The Effect of Water Spinach on the Water Quality, Antioxidant System, Non-Specific Immune Response, Growth Performance, and Carbon Balance in Red Tilapia Production
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pond and Tilapia-Water Spinach Raft Aquaponics System Design
2.2. Measurement of Water Quality
2.3. Monitoring and Growth Performance of Red Tilapia
2.4. Antioxidant Status and Non-Specific Immune Responses
2.5. The Carbon Balance of Payments
2.5.1. Determination of the Solid Carbon Content
2.5.2. TOC Determination of the Water Body
2.6. Statistical Analyses
3. Results
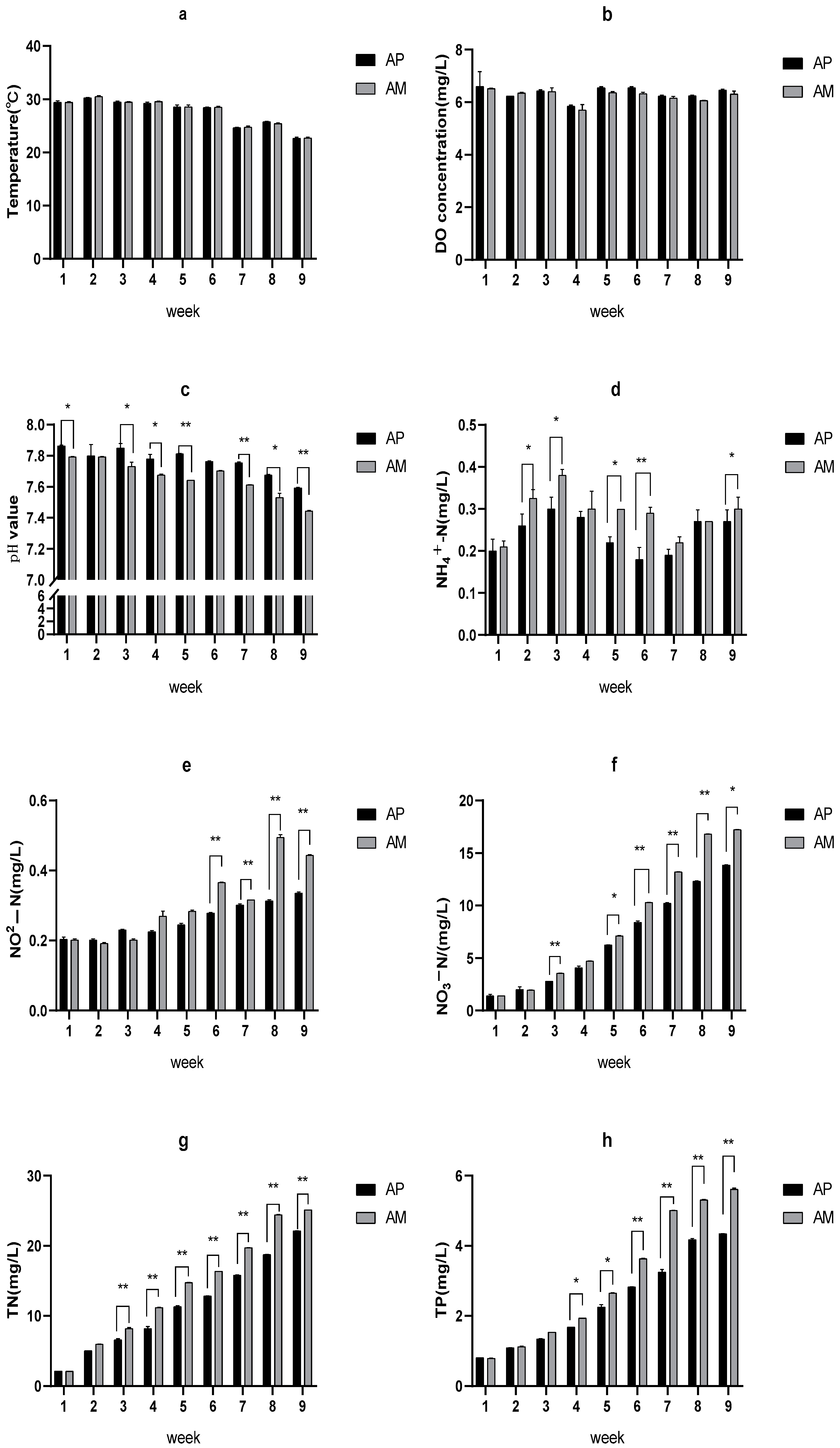
3.1. Modulation of Water Quality in the AP and AM System
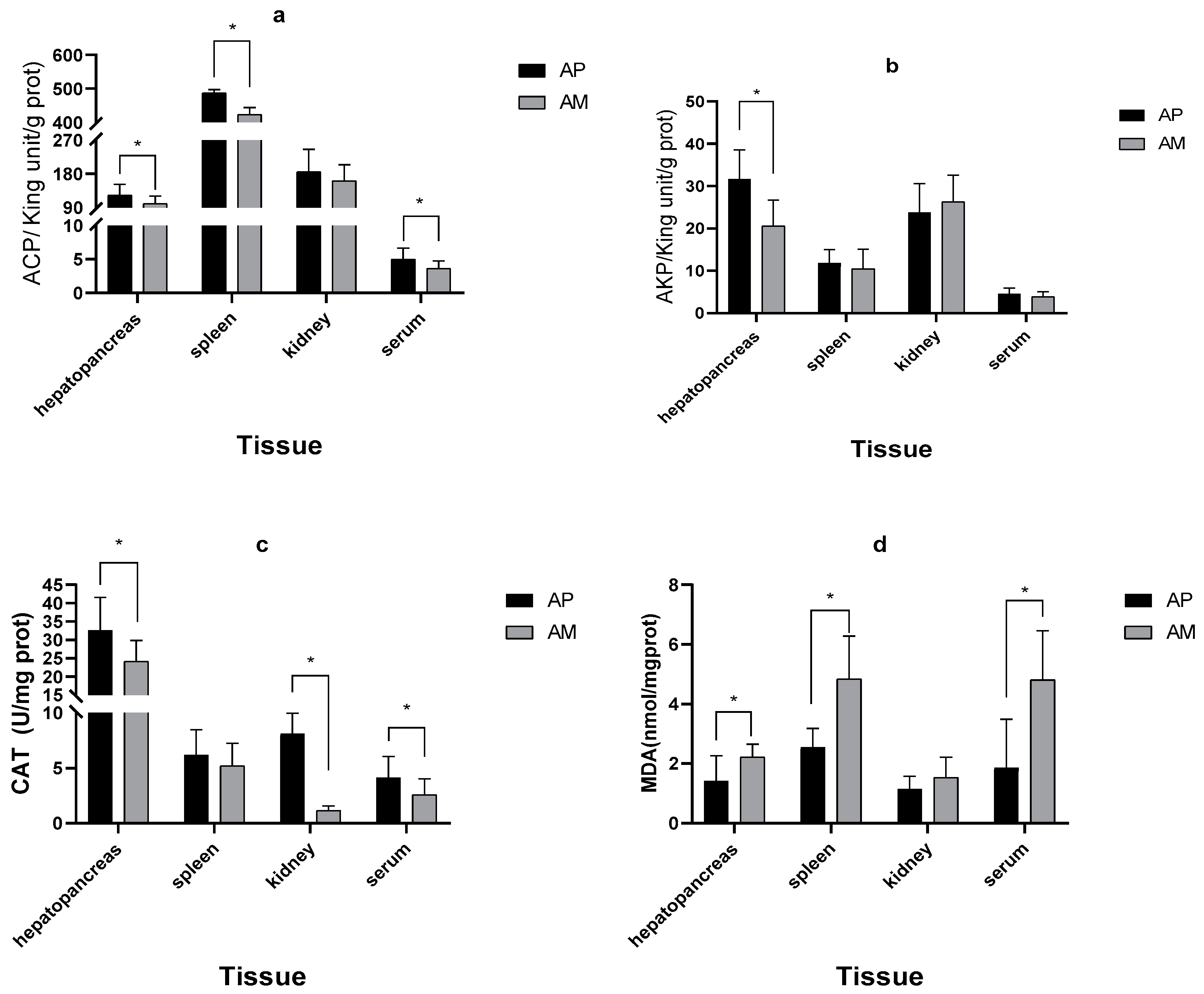
3.2. The AP System Improved the Antioxidant and Non-Specific Immune Response of Red Tilapia
3.3. The Red Tilapia-Water Spinach Raft Aquaponics System Improved the Production Performance of Red Tilapia
3.4. Water Spinach Is a Carbon Sink in the Red Tilapia-Water Spinach Raft Aquaponics System
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shen, X.; Xu, M.; Li, M.; Zhao, Y.; Shao, X. Response of sediment bacterial communities to the drainage of wastewater from aquaculture ponds in different seasons. Sci. Total Environ. 2020, 717, 137180. [Google Scholar] [CrossRef] [PubMed]
- Cashion, T.; Le Manach, F.; Zeller, D.; Pauly, D. Most fish destined for fishmeal production are food-grade fish. Fish Fish. 2017, 18, 837–844. [Google Scholar] [CrossRef]
- Crab, R.; Avnimelech, Y.; Defoirdt, T.; Bossier, P.; Verstraete, W. Nitrogen removal techniques in aquaculture for a sustainable production. Aquaculture 2007, 270, 1–14. [Google Scholar] [CrossRef]
- Buck, B.H.; Troell, M.F.; Krause, G.; Angel, D.L.; Grote, B.; Chopin, T. State of the Art and Challenges for Offshore Integrated Multi-Trophic Aquaculture (IMTA). Front. Mar. Sci. 2018, 5, 165. [Google Scholar] [CrossRef]
- Hu, Z.; Lee, J.W.; Chandran, K.; Kim, S.; Brotto, A.C.; Khanal, S.K. Effect of plant species on nitrogen recovery in aquaponics. Bioresour. Technol. 2015, 188, 92–98. [Google Scholar] [CrossRef]
- Ke, X.; Yi, M.; Li, Q.; Liu, Z.; Wang, M.; Cao, J.; Gao, F.; Lu, M. Effect of the herbal Houttuynia cordata floating bed on the Nile tilapia pond culturing system. Aquac. Rep. 2021, 20, 100680. [Google Scholar] [CrossRef]
