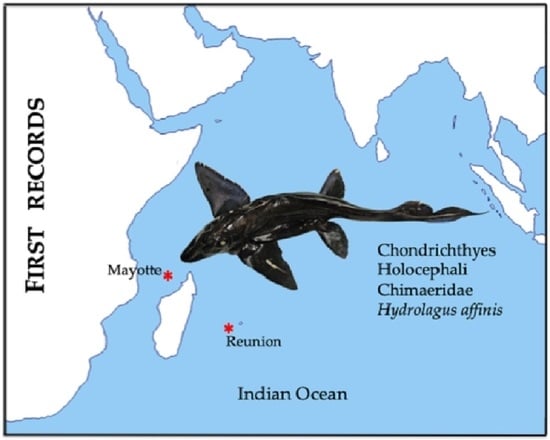
First Records of a Hydrolagus Species (Holocephali: Chimaeridae) from Reunion Island and Mayotte (Southwestern Indian Ocean)
Abstract
:1. Introduction
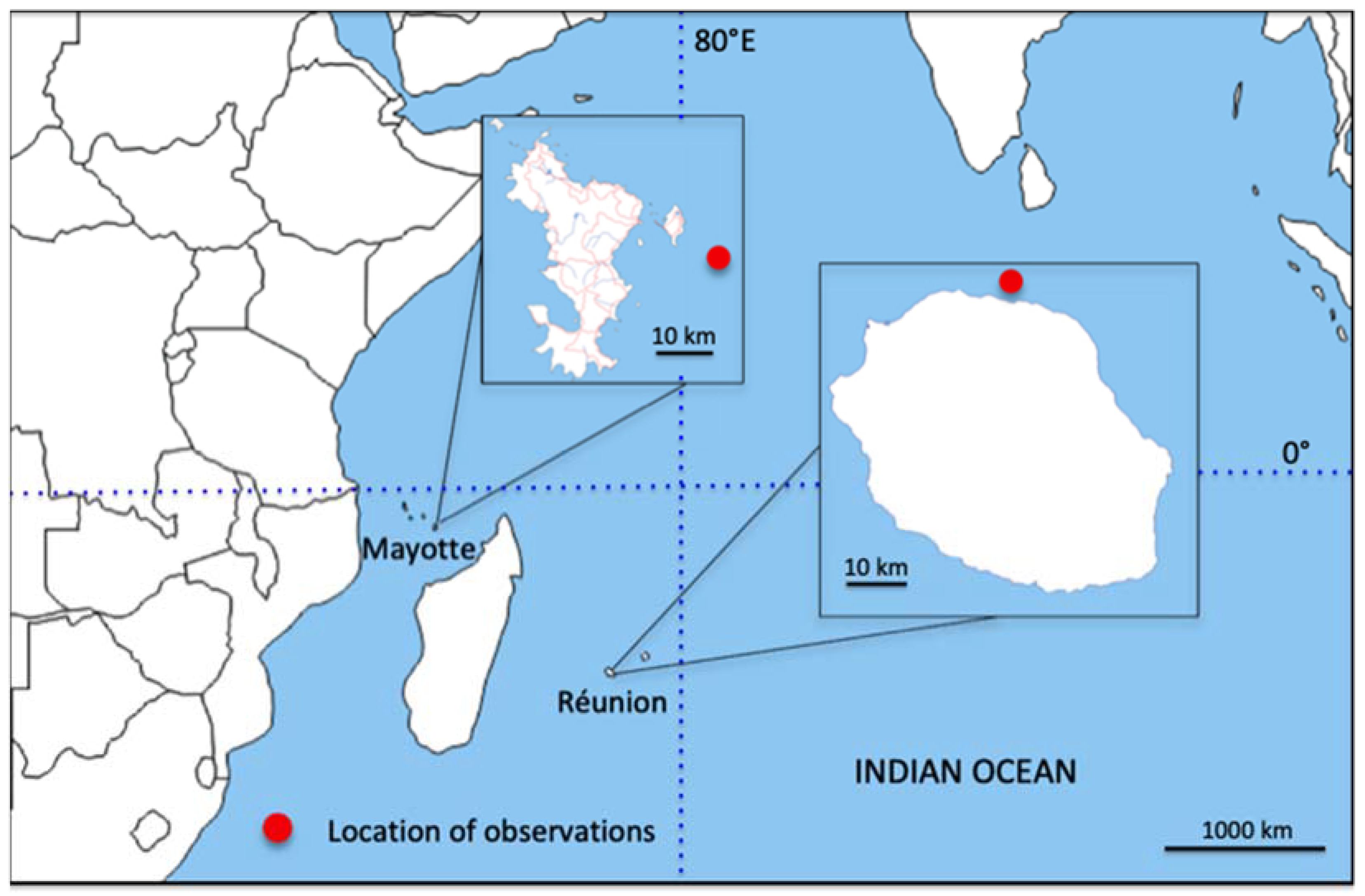
2. Materials and Methods
3. Results
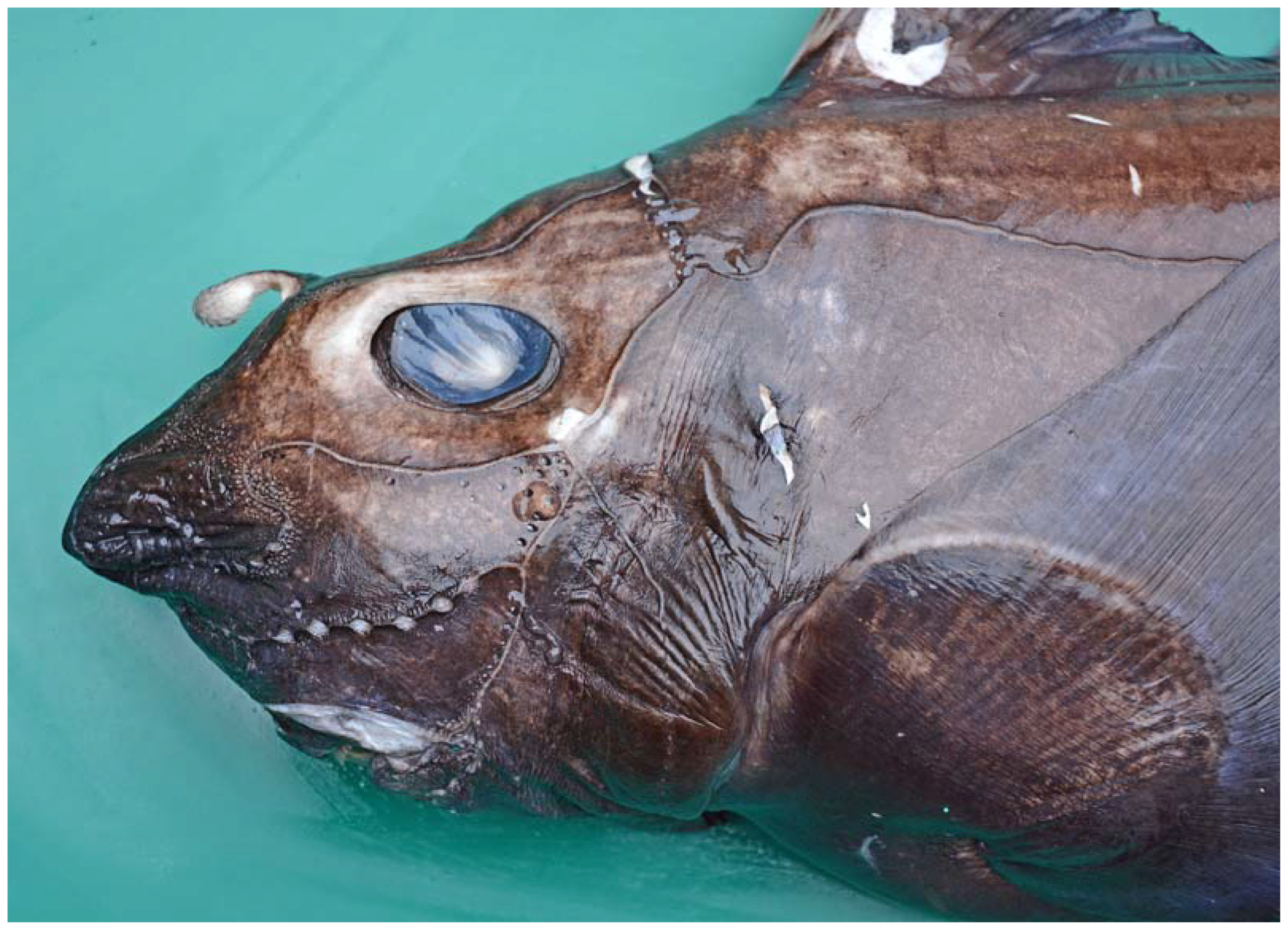
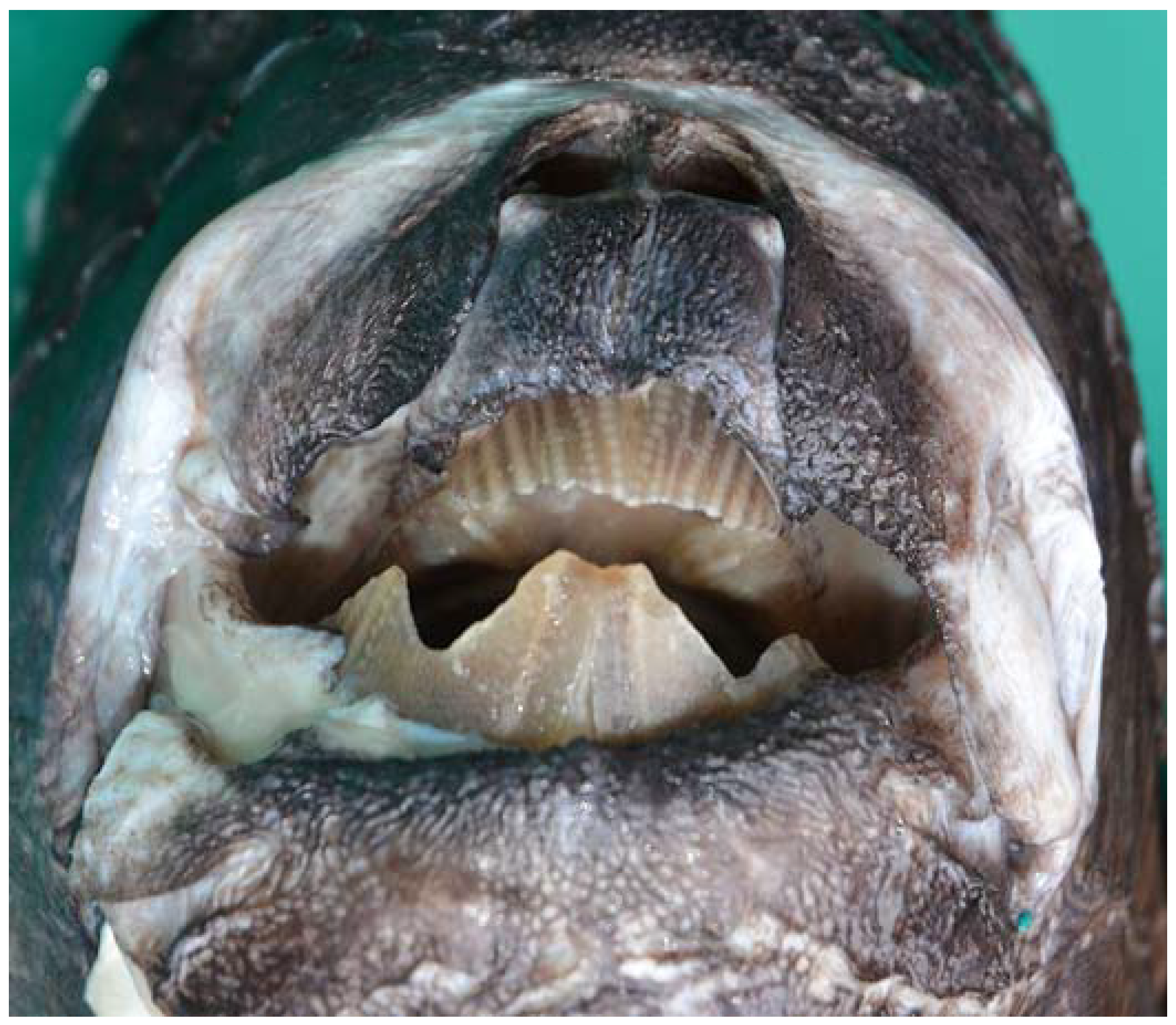
3.1. Description of the Specimens
3.2. Species Comparisons
3.2.1. Comparison with H. affinis
3.2.2. Comparison with H. erithacus
3.2.3. Comparison with H. melanophasma
3.2.4. Comparison with H. homonycteris
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Linnaeus, C. Systema Naturae per Regna Tria Naturae, Secundum Classes, Ordines, Genera, Species, Cum Characteribus, Differentiis, Synonymis, Locis, Editio decima, Reformata. Typis Ioannis Thomae Trattner. Ed. Decima Reformata 1758, 1, 824. [Google Scholar]
- Didier, D.A. Two new species of chimaeroid fishes from the southwestern Pacific Ocean (Holocephali, Chimaeridae). Ichthyol. Res. 2002, 49, 299–306. [Google Scholar] [CrossRef]
- Didier, D.A. Phylogeny and classification of extant holocephali. In Biology of Sharks and their Relatives; Carrier, J.C., Musick, J.A., Heithaus, M.R., Eds.; CRC Press: Boca Raton, FL, USA, 2004; pp. 115–135. [Google Scholar]
- Didier, D.A.; Séret, B. Chimaeroid fishes of New Caledonia with description of a new species of Hydrolagus (Chondrichthyes, Holocephali). Cybium 2002, 26, 225–233. [Google Scholar]
- Didier, D.A.; Kemper, J.M.; Ebert, D.A. Phylogeny, biology, and classification of extant holocephalans. In Biology of Sharks and their Relatives; Carrier, J.C., Musick, J.A., Heithaus, M.R., Eds.; CRC Press: Boca Raton, FL, USA, 2012; pp. 97–122. [Google Scholar]
- Finucci, B.; Cheok, J.; Ebert, D.A.; Herman, K.; Kyne, P.M.; Dulvy, N.K. Ghosts of the deep—Biodiversity, fisheries, and extinction risk of ghost sharks. Fish Fish. 2020, 22, 391–412. [Google Scholar] [CrossRef]
- Kemper, J.M.; Ebert, D.A.; Compagno, L.J.V.; Didier, D.A. Chimaera notafricana sp. nov. (Chondrichthys: Chimaeriformes: Chimaeridae), a new species of chimaera from southern Africa. Zootaxa 2010, 2532, 55–63. [Google Scholar] [CrossRef]
- Kemper, J.M.; Ebert, D.A.; Didier, D.A.; Compagno, L.J.V. Description of a new species of chimaerid, Chimaera bahamaensis from the Bahamas (Holocephali: Chimaeridae). Bull. Mar. Sci. 2010, 86, 649–659. [Google Scholar]
- Last, P.R.; Stevens, J.D. Shark and Rays of Australia, 2nd ed.; CSIRO Publishing: Melbourne, Australia, 2009; p. 519. [Google Scholar]
- Walovich, K. Taxonomic revision of the short nose chimaeras (Genus Hydrolagus) from the southern African region. Master’s Thesis, San Jose State University, San Jose, CA, USA, 2017; p. 116. [Google Scholar]
