Complete Mitogenome and Phylogenetic Analysis of a Marine Ray-Finned Fish, Alcichthys elongatus (Perciformes: Cottidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Collection and DNA Isolation
2.2. Whole Genome Sequencing
2.3. Mitogenome Assembly and Annotation
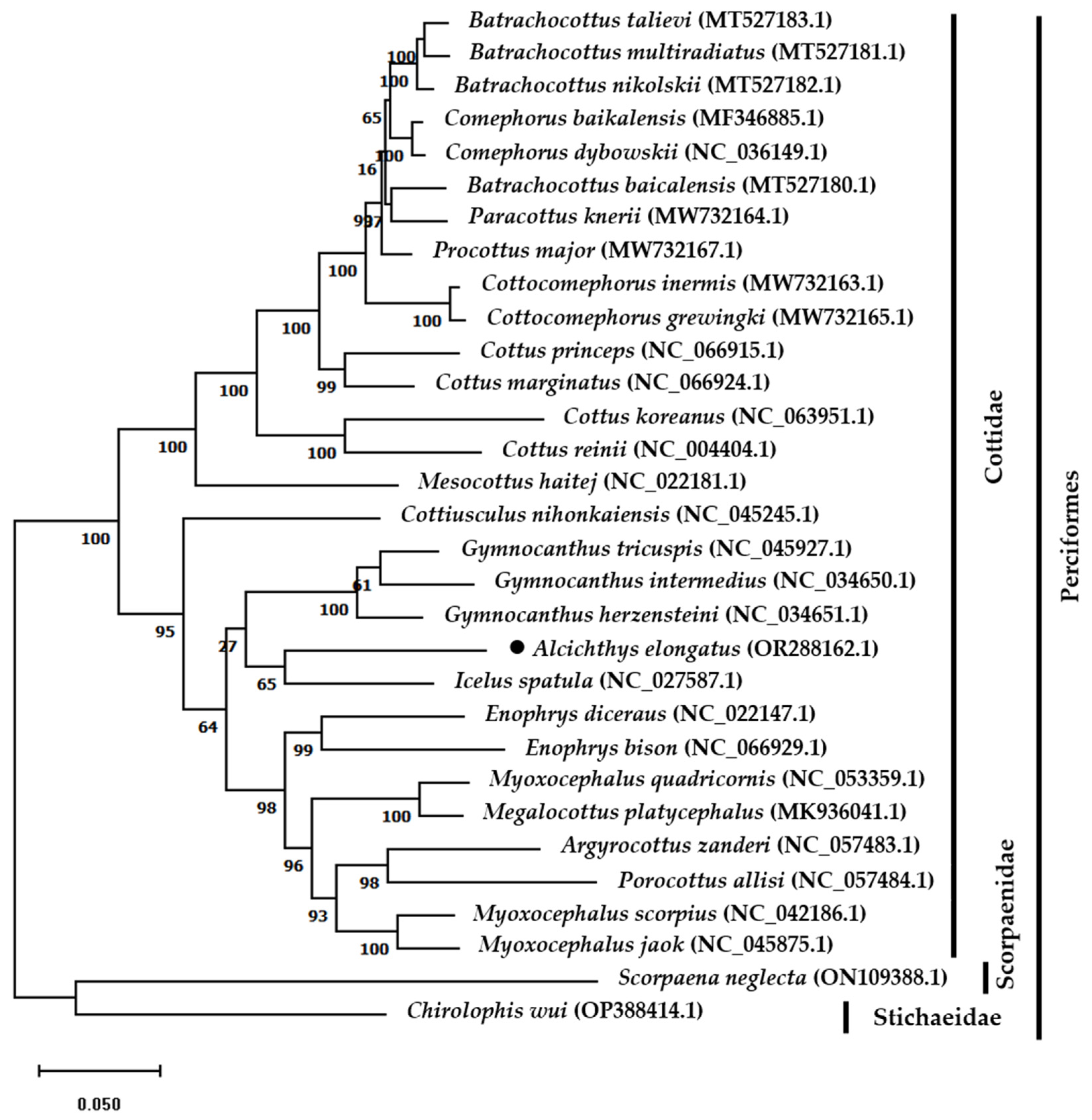
2.4. Phylogenetic Tree Construction
3. Results and Discussion
3.1. Genome Size and Organization
3.2. Protein Coding Genes
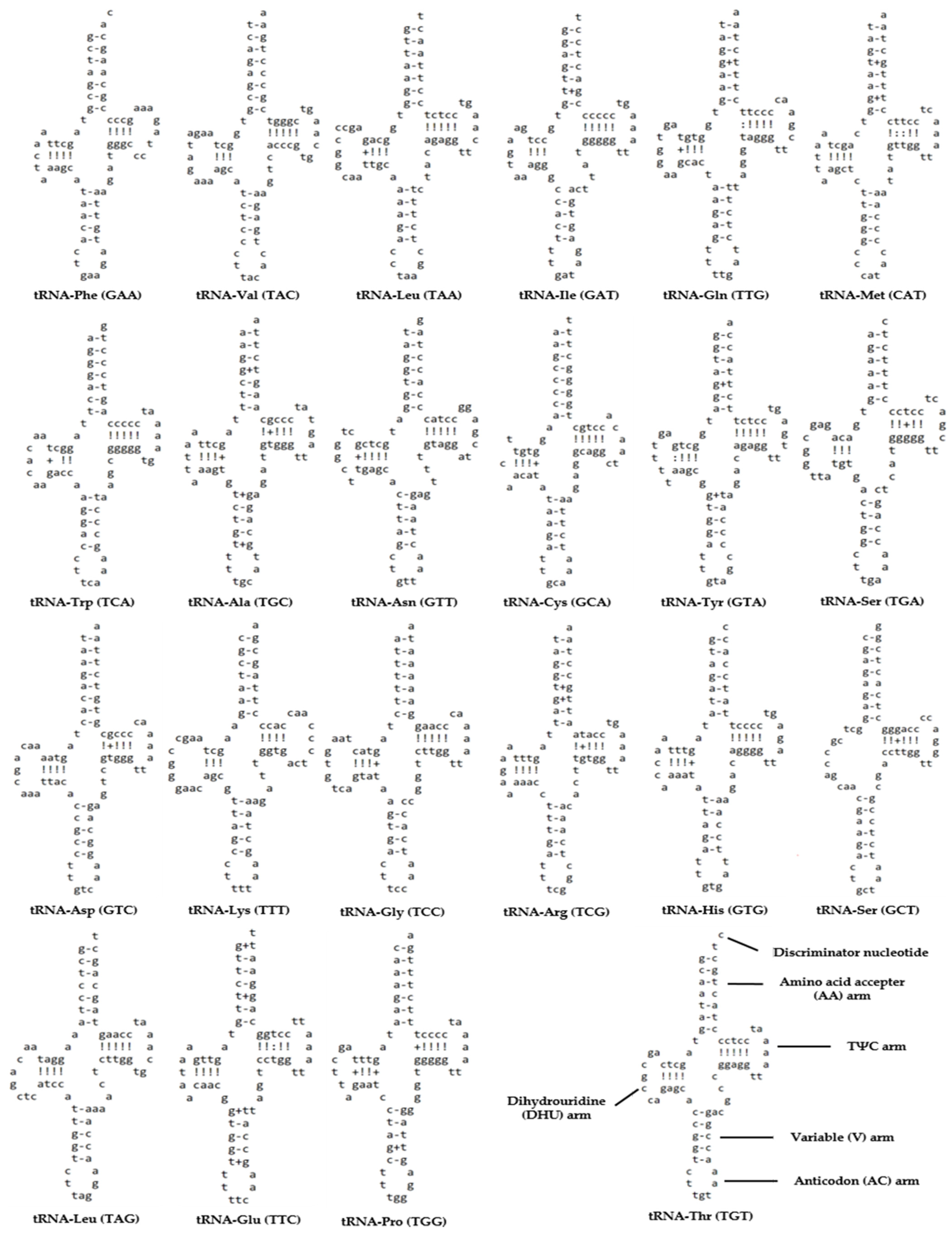
3.3. Transfer RNA and Ribosomal RNA Genes
3.4. Overlapping and Intergenic Spacer Regions
3.5. Phylogenetic Relationship
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mecklenburg, C.W.; Mecklenburg, T.A.; Thorsteinson, L.K. Fishes of Alaska; American Fisheries Society: Bethesda, MD, USA, 2002; p. 1037. [Google Scholar]
- Jackson, K.L. Contributions to the Systematics of Cottoid Fishes (Teleostei: Scorpaeniformes). Ph.D. Thesis, Department of Biological Sciences, University of Alberta, Edmonton, AB, Canada, 2003; p. 16. [Google Scholar]
- Knope, M.L. Phylogenetics of the marine sculpins (Teleostei: Cottidae) of the North American Pacific coast. Mol. Phylo. Evol. 2013, 66, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Im, S.-O. Feeding Relationship and Trophic Partitioning of Demersal Fish Assemblage in the Coastal Waters of East Sea. Ph.D. Thesis, Pukyong National University, Busan, Republic of Korea, 2019; p. 15. [Google Scholar]
- Shinohara, G.; Nakae, M.; Ueda, Y.; Kojima, S.; Matsuura, K. Annotated checklist of deep-sea fishes of the Sea of Japan. Natl. Mus. Nat. Sci. Monogr. 2014, 44, 225–291. [Google Scholar]
- Panchenko, V.V.; Pushchina, O.I.; Antonenko, D.V.; Solomatov, S.F.; Kalchugin, P.V. Distribution and some traits of biology of the elongated sculpin Alcichthys elongatus in the north-western part of the Sea of Japan. J. Ichthyol. 2011, 51, 217–226. [Google Scholar] [CrossRef]
- Nakabo, T. Fishes of Japan with Pictorial Keys to the Species; English Edition I; Tokai University Press: Tokyo, Japan, 2002; p. v-866. [Google Scholar]
