jigging Vessels in the Northwest Pacific Ocean Derived from AIS Data. Fishes, 8(10), 530. https://doi.org/10.3390/fishes8100530