- Walovich, K.A.; Ebert, D.A.; Kemper, J.M. Hydrolagus erithacus sp. nov. (Chimaeriformes: Chimaeridae), a new species of chimaerid from the southeastern Atlantic and southwestern Indian oceans. Zootaxa 2017, 4226, 509–520. [Google Scholar] [CrossRef]
- Weigmann, S. Annotated checklist of the living sharks, batoids and chimaeras (Chondrichthyes) of the world, with a focus on biogeographical diversity. J. Fish. Biol. 2016, 88, 837–1037. [Google Scholar] [CrossRef]
- Jordan, D.S.; Hubbs, C.J. Record of fishes obtained by David Starr Jordan in Japan, 1922. Mem. Carnegie Mus. 1925, 10, 93–346. [Google Scholar] [CrossRef]
- Waite, E.R. Report upon Trawling Operations off the Coast of New South Wales, Between the Manning River and Jervis Bay, Carried on by H. M. C. S. "Thetis"; Scientific Report on the Fishes; New South Wales Sea Fisheries, Report: Sydney, Australia, 1898; pp. 1–62. [Google Scholar]
- Jordan, D.S.; Snyder, J.O. A list of fishes collected in Japan by Keinosuke Otaki, and by the United States steamer Albatross, with descriptions of fourteen new species. Proc. U. S. Natl. Mus. 1900, 23, 335–380. [Google Scholar] [CrossRef]
- Tanaka, S. On two new species of Chimaera. J. Coll. Sci. Imp. Univ. Tokyo 1905, 20, 1–14. [Google Scholar]
- Howell Rivero, L. Some new, rare and little-known fishes from Cuba. Proc. Boston Soc. Nat. Hist. 1936, 41, 41–76. [Google Scholar]
- Didier, D.A. The leopard Chimaera, a new species of chimaeroid fish from New Zealand (Holocephali, Chimaeriformes, Chimaeridae). Ichthyol. Res. 1998, 45, 281–289. [Google Scholar] [CrossRef]
- Last, P.R.; White, W.T.; Pogonoski, J.J. Chimaera argiloba sp. nov., a new species of chimaerid (Chimaeriformes: Chimaeridae) from northwestern Australia. In Descriptions of New Australian Chondrichthyans; CSIRO Marine and Atmosphere Research Report; CSIRO Publishing: Clayton, Australia, 2008; Volume 022, pp. 341–348. [Google Scholar]
- Didier, D.A.; Last, P.R.; White, W.T. Three new species of the genus Chimaera Linnaeus (Chimariformes: Chimaeridae) from Australia. In Descriptions of New Australian Chondrichthyans; CSIRO Marine and Atmosphere Research Report; CSIRO Publishing: Clayton, Australia, 2008; Volume 022, pp. 327–339. [Google Scholar]
- Luchetti, E.A.; Iglésias, S.P.; Sellos, D.Y. Chimaera opalescens n. sp., a new chimaeroid (Chondrichthyes: Holocephali) from the north-eastern Atlantic Ocean. J. Fish. Biol. 2011, 79, 399–417. [Google Scholar] [CrossRef] [PubMed]
- Kemper, J.M.; Ebert, D.A.; Naylor, G.J.P.; Didier, D.A. Chimaera carophila (Chondrichthyes: Chimaeriformes: Chimaeridae), a new species of chimaera from New Zealand. Bull. Mar. Sci. 2014, 91, 63–81. [Google Scholar] [CrossRef]