- Forchino, A.A.; Lourguioui, H.; Brigolin, D.; Pastres, R. Aquaponics and sustainability: The comparison of two different aquaponic techniques using the Life Cycle Assessment (LCA). Aquac. Eng. 2017, 77, 80–88. [Google Scholar] [CrossRef]
- Bartelme, R.P.; Oyserman, B.O.; Blom, J.E.; Sepulveda-Villet, O.J.; Newton, R.J. Stripping Away the Soil: Plant Growth Promoting Microbiology Opportunities in Aquaponics. Front. Microbiol. 2018, 9, 8. [Google Scholar] [CrossRef]
- Xiong, J.B.; Guo, G.L.; Mahmood, Q.; Yue, M. Nitrogen removal from secondary effluent by using integrated constructed wetland system. Ecol. Eng. 2011, 37, 659–662. [Google Scholar] [CrossRef]
- Dong, H.T.; Senapin, S.; Jeamkunakorn, C.; Nguyen, V.V.; Nguyen, N.T.; Rodkhum, C.; Khunrae, P.; Rattanarojpong, T. Natural occurrence of edwardsiellosis caused by Edwardsiella ictaluri in farmed hybrid red tilapia (Oreochromis sp.) in Southeast Asia. Aquaculture 2019, 499, 17–23. [Google Scholar] [CrossRef]
- Phuoc, N.N.; Linh, N.T.H.; Crestani, C.; Zadoks, R.N. Effect of strain and enviromental conditions on the virulence of Streptococcus agalactiae (Group B Streptococcus; GBS) in red tilapia (Oreochromis sp.). Aquaculture 2021, 534, 756256. [Google Scholar] [CrossRef]
- Hu, M.H.; Ao, Y.S.; Yang, X.E.; Li, T.Q. Treating eutrophic water for nutrient reduction using an aquatic macrophyte (Ipomoea aquatica Forsskal) in a deep flow technique system. Agric. Water Manag. 2008, 95, 607–615. [Google Scholar] [CrossRef]
- Li, W.; Li, Z. In situ nutrient removal from aquaculture wastewater by aquatic vegetable Ipomoea aquatica on floating beds. Water Sci. Technol. 2009, 59, 1937–1943. [Google Scholar] [CrossRef]
- Liang, J.-Y.; Chien, Y.-H. Effects of feeding frequency and photoperiod on water quality and crop production in a tilapia–water spinach raft aquaponics system. Int. Biodeterior. Biodegrad. 2013, 85, 693–700. [Google Scholar] [CrossRef]
- Effendi, H.; Utomo, B.A.; Darmawangsa, G.M. Phytoremediation of freshwater crayfish (Cherax quadricarinatus) culture wastewater with spinach (Ipomoea aquatica) in aquaponic system. AACL Bioflux 2015, 8, 421–430. [Google Scholar]
- Li, M.; Chen, L.; Qin, J.G.; Li, E.; Yu, N.; Du, Z. Growth performance, antioxidant status and immune response in darkbarbel catfish Pelteobagrus vachelli fed different PUFA/vitamin E dietary levels and exposed to high or low ammonia. Aquaculture 2013, 406–407, 18–27. [Google Scholar] [CrossRef]