- Froese, R.; Pauly, D. FishBase. Alcichthys elongatus (Steindachner, 1881). World Register of Marine Species. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=279545 (accessed on 3 August 2023).
- Radchenko, O.A.; Moreva, I.N.; Petrovskaya, A.V. The subfamily Myoxocephalinae of cottid fishes (Cottidae): Genetic divergence and phylogenetic relationships. J. Fish Biol. 2021, 99, 1857–1868. [Google Scholar] [CrossRef]
- Avise, J.C.; Arnold, J.; Ball, R.M.; Bermingham, E.; Lamb, T.; Neigel, J.E.; Reeb, C.A.; Saunders, N.C. Intraspecific phylogeography: The mitochondrial DNA bridge between population genetics and systematics. Annu. Rev. Ecol. Syst. 1987, 18, 489–522. [Google Scholar] [CrossRef]
- Crampton-Platt, A.; Yu, D.W.; Zhou, X.; Vogler, A.P. Mitochondrial metagenomics: Letting the genes out of the bottle. GigaScience 2016, 5, s13742-016-0120-y. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data; Babraham Institute: Cambridge, UK, 2010. [Google Scholar]
- Chikhi, R.; Medvedev, P. Informed and automated k-mer size selection for genome assembly. Bioinformatics 2014, 30, 31–37. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Zhu, T.; Sato, Y.; Sado, T.; Miya, M.; Iwasaki, W. MitoFish, MitoAnnotator, and MiFish pipeline: Updates in 10 years. Mol. Biol. Evol. 2023, 40, msad035. [Google Scholar] [CrossRef]
- Bernt, M.; Donath, A.; Jühling, F.; Externbrink, F.; Florentz, C.; Fritzsch, G.; Pütz, J.; Middendorf, M.; Stadler, P.F. MITOS: Improved de novo metazoan mitochondrial genome annotation. Mol. Phylo. Evol. 2013, 69, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Gish, W.; States, D.J. Identification of protein coding regions by database similarity search. Nat. Genet. 1993, 3, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Lowe, T.M.; Chan, P.P. tRNAscan-SE online and contextual analysis of transfer RNA genes. Nucleic Acids Res. 2016, 44, W54–W57. [Google Scholar] [CrossRef] [PubMed]
- Laslett, D.; Canbäck, B. ARWEN: A program to detect tRNA genes in metazoan mitochondrial nucleotide sequences. Bioinformatics 2008, 24, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef]