- Angulo, A.; López, M.I.; Bussing, W.A.; Murase, A. Records of chimaeroid fishes (Holocephali: Chimaeriformes) from the Pacific coast of Costa Rica, with the description of a new species of Chimaera (Chimaeridae) from the eastern Pacific Ocean. Zootaxa 2014, 3861, 554–574. [Google Scholar] [CrossRef]
- Clerkin, P.J.; Ebert, D.A.; Kemper, J.M. New species of Chimaera (Chondrichthyes: Holocephali: Chimaeriformes: Chimaeridae) from the southwestern Indian Ocean. Zootaxa 2017, 4312, 1–37. [Google Scholar] [CrossRef]
- Iglésias, S.P.; Kemper, J.M.; Naylor, G.J.P. Chimaera compacta, a new species from southern Indian Ocean, and an estimate of phylogenetic relationships within the genus Chimaera (Chondrichthyes: Chimaeridae). Ichthyol. Res. 2021, 69, 31–45. [Google Scholar] [CrossRef]
- Lay, G.T.; Bennett, E.T. Fishes. In The Zoology of Captain Beechey’s Voyage, Comp. from The Collections... to the Pacific And Behring’s Straits... in 1825–28; Beechey, F.W., Ed.; H. G. Bohn: London, UK, 1839; pp. 41–75. [Google Scholar]
- De Brito Capello, F. Descripção de dois peixes novos provenientes dos mares de Portugal. J. Sci. Math. Phys. Nat. Lisboa 1868, 1, 314–317. [Google Scholar]
- Collett, R. Diagnoses of four hitherto undescribed fishes from the depths south of the Faroe Islands. Forh. Vidensk. Selsk. Christiania 1904, 9, 1–7. [Google Scholar]
- Jordan, D.S.; Snyder, J.O. On the species of white chimaera from Japan. Proc. U. S. Natl. Mus. 1904, 27, 223–226. [Google Scholar] [CrossRef]
- Gilbert, C.H. The deep-sea fishes of the Hawaiian Islands. In: The aquatic resources of the Hawaiian Islands. Bull. U. S. Fish Comm. 1905, 23, 577–713. [Google Scholar]
- Garman, S. New Plagiostomia and Chismopnea. Bull. Mus. Comp. Zool. 1908, 51, 249–256. [Google Scholar]
- Fowler, H.W. Notes on chimaeroid and ganoid fishes. Proc. Acad. Nat. Sci. Phila. 1911, 62, 603–612. [Google Scholar]
- Smith, H.M.; Radcliffe, L. The chimaeroid fishes of the Philippine Islands, with description of a new species. [Scientific results of the Philippine cruise of the Fisheries steamer “Albatross”, 1907–1910 No. 18]. Proc. U. S. Natl. Mus. 1912, 42, 231–232. [Google Scholar] [CrossRef]
- Gilchrist, J.D.F. Deep-sea fishes procured by the S.S. “Pickle” (Part I). Proc. U. S. Natl. Mus. 1922, 2, 41–79. [Google Scholar]
- Bigelow, H.B.; Schroeder, W.C. Three new skates and a new chimaerid fish from the Gulf of Mexico. J. Wash. Acad. Sci. 1951, 41, 383–392. [Google Scholar]
- De Buen, F. Notas preliminares sobre la fauna marina preabismal de Chile, con descripción de una familia de rayas, dos géneros y siete especies nuevos. Bol. Mus. Nac. Chile 1949, 27, 171–201. [Google Scholar] [CrossRef]