- Sun, H.; Lu, K.; Minter, E.J.; Chen, Y.; Yang, Z.; Montagnes, D.J. Combined effects of ammonia and microcystin on survival, growth, antioxidant responses, and lipid peroxidation of bighead carp Hypophthalmythys nobilis larvae. J. Hazard Mater. 2012, 221–222, 213–219. [Google Scholar] [CrossRef]
- Qi, X.Z.; Xue, M.Y.; Yang, S.B.; Zha, J.W.; Wang, G.X.; Ling, F. Ammonia exposure alters the expression of immune-related and antioxidant enzymes-related genes and the gut microbial community of crucian carp (Carassius auratus). Fish Shellfish Immunol. 2017, 70, 485–492. [Google Scholar] [CrossRef]
- Sun, Y.; Yin, Y.; Zhang, J.; Yu, H.; Wang, X.; Wu, J.; Xue, Y. Hydroxyl radical generation and oxidative stress in Carassius auratus liver, exposed to pyrene. Ecotoxicol. Environ. Saf. 2008, 71, 446–453. [Google Scholar] [CrossRef]
- Du, J.; Zhu, H.; Liu, P.; Chen, J.; Xiu, Y.; Yao, W.; Wu, T.; Ren, Q.; Meng, Q.; Gu, W.; et al. Immune responses and gene expression in hepatopancreas from Macrobrachium rosenbergii challenged by a novel pathogen spiroplasma MR-1008. Fish Shellfish Immunol. 2013, 34, 315–323. [Google Scholar] [CrossRef]
- Wei, F.S. Methods of Examination of Water and Wastewater; China Environmental Science Processing: Beijing, China, 2002; pp. 189–193. [Google Scholar]
- Maucieri, C.; Nicoletto, C.; Zanin, G.; Birolo, M.; Trocino, A.; Sambo, P.; Borin, M.; Xiccato, G. Effect of stocking density of fish on water quality and growth performance of European Carp and leafy vegetables in a low-tech aquaponic system. PLoS ONE 2019, 14, e0217561. [Google Scholar] [CrossRef] [PubMed]
- Hegazi, M.M.; Attia, Z.I.; Ashour, O.A. Oxidative stress and antioxidant enzymes in liver and white muscle of Nile tilapia juveniles in chronic ammonia exposure. Aquat. Toxicol. 2010, 99, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Abbink, W.; Garcia, A.B.; Roques, J.A.C.; Partridge, G.J.; Kloet, K.; Schneider, O. The effect of temperature and pH on the growth and physiological response of juvenile yellowtail kingfish Seriola lalandi in recirculating aquaculture systems. Aquaculture 2012, 330, 130–135. [Google Scholar] [CrossRef]