- Greiner, S.; Lehwark, P.; Bock, R. OrganellarGenomeDRAW (OGDRAW) version 1.3. 1: Expanded toolkit for the graphical visualization of organellar genomes. Nucleic Acids Res. 2019, 47, W59–W64. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]
- Stothard, P. The sequence manipulation suite: JavaScript programs for analyzing and formatting protein and DNA sequences. Biotechniques 2000, 28, 1102–1104. [Google Scholar] [CrossRef]
- Perna, N.T.; Kocher, T.D. Patterns of nucleotide composition at fourfold degenerate sites of animal mitochondrial genomes. J. Mol. Evol. 1995, 41, 353–358. [Google Scholar] [CrossRef]
- Thompson, J.D.; Gibson, T.J.; Higgins, D.G. Multiple sequence alignment using ClustalW and ClustalX. Curr. Protoc. Bioinform. 2003, 1, 2–3. [Google Scholar] [CrossRef]
- Felsenstein, J. Evolutionary trees from DNA sequences: A maximum likelihood approach. J. Mol. Evol. 1981, 17, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Balakirev, E.S.; Kravchenko, A.Y.; Semenchenko, A.A. Genetic evidence for a mixed composition of the genus Myoxocephalus (Cottoidei: Cottidae) necessitates generic realignment. Genes 2020, 11, 1071. [Google Scholar] [CrossRef] [PubMed]
- Teterina, V.; Bogdanov, B.; Kirilchik, S. Complete mitochondrial genomes and phylogenetic analysis of four Baikal endemic Batrachocottus species (Scorpaeniformes: Cottoidei). Mitochondrial DNA Part B Resour. 2022, 7, 123–124. [Google Scholar] [CrossRef] [PubMed]
- Sandel, M.W.; Aguilar, A.; Fast, K.; O’Brien, S.; Lapidus, A.; Allison, D.B.; Teterina, V.; Kirilchik, S. Complete mitochondrial genomes of Baikal oilfishes (Perciformes: Cottoidei), earth’s deepest-swimming freshwater fishes. Mitochondrial DNA Part B Resour. 2017, 2, 773–775. [Google Scholar] [CrossRef]
- Yang, L.; Zhu, K.; Fang, J.; Liu, L.; Lü, Z. Characterization of the complete mitochondrial genome of Cottiusculus nihonkaiensis (Scorpaeniformes, Cottidae) and phylogenetic studies of Scorpaeniformes. Mitochondrial DNA Part B Resour. 2021, 6, 358–360. [Google Scholar] [CrossRef]