- Hardy, G.S.; Stehmann, M. A new deep-water ghost shark, Hydrolagus pallidus n. sp. (Holocephali, Chimaeridae), from the Eastern North Atlantic, and redescription of Hydrolagus affinis (Brito Capello, 1867). Arch Fisch. 1990, 40, 229–248. [Google Scholar]
- Soto, J.M.R.; Vooren, C.M. Hydrolagus matallanasi sp. nov. (Holocephali, Chimaeridae) a new species of rabbitfish from southern Brazil. Zootaxa 2004, 687, 1–10. [Google Scholar] [CrossRef]
- Moura, T.; Figueiredo, I.; Bordalo-Machado, P.; Almeida, C.; Gordo, L.S. A new deep-water chimaerid species, Hydrolagus lusitanicus n. sp., from off mainland Portugal with a proposal of a new identification key for the genus Hydrolagus (Holocephali: Chimaeridae) in the north-east Atlantic. J. Fish. Biol. 2005, 67, 742–751. [Google Scholar] [CrossRef]
- Quaranta, K.L.; Didier, D.A.; Long, D.J.; Ebert, D.A. A new species of chimaeroid, Hydrolagus alphus sp. nov. (Chimaeriformes: Chimaeridae) from the Galapagos Islands. Zootaxa 2006, 1377, 33–45. [Google Scholar]
- Barnett, L.A.K.; Didier, D.A.; Long, D.J.; Ebert, D.A. Hydrolagus mccoskeri sp. nov., a new species of chimaeroid fish from the Galápagos Islands (Holocephali: Chimaeriformes: Chimaeridae). Zootaxa 2006, 1328, 27–38. [Google Scholar] [CrossRef]
- Didier, D.A. Two new species of the genus Hydrolagus Gill (Holocephali: Chimaeridae) from Australia. CSIRO Mar. Atmos. Res. Pap. 2008, 022, 349–356. [Google Scholar]
- James, K.C.; Ebert, D.A.; Long, D.J.; Didier, D.A. A new species of chimaera, Hydrologus melanophasma sp. nov. (Chondrichthyes: Chimaeriformes: Chimaeridae), from the eastern North Pacific. Zootaxa 2009, 2218, 59–68. [Google Scholar] [CrossRef]
- Séret, B.; Quod, J.P.; von Arnim, N. Des chimères signalées à La Réunion pour la première fois. Diodon (Bull. Mauritius Mar. Cons. Soc.) 2013, 34, 22–23. [Google Scholar]
- Araya, J.F.; Reyes, P.; Hüne, M. On the presence of the Eastern Pacific Black Ghostshark Hydrolagus melanophasma (Chondrichthyes: Chimaeridae) in northern Chile, with notes on its distribution in the Eastern Pacific. Thalassa 2020, 36, 565–572. [Google Scholar] [CrossRef]
- Bustamante, C.; Flores, H.; Concha-Perez, Y.; Vargas-Caro, C.; Lamilla, J.; Bennette, M. First record of Hydrolagus melanophasma James, Ebert, Long & Didier, 2009 (Chondrichthyes, Chimaeriformes, Holocephali) from the southeastern Pacific ocean. Lat. Am. J. Aquat. Res. 2012, 40, 236–242. [Google Scholar]
- Walovich, K.; Ebert, D.A.; Long, D.J.; Didier, D.A. Redescription of Hydrolagus africanus (Gilchrist, 1922) (Chimaeriformes: Chimaeridae), with a review of southern African chimaeroids and a key to their identification. Afr. J. Mar. Sci. 2015, 37, 157–165. [Google Scholar] [CrossRef]