- Wang, C.; Li, Z.; Pan, Z.; Li, D. A High-Performance Optoelectronic Sensor Device for Nitrate Nitrogen in Recirculating Aquaculture Systems. Sensors 2018, 18, 3382. [Google Scholar] [CrossRef] [PubMed]
- Hornstrom, E. Phytoplankton in 63 limed lakes in comparison with the distribution in 500 untreated lakes with varying pH. Hydrobiologia 2002, 470, 115–126. [Google Scholar] [CrossRef]
- Körner, S.; Das, S.K.; Veenstra, S.; Vermaat, J.E. The effect of pH variation at the ammonium/ammonia equilibrium in wastewater and its toxicity to Lemna gibba. Aquat. Bot. 2001, 71, 71–78. [Google Scholar] [CrossRef]
- Ramli, N.M.; Giatsis, C.; Yusoff, F.M.; Verreth, J.; Verdegem, M. Resistance and resilience of small-scale recirculating aquaculture systems (RAS) with or without algae to pH perturbation. PLoS ONE 2018, 13, e0195862. [Google Scholar]
- Tyson, R.V.; Simonne, E.H.; White, J.M.; Lamb, E. Reconciling water quality parameters impacting nitrification in aquaponics: The pH levels. Proc. Fla. State Hortic. Soc. 2004, 117, 79–83. [Google Scholar]
- Ebeling, J.M.; Sibrell, P.L.; Ogden, S.R.; Summerfelt, S.T. Evaluation of chemical coagulation-flocculation aids for the removal of suspended solids and phosphorus from intensive recirculating aquaculture effluent discharge. Aquac. Eng. 2003, 29, 23–42. [Google Scholar] [CrossRef]
- Cong, M.; Wu, H.; Yang, H.; Zhao, J.; Lv, J. Gill damage and neurotoxicity of ammonia nitrogen on the clam Ruditapes philippinarum. Ecotoxicology 2017, 26, 459–469. [Google Scholar] [CrossRef]
- Zou, Y.; Hu, Z.; Zhang, J.; Xie, H.; Guimbaud, C.; Fang, Y. Effects of pH on nitrogen transformations in media-based aquaponics. Bioresour. Technol. 2016, 210, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, L.; Yao, C.; Qiu, L.; Zhang, H.; Zhi, Z.; Song, L. Alternation of immune parameters and cellular energy allocation of Chlamys farreri under ammonia-N exposure and Vibrio anguillarum challenge. Fish Shellfish Immunol. 2012, 32, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Payen, S.; Cosme, N.; Elliott, A.H. Freshwater eutrophication: Spatially explicit fate factors for nitrogen and phosphorus emissions at the global scale. Int. J. Life Cycle Ass. 2021, 26, 388–401. [Google Scholar] [CrossRef]
- Zhao, F.L.; Xi, S.; Yang, X.E.; Yang, W.D.; Li, J.J.; Gu, B.H.; He, Z.L. Purifying eutrophic river waters with integrated floating island systems. Ecol. Eng. 2012, 40, 53–60. [Google Scholar] [CrossRef]