- Mugue, N.; Barmintseva, A.; Etingova, A.; Didorenko, S.; Selifanova, M.; Mugue, L.; Popov, A.; Bulakhov, A.; Kupchinskiy, A. Complete mitochondrial genomes of representatives of two endemic sculpin families (Perciformes: Cottoidei) from Baikal–the world’s largest and deepest lake. Mitochondrial DNA Part B Resour. 2021, 6, 3190–3192. [Google Scholar] [CrossRef]
- Miya, M.; Takeshima, H.; Endo, H.; Ishiguro, N.B.; Inoue, J.G.; Mukai, T.; Satoh, T.P.; Yamaguchi, M.; Kawaguchi, A.; Mabuchi, K.; et al. Major patterns of higher teleostean phylogenies: A new perspective based on 100 complete mitochondrial DNA sequences. Mol. Phylo. Evol. 2003, 26, 121–138. [Google Scholar] [CrossRef]
- Li, A.; Munehara, H. Complete mitochondrial genome of the Antlered sculpin Enophrys diceraus (Scorpaeniformes, Cottidae). Mitochondrial DNA Part B Resour. 2015, 26, 125–126. [Google Scholar] [CrossRef]
- Yi, C.H.; Kwun, H.J.; Song, Y.S.; Kim, Y.K.; Kim, W.; Kim, I.H. Complete mitochondrial genome of Gymnocanthus intermedius and Gymnocanthus herzensteini (Scorpaeniformes: Cottidae). Mitochondrial DNA Part B Resour. 2019, 4, 2660–2661. [Google Scholar] [CrossRef]
- Song, P.; Zhang, N.; Feng, J.; Li, Y.; Lin, L. The complete mitochondrial genome of the Arctic staghorn sculpin Gymnocanthus tricuspis (Scorpaeniformes: Cottidae). Mitochondrial DNA Part B Resour. 2019, 4, 1400–1401. [Google Scholar] [CrossRef]
- Swanburg, T.; Horne, J.B.; Baillie, S.; King, S.D.; McBride, M.C.; Mackley, M.P.; Paterson, I.G.; Bradbury, I.R.; Bentzen, P. Complete mitochondrial genomes for Icelus spatula, Aspidophoroides olrikii and Leptoclinus maculatus: Pan-Arctic marine fishes from Canadian waters. Mitochondrial DNA Part A 2016, 27, 2982–2983. [Google Scholar] [CrossRef] [PubMed]
- Balakirev, E.S.; Kravchenko, A.Y.; Cherepkova, E.V.; Saveliev, P.A.; Semenchenko, A.A.; Ayala, F.J. Complete mitochondrial genome of the Belligerent sculpin Megalocottus platycephalus (Cottoidei: Cottidae). Mitochondrial DNA Part B Resour. 2019, 4, 2980–2981. [Google Scholar] [CrossRef] [PubMed]
- Balakirev, E.S.; Kravchenko, A.Y.; Cherepkova, E.V.; Saveliev, P.A.; Semenchenko, A.A.; Ayala, F.J. Complete mitochondrial genome of the plain sculpin Myoxocephalus jaok (Cottoidei: Cottidae). Mitochondrial DNA Part B Resour. 2020, 5, 1295–1296. [Google Scholar] [CrossRef]