- Bigelow, H.B.; Schroeder, W.C. Part two. Chapter one. Sawfishes, guitarfishes, skates and rays. In Fishes of the Western North Atlantic; Memoirs of the Sears Foundation of Marine Research; Sears Foundation for Marine Research: New Haven, CT, USA, 1953; pp. 1–514. [Google Scholar]
- Heemstra, P.C. Additions and corrcetions for the 1995 impression. In Smiths’Sea Fishes, Revised ed.; Smith, M.M., Heemstra, P.C., Eds.; Springer: Berlin, Germany, 1995; pp. v–xv. [Google Scholar]
- Gates, A.R. Deep-Sea Life of Tanzania; SERPENT Project (Scientific and Environmantal ROV Partnership Using Existing Industrial Technology); National Oceanography Centre: Southampton, UK, 2016; p. 88. [Google Scholar]
- Gates, A.R.; Durden, J.M.; Richmond, M.D.; Muhando, C.A.; Khamis, Z.A.; Jones, D.O.B. Ecological considerations for marine spatial management in deep-water Tanzania. Ocean. Coast. Manag. 2021, 210, 105703. [Google Scholar] [CrossRef]
- Marquez-Farias, J.F.; Lara-Mendoza, R.E. Notes about the reproductive tract of the chimaera Hydrolagus melanophasma (Chondrichthyes, Holocephali) from the west coast of Baja California, Mexico. Hidrobiológica 2014, 24, 151–158. [Google Scholar]













| Species | Authors | English Name | Distribution | Depth Range | TL | BDL | Red List |
|---|---|---|---|---|---|---|---|
| Chimaera monstrosa | [1] | Rabbitfish | Eastern North Atlantic: Iceland to Morocco | 50–1742 m | 119 cm | 59 cm | VU |
| Chimaera ogilbyi (=lemures) | [14] | Ogilbys ghost shark | Indo-Australia: Australia, Indonesia, New Guinea | 120–872 m | 103 cm | 55 cm | NT |
| Chimaera phantasma | [15] | Silver chimaera | Western Pacific: Japan to Philippines | 20–962 m | 110 cm | VU | |
| Chimaera jordani | [16] | Jordan’s chimaera | NW Pacific: Japan | 716–780 m | 93 cm | DD | |
| Chimaera owstoni | [16] | Owston’s chimaera | NW Pacific: Japan | 650–900 m | 80 cm | DD | |
| Chimaera cubana | [17] | Chimaera | WC Atlantic: Cuba | 180–1050 m | 80 cm | LC | |
| Chimaera panthera | [18] | Leopard chimaera | SW Pacific: New Zealand | 327–1020 m | 129 cm | LC | |
| Chimaera lignaria | [2] | Giant chimaera | Southern Pacific: New Zealand + Tasmania | 400–1800 m | 142 cm | LC | |
| Chimaera argiloba | [19] | Whitefin chimaera | Eastern Indian Ocean: Western Australia | 370–520 m | 91 cm | LC | |
| Chimaera fulva | [20] | Southern chimaera | Southern Australia | 780–1095 m | 118 cm | LC | |
| Chimaera macrospina | [20] | Longspine chimaera | Indo-West Pacific: Australia east and west | 435–1190 m | 103 cm LL | LC | |
| Chimaera obscura | [20] | Shortspine chimaera | SW Pacific: eastern Australia | 450–1080 m | 95 cm | LC | |
| Chimaera bahamaensis | [8] | Bahamas ghost shark | North Atlantic: Bahamas | 732–1506 m | 88 cm | LC | |