- Zhao, F.L.; Yang, W.D.; Zeng, Z.; Li, H.; Yang, X.E.; He, Z.L.; Gu, B.H.; Rafiq, M.T.; Peng, H.Y. Nutrient removal efficiency and biomass production of different bioenergy plants in hypereutrophic water. Biomass Bioenerg. 2012, 42, 212–218. [Google Scholar] [CrossRef]
- Sooknah, R.; Wilkie, A. Nutrient Removal by Floating Aquatic Macrophytes Cultured in Anaerobically Digested Flushed Dairy Manure Wastewater. Ecol. Eng. 2004, 22, 27–42. [Google Scholar] [CrossRef]
- Grunert, O.; Hernandez-Sanabria, E.; Buysens, S.; De Neve, S.; Van Labeke, M.C.; Reheul, D.; Boon, N. In-Depth Observation on the Microbial and Fungal Community Structure of Four Contrasting Tomato Cultivation Systems in Soil Based and Soilless Culture Systems. Front. Plant Sci. 2020, 11, 520834. [Google Scholar] [CrossRef]
- Addy, M.M.; Kabir, F.; Zhang, R.; Lu, Q.; Deng, X.; Current, D.; Griffith, R.; Ma, Y.; Zhou, W.; Chen, P.; et al. Co-cultivation of microalgae in aquaponic systems. Bioresour. Technol. 2017, 245 Pt A, 27–34. [Google Scholar] [CrossRef]
- Fang, Y.; Hu, Z.; Zou, Y.; Zhang, J.; Zhu, Z.; Zhang, J.; Nie, L. Improving nitrogen utilization efficiency of aquaponics by introducing algal-bacterial consortia. Bioresour. Technol. 2017, 245 Pt A, 358–364. [Google Scholar] [CrossRef]
- Martinez-Alvarez, R.M.; Morales, A.E.; Sanz, A. Antioxidant defenses in fish: Biotic and abiotic factors. Rev. Fish Biol. Fish. 2005, 15, 75–88. [Google Scholar] [CrossRef]
- Prieto, A.I.; Jos, A.; Pichardo, S.; Moreno, I.; de Sotomayor, M.A.; Moyano, R.; Blanco, A.; Camean, A.M. Time-dependent protective efficacy of Trolox (vitamin E analog) against microcystin-induced toxicity in tilapia (Oreochromis niloticus). Environ. Toxicol. 2009, 24, 563–579. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Xie, P.; Zhang, X. Oxidative stress response after prolonged exposure of domestic rabbit to a lower dosage of extracted microcystins. Environ. Toxicol. Pharmacol. 2009, 27, 195–199. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.Y.; Geng, J.J.; Ren, H.Q.; Wang, X.R. Hydroxyl radical generation and oxidative stress in Carassius auratus exposed to glyphosate and its formulation. Toxicol. Environ. Chem. 2013, 95, 1183–1191. [Google Scholar] [CrossRef]