- Kim, B.M.; Kihm, J.H.; Park, T.Y.S. The complete mitochondrial genome of the fourhorn sculpin Triglopsis quadricornis (Perciformes, Cottidae) from Sirius Passet, North Greenland. Ocean Polar Res. 2021, 43, 371–374. [Google Scholar] [CrossRef]
- Li, Y.; Song, P.; Feng, J.; Zhang, N.; Zhang, R.; Lin, L. Complete mitochondrial genome sequence and phylogenetic analysis of Myoxocephalus scorpius (Linnaeus, 1758). Mitochondrial DNA Part B Resour. 2019, 4, 862–863. [Google Scholar] [CrossRef]
- Patil, M.P.; Kim, J.O.; Seo, Y.B.; Shin, J.; Yang, J.Y.; Kim, G.D. Complete mitochondrial genome of scorpionfish Scorpaena neglecta (Actinopterygii). Mitochondrial DNA Part B Resour. 2022, 7, 1375–1376. [Google Scholar] [CrossRef]
- Lee, Y.S.; Patil, M.P.; Kim, J.-O.; Lee, Y.J.; Seo, Y.B.; Kim, J.K.; Mahale, K.R.; Kim, G.-D. Complete Mitochondrial Genome and Phylogenetic Position of Chirolophis wui (Perciformes: Stichaeidae). Fishes 2023, 8, 165. [Google Scholar] [CrossRef]
- Satoh, T.P.; Miya, M.; Mabuchi, K.; Nishida, M. Structure and variation of the mitochondrial genome of fishes. BMC Genom. 2016, 17, 719. [Google Scholar] [CrossRef]
- Janke, A.; Pääbo, S. Editing of a tRNA anticodon in marsupial mitochondria changes its codon recognition. Nucleic Acids Res. 1993, 21, 1523–1525. [Google Scholar] [CrossRef]
- Boore, J.L. Animal mitochondrial genomes. Nucleic Acids Res. 1999, 27, 1767–1780. [Google Scholar] [CrossRef]


| Name | Accession Number | Size (bp) | In Percentage | AT-Skew | GC-Skew | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| G | A | T | C | A + T | G + C | ||||||
| Alcichthys elongatus | OR288162 | 16,712 | 17.48 | 26.43 | 25.90 | 30.14 | 52.33 | 47.62 | 0.0101 | −0.2659 | This study |
| Argyrocottus zanderi | NC_057483 | 16,608 | 17.09 | 26.99 | 26.47 | 29.44 | 53.46 | 46.53 | 0.0097 | −0.2654 | [28] |
| Batrachocottus baicalensis | MT527180 | 16,523 | 17.53 | 26.47 | 25.87 | 30.13 | 52.33 | 47.67 | 0.0115 | −0.2644 | [29] |
| Batrachocottus multiradiatus | MT527181 | 16,532 | 17.51 | 26.34 | 25.96 | 30.19 | 52.30 | 47.70 | 0.0073 | −0.2658 | [29] |