| Chimaera notafricana | [7] | Cape chimaera | Southern Atlantic: Namibia, South Africa | 680–1000 m | 93 cm | LC | |
| Chimaera opalescens | [21] | Opal chimaera | NE Atlantic | 800–1975 m | 110 cm | DD | |
| Chimaera carophila | [22] | Brown chimaera | SW Pacific: New Zealand | 846–1350 m | 104 cm | 60 cm | LC |
| Chimaera orientalis | [23] | Eastern Pacific black chimaera | Eastern Pacific: Costa Rica, Peru | 560–1138 m | 86 cm | 50 cm | DD |
| Chimaera buccanigella | [24] | Dark-mouth chimaera | SW Indian Ocean Ridge | 495–960 m | 86 cm | DD | |
| Chimaera didierae | [24] | Falkor chimaera | SW Indian Ocean: Walters Shoal | 1000–1100 m | 155 cm | DD | |
| Chimaera willwatchi | [24] | Seafarer’s ghost shark | SW Indian Ocean: Madagascar Ridge | 89–1375 m | 97 cm | DD | |
| Chimaera compacta | [25] | Stubby chimaera | Southern Indian Ocean: Amsterdam | 595–655 m | 84 cm | ||
| Hydrolagus colliei | [26] | White spotted chimaera | NE Pacific: Alaska to Costa Rica | 0–1029 m | 60 cm | LC | |
| Hydrolagus affinis | [27] | Small-eyed rabbitfish | NE + NW Atlantic + WIO (South Africa) | 300–3000 m | 147 cm | 96 cm | LC |
| Hydrolagus mirabilis | [28] | Large-eyed rabbitfish | EN Atlantic to Morocco + WN Atlantic | 450–2058 m | 80 cm | 35 cm | LC |
| Hydrolagus mitsukurii | [29] | Spookfish / Mitsukuri’s chimaera | NW Pacific: Japan, Philippines, New Guinea | 325–830 m | 79 cm | 37 cm | NT |
| Hydrolagus purpurescens | [30] | Purple chimaera | Pacific: Japan, Hawaii; Sakhalin | 920–1951 m | 138 cm + | LC | |
| Hydrolagus barbouri | [31] | Ninepsoy chimaera | NW Pacific: Japan, China | 250–1100 m | 86 cm | 48 cm | DD |
| Hydrolagus novaezealandiae | [32] | Dark ghost shark | SW Pacific: New Zealand | 25–950 m | 96 cm | LC | |
| Hydrolagus deani (? = H. misukurii) | [33] | Philippine chimaera | WC Pacific: Philippines | 469–770 m | 73 cm | ||
| Hydrolagus africanus | [34] | African chimaera | W Indian Ocean | 303–1470 | 98 cm | 46 cm | LC |
| Hydrolagus alberti | [35] | Gulf chimaera | Western Atlantic: Gulf Mexico + Caribbean | 348–1470 m | 100 cm | 45 cm | LC |
| Hydrolagus macrophthalmus | [36] | Bigeye chimaera | Eastern Pacific: Mexico to Chile | 590–1160 m | 64 cm | 35 cm | LC |
| Hydrolagus pallidus | [37] | Pale chimaera | North Atlantic: Iceland to Newfoundland | 883–2619 m | 138 cm | 91 cm | LC |
| Hydrolagus bemisi | [2] | Pale ghost shark | SW Pacific: New Zealand | 400–1100 m | 112 cm | 57 cm | LC |
| Hydrolagus trolli | [4] | Pointy-nosed blue chimaera | Western Pacific: New Caledonia, New Zealand | 612–2000 m | 120 cm | 79 cm | LC |
| Hydrolagus matallanasi | [38] | Striped rabbitfish | SW Atlantic: Brazil | 416–73 m | 70 cm | 31 cm | VU |
| Hydrolagus lusitanicus | [39] | Portuguese rabbitfish / coelho | NE Atlantic: Portugal | 1600–2410 m | 118 cm PCL | LC | |