- Abhijith, B.D.; Ramesh, M.; Poopal, R.K. Responses of metabolic and antioxidant enzymatic activities in gill, liver and plasma of Catla catla during methyl parathion exposure. J. Basic Appl. Zool. 2016, 77, 31–40. [Google Scholar] [CrossRef]
- Pinho, G.L.; da Rosa, C.M.; Maciel, F.E.; Bianchini, A.; Yunes, J.S.; Proenca, L.A.; Monserrat, J.M. Antioxidant responses and oxidative stress after microcystin exposure in the hepatopancreas of an estuarine crab species. Ecotoxicol. Environ. Saf. 2005, 61, 353–360. [Google Scholar] [CrossRef]
- Cheng, C.H.; Yang, F.F.; Ling, R.Z.; Liao, S.A.; Miao, Y.T.; Ye, C.X.; Wang, A.L. Effects of ammonia exposure on apoptosis, oxidative stress and immune response in pufferfish (Takifugu obscurus). Aquat. Toxicol. 2015, 164, 61–71. [Google Scholar] [CrossRef]
- Chen, J.; Liu, N.; Li, B.; Zhang, H.; Zhao, Y.; Cao, X. The effects of fipronil exposure on oxidative stress, non-specific immunity, autophagy, and apoptosis in the common carp. Environ. Sci. Pollut. Res. Int. 2021, 28, 27799–27810. [Google Scholar] [CrossRef]
- Xia, Z.; Wu, S. Effects of glutathione on the survival, growth performance and non-specific immunity of white shrimps (Litopenaeus vannamei). Fish Shellfish Immunol. 2018, 73, 141–144. [Google Scholar] [CrossRef]
- Gisbert, E.; Nolasco, H.; Solovyev, M. Towards the standardization of brush border purification and intestinal alkaline phosphatase quantification in fish with notes on other digestive enzymes. Aquaculture 2018, 487, 102–108. [Google Scholar] [CrossRef]
- Wang, G.; Sun, C.-B.; Chan, S. Effects of artificial infection of Litopenaeus vannamei by Micrococcus ysodeikticus and WSSV on the activity of immunity related enzymes. Fish Shellfish. Immunol. 2015, 46, 778–786. [Google Scholar]
- Zheng, Y.; Hu, G.D.; Qiu, L.P.; Zhao, Z.X.; Chao, S.; Fan, L.; Meng, S.L.; Xu, P.; Chen, J.Z. Effects of cultivation of Houttuynia cordata on floating beds on serumal immune factors of GIFT tilapia. J. Ecol. Rural. Environ. 2017, 33, 950–954. [Google Scholar]
- Oliveira, V.; Martins, P.; Marques, B.; Cleary, D.F.R.; Lillebo, A.I.; Calado, R. Aquaponics using a fish farm effluent shifts bacterial communities profile in halophytes rhizosphere and endosphere. Sci. Rep. 2020, 10, 10023. [Google Scholar] [CrossRef] [PubMed]