| Batrachocottus nikolskii | MT527182 | 16,535 | 17.41 | 26.42 | 26.01 | 30.17 | 52.42 | 47.58 | 0.0078 | −0.2682 | [29] |
| Batrachocottus talievi | MT527183 | 16,530 | 17.41 | 26.38 | 26.04 | 30.16 | 52.43 | 47.57 | 0.0065 | −0.2680 | [29] |
| Comephorus baikalensis | MF346885 | 16,538 | 17.17 | 26.74 | 26.05 | 30.00 | 52.79 | 47.18 | 0.0131 | −0.2720 | [30] |
| Comephorus dybowskii | NC_036149 | 16,527 | 17.20 | 26.73 | 26.19 | 29.88 | 52.92 | 47.08 | 0.0102 | −0.2693 | [30] |
| Cottiusculus nihonkaiensis | NC_045245 | 16,612 | 17.44 | 26.32 | 24.75 | 31.50 | 51.07 | 48.93 | 0.0307 | −0.2873 | [31] |
| Cottocomephorus grewingki | MW732165 | 16,590 | 17.15 | 27.13 | 26.60 | 29.10 | 53.73 | 46.24 | 0.0099 | −0.2584 | [32] |
| Cottocomephorus inermis | MW732163 | 16,510 | 17.14 | 27.10 | 26.58 | 29.17 | 53.68 | 46.31 | 0.0097 | −0.2598 | [32] |
| Cottus koreanus | NC_063951 | 16,558 | 17.62 | 26.48 | 26.02 | 29.89 | 52.49 | 47.51 | 0.0088 | −0.2583 | - |
| Cottus marginatus | NC_066924 | 16,603 | 16.68 | 27.28 | 26.10 | 29.93 | 53.39 | 46.61 | 0.0221 | −0.2843 | - |
| Cottus princeps | NC_066915 | 16,561 | 16.32 | 27.83 | 26.44 | 29.41 | 54.27 | 45.73 | 0.0256 | −0.2862 | - |
| Cottus reinii | NC_004404 | 16,561 | 17.63 | 26.30 | 25.78 | 30.28 | 52.09 | 47.91 | 0.0100 | −0.2640 | [33] |
| Enophrys bison | NC_066929 | 16,888 | 16.88 | 27.19 | 26.69 | 29.23 | 53.88 | 46.12 | 0.0092 | −0.2678 | - |
| Enophrys diceraus | NC_022147 | 16,976 | 16.65 | 27.53 | 27.19 | 28.64 | 54.71 | 45.29 | 0.0062 | −0.2647 | [34] |
| Gymnocanthus herzensteini | NC_034651 | 16,691 | 17.46 | 26.54 | 25.92 | 30.01 | 52.46 | 47.47 | 0.0118 | −0.2644 | [35] |
| Gymnocanthus intermedius | NC_034650 | 16,639 | 17.65 | 26.40 | 25.52 | 30.42 | 51.92 | 48.06 | 0.0169 | −0.2657 | [35] |
| Gymnocanthus tricuspis | NC_045927 | 16,570 | 17.36 | 26.74 | 25.76 | 30.14 | 52.49 | 47.51 | 0.0187 | −0.2691 | [36] |
| Icelus spatula | NC_027587 | 16,384 | 17.43 | 26.43 | 26.03 | 30.04 | 52.46 | 47.47 | 0.0076 | −0.2656 | [37] |
| Megalocottus platycephalus | MK936041 | 16,673 | 17.14 | 27.03 | 26.53 | 29.29 | 53.57 | 46.43 | 0.0093 | −0.2617 | [38] |