| Hydrolagus alphus | [40] | Whitespot ghost shark | EC Pacific: Galapagos | 630–907 m | >48 cm | >25 cm | LC |
| Hydrolagus mccoskeri | [41] | Galápagos ghost shark | EC Pacific: Galapagos | 396–606 m | 38 cm | 37 cm | LC |
| Hydrolagus homonycteris | [42] | Black ghost shark | Southern Ocean: Australia, Tasmania + New Zealand | 400–1450 m | 109 cm | 66 cm | LC |
| Hydrolagus marmoratus | [42] | Marbled ghost shark | Western Pacific: eastern Australia | 548–995 m | 80 cm | LC | |
| Hydrolagus melanophasma | [43] | Eastern Pacific black ghost shark | Eastern Pacific: California to Chile | 30–1800 m | 128 cm | 92 cm | LC |
| Hydrolagus erithacus | [11] | Robin’s ghost shark | SE Atlantic and SW Indian Oceans | 470–1000 m | 144 cm | 91 cm | DD |
| MNHN 2015-0097 Mayotte Male ad. 710 mm BDL | MNHN 2015-0098 La Reunion Female ad. 870 mm BDL | H. africanus 65 Specimens 221–465 mm BDL [47] | H. erithacus 9 Types 765–945 mm BDL [11] | H. melanophasma 2 Types + 6 Specimens 577–918 mm BDL Data from the Literature (cf Legend) | H. homonycteris 12 Types 235–630 mm BDL [42] | ||
|---|---|---|---|---|---|---|---|
| TL | total length | 157.7 | 149.4 | 117.2–293.8 | 151–163 | 138.9–160.5 | 140–202 |
| PCL | precaudal length | 125.4 | 124.1 | 116.1–130.0 | 121–132 | 121.8–130.6 | 120–136 |
| BDL | body length | 100% | 100% | 100% | 100% | 100% | 100% |
| SVL | snout–vent length | 60.6 | 72.4 | 51.9–77.9 | 62–70 | 56.1–73.1 | 53–71 |
| TRL | trunk length | 35.9 | 48.3 | 30.9–46.8 | 35–44 | 31.9–39.7 | 33–42 |
| PD2 | pre-D2 length | 47.2 | 50.0 | 41.1–57.7 | 53–57 | 41.7–53.4 | 42–60 |
| PD1 | pre-D1 length | 27.3 | 34.5 | 20.3–33.9 | 28–35 | 25.9–29.1 | 24–40 |
| POB | preorbital length | 11.1 | 15.5 | 8.5–15.0 | 15–18 | 8.4–14.2 | 11–20 |
| POR | preoral length | 8.0 | 14.6 | 5–15 | 8.3–11.5 | ||
| PRN | prenarial length | 7.8 | 12.1 | 4–12 | 6.4–12.1 | ||
| D2B | D2 base length | 84.5 | 74.7 | 69.6–86.7 | 71–81 | 77.3–82.2 | 74–82 |
| D2AH | maxi height at ant. 1/3 D2 | 3.9 | 4.4 | 4.3–7.5 | 3–5 | 4.0–5.2 | 4–8 |
| D2PH | maxi height at post. 1/3 D2 | 4.6 | 4.6 | 3.4–6.5 | 3–5 | 4.2–7 | |
| D1B | D1 base length | 21.5 | 19.9 | 9.0–18.9 | 12–16 | 13.9–20.0 | 9–18 |
| DSA | ant. spine to D1 | 18.6–28.3 | 21–25 | 17.6–29.9 | 9–25 | ||
| D1H | max. height D1 | 19.0 | 17.6 | 11.8–20.5 | 11–15 | 18.1–26.3 | 14–17 |
| CDM | dorsal caudal margin length | 20.1 | 19.5 | 16.0–25.6 | 21–25 | 18.5–23.1 | 16–24 |
| CDH | max. height caudal dorsal lobe | 3.2 | 3.5 | 2.3–5.0 | 3–5 | 2.0–3.3 | 3–4 |
| CTL | total caudal length | 31.7 | 28.2 | 33.8-xx | 25–32 | 25.3–34.5 | 34–79 |
| CVM | ventral caudal margin length | 23.1 | 33.3 | 22.8–44.1 | 30–36 | 24.4–43.6 | 26–34 |