- Viadero, R.C. Factors Affecting Fish Growth and Production. In Water Encyclopedia; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Shilta, M.T.; Chadha, N.K.; Pandey, P.K.; Sawant, P.B. Effect of biofilm on water quality and growth of Etroplus suratensis (Bloch, 1790). Aquac. Int. 2015, 24, 661–674. [Google Scholar] [CrossRef]

| AP | AM | |
|---|---|---|
| Initial stock (g) | 600 | 600 |
| Final stock (g) | 581 | 573 |
| Survival rate (%) | 96.83 ± 2.05 | 95.50 ± 1.80 |
| Initial body weight (g) | 18.54 ± 1.78 | 18.54 ± 1.78 |
| Final average body weight (g) | 47.52 ± 1.93 * | 40.56 ± 1.16 |
| Average daily gain (g/d) | 0.51 ± 0.003 * | 0.39 ± 0.011 |
| Specific growth rate (SGR) | 1.65 ± 0.12 * | 1.37 ± 0.09 |
| Items | Dry Matter/% | Carbon Content (Dry Weight)/% | ||
|---|---|---|---|---|
| Stocking | Harvesting | Stocking | Harvesting | |
| red tilapia in experimental group | 26.47 | 27.19 | 48.61 | 46.88 |
| red tilapia in control group | 26.47 | 28.26 | 48.61 | 45.96 |
| water spinach | 7.05 | 6.56 | 31.95 | 39.11 |
| feed | 91.25 | 45.72 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Y.-Y.; Chen, X.-C.; Xie, R.-L.; Ruan, Z.-H.; Lu, Z.-Q.; Jiang, L.-S.; Li, Y.-F.; Liu, W.-S. The Effect of Water Spinach on the Water Quality, Antioxidant System, Non-Specific Immune Response, Growth Performance, and Carbon Balance in Red Tilapia Production. Fishes 2023, 8, 515. https://doi.org/10.3390/fishes8100515
Luo Y-Y, Chen X-C, Xie R-L, Ruan Z-H, Lu Z-Q, Jiang L-S, Li Y-F, Liu W-S. The Effect of Water Spinach on the Water Quality, Antioxidant System, Non-Specific Immune Response, Growth Performance, and Carbon Balance in Red Tilapia Production. Fishes. 2023; 8(10):515. https://doi.org/10.3390/fishes8100515
Chicago/Turabian StyleLuo, Yuan-Yuan, Xian-Can Chen, Rui-Lin Xie, Zhuo-Hao Ruan, Zhi-Qiang Lu, Liang-Sen Jiang, Yi-Fu Li, and Wen-Sheng Liu. 2023. "The Effect of Water Spinach on the Water Quality, Antioxidant System, Non-Specific Immune Response, Growth Performance, and Carbon Balance in Red Tilapia Production" Fishes 8, no. 10: 515. https://doi.org/10.3390/fishes8100515
APA StyleLuo, Y.-Y., Chen, X.-C., Xie, R.-L., Ruan, Z.-H., Lu, Z.-Q., Jiang, L.-S., Li, Y.-F., & Liu, W.-S. (2023). The Effect of Water Spinach on the Water Quality, Antioxidant System, Non-Specific Immune Response, Growth Performance, and Carbon Balance in Red Tilapia Production. Fishes, 8(10), 515. https://doi.org/10.3390/fishes8100515