| CVH | max. height caudal ventral lobe | 2.9 | 3.0 | 2.0–5.0 | 3–4 | 2.0–2.9 | 3–4 |
| HDL | head length | 24.5 | 28.4 | 17.9–31.3 | 27–31 | 21.8–30.6 | 21–34 |
| P1A | pectoral ant. margin length | 35.2 | 34.5 | 29.3–41.4 | 29–42 | 32.0–41.5 | 32–40 |
| P2A | pelvic ant. margin length | 19.0 | 19.8 | 16.3–23.5 | 18–22 | 17.8–21.0 | 13–18 |
| IDS | interdorsal space | 2.3 | 0.0 | 2.1–12.6 | 8–12 | 2.6–10.6 | 3–14 |
| DCS | dorsal–caudal space | 0.0 | 0.0 | 0.0–1.3 | 1–3 | 2–3 | |
| D1P1 | ant. D1 to ant. pectoral base | 24.2 | 20.0 | 14.4–21.9 | 19–28 | 16.4–22.4 | 16–20 |
| D1P2 | ant. D1 to ant. pelvic base | 45.1 | 39.1 | 25.6–44.0 | 43–49 | 27.2–43.9 | 37–43 |
| EYL | eye length | 6.1 | 6.0 | 5.1–9.7 | 5–7 | 5.0–7.1 | 5–7 |
| EYH | eye height | 4.1 | 4.0 | 2.9–5.9 | 3–5 | 3.7–4.8 | 4–6 |
| CLT | clasper length from pelvic base | 13.4 | 0.0 | 3.8–14.2 | 19–20 | 13.7–15.0 | 11 |
| CLM | clasper medial branch from fork to tip | 5.1 | 0.0 | 4.5–7.7 | 3–4 | 3.8–4.0 | 3 |
| CLL | clasper lateral branch from fork to tip | 4.0 | 0.0 | 4.3–7.2 | 3–4 | 3.9–4.2 | 3 |
| % HDL | % HDL | % HDL calcul. | % HDL | ||||
| ONC | oronasal fold to centre of nasal canal | 8.4 | 8.9 | 10.0–13.3 | 8.2–12.5 | ||
| LRC | rostral canal length | 3.7 | 3.9 | 3.3–10.0 | 3.8–6.5 | ||
| LNC | straight nasal canal length | 24.1 | 21.5 | 10.0–23.3 | 23.6–31.5 | ||
| IOA | infraorbital to oro-angular junction | 15.1 | 15.1 | 3.3–13.3 | 12.9–16.1 | ||
| OTM | preopercular to infraorbital distance | 29.2 | 32.4 | 26.7–36.7 | 28.1–32.9 | ||
| OCL | infraorbital to postorbital distance | 15.2 | 13.8 | 13.3–20.0 | 11.1–14.0 | ||
| STL | supratemporal to postorbital distance | 15.7 | 15.3 | 16.7–26.7 | 14.5–27.0 | ||
| SPS | spine to supratemporal distance | 19.9 | 21.0 | 13.3–30.0 | 14.9–18.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Séret, B.; Quod, J.-P. First Records of a Hydrolagus Species (Holocephali: Chimaeridae) from Reunion Island and Mayotte (Southwestern Indian Ocean). Fishes 2023, 8, 522. https://doi.org/10.3390/fishes8100522
Séret B, Quod J-P. First Records of a Hydrolagus Species (Holocephali: Chimaeridae) from Reunion Island and Mayotte (Southwestern Indian Ocean). Fishes. 2023; 8(10):522. https://doi.org/10.3390/fishes8100522
Chicago/Turabian StyleSéret, Bernard, and Jean-Pascal Quod. 2023. "First Records of a Hydrolagus Species (Holocephali: Chimaeridae) from Reunion Island and Mayotte (Southwestern Indian Ocean)" Fishes 8, no. 10: 522. https://doi.org/10.3390/fishes8100522
APA StyleSéret, B., & Quod, J.-P. (2023). First Records of a Hydrolagus Species (Holocephali: Chimaeridae) from Reunion Island and Mayotte (Southwestern Indian Ocean). Fishes, 8(10), 522. https://doi.org/10.3390/fishes8100522