| Mesocottus haitej | NC_022181 | 16,527 | 17.35 | 26.64 | 26.12 | 29.88 | 52.76 | 47.24 | 0.0099 | −0.2653 | - |
| Myoxocephalus jaok | NC_045875 | 16,653 | 16.89 | 27.08 | 26.61 | 29.43 | 53.68 | 46.32 | 0.0088 | −0.2707 | [39] |
| Myoxocephalus quadricornis | NC_053359 | 16,736 | 17.42 | 26.83 | 26.42 | 29.33 | 53.25 | 46.75 | 0.0077 | −0.2548 | [40] |
| Myoxocephalus scorpius | NC_042186 | 16,626 | 16.83 | 27.22 | 26.78 | 29.18 | 53.99 | 46.01 | 0.0081 | −0.2684 | [41] |
| Paracottus knerii | MW732164 | 16,550 | 17.43 | 26.62 | 26.04 | 29.92 | 52.65 | 47.35 | 0.0110 | −0.2638 | [32] |
| Porocottus allisi | NC_057484 | 16,369 | 17.44 | 26.15 | 25.24 | 31.17 | 51.39 | 48.61 | 0.0177 | −0.2825 | [28] |
| Procottus major | MW732167 | 16,512 | 17.14 | 26.94 | 26.18 | 29.74 | 53.12 | 46.88 | 0.0143 | −0.2688 | [32] |
| Scorpaena neglecta | ON109388 | 17,202 | 17.45 | 28.36 | 26.40 | 27.79 | 54.76 | 45.24 | 0.0358 | −0.2286 | [42] |
| Chirolophis wui | OP388414 | 16,522 | 18.28 | 25.52 | 28.53 | 27.67 | 54.05 | 45.95 | −0.0556 | −0.2043 | [43] |
| Group | Group of Genes | Gene | Three Letter Code | Sequence | Size (bp) | Strand | No. of Amino Acids | Start Codon | Stop Codon | Anti-Codon | Intergenic Nucleotides * | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Start | End | |||||||||||
| PCGs | NADH dehydrogenase subunit | ND1 | - | 2852 | 3826 | 975 | H | 324 | ATG | TAG | - | 4 |
| ND2 | - | 4039 | 5084 | 1046 | H | 348 | ATG | TA- | - | 0 | ||
| ND3 | - | 9645 | 9993 | 349 | H | 116 | ATG | T-- | - | 0 | ||
| ND4L | - | 10,063 | 10,359 | 297 | H | 93 | ATG | TAA | - | −7 | ||
| ND4 | - | 10,353 | 11,733 | 1381 | H | 460 | ATG | T-- | - | 0 | ||
| ND5 | - | 11,948 | 13,786 | 1839 | H | 613 | ATG | TAA | - | −4 | ||
| ND6 | - | 13,783 | 14,304 | 522 | L | 174 | ATG | TAG | - | 0 | ||
| Cytochrome c oxidase subunit | COXI | - | 5475 | 7025 | 1551 | H | 516 | GTG | TAA | - | 0 | |
| COXII | - | 7180 | 7870 | 691 | H | 230 | ATG | T-- | - | 0 | ||
| COXIII | - | 8787 | 9571 | 785 | H | 261 | ATG | TA- | - | 0 | ||
| ATP synthase subunit | ATP8 | - | 7946 | 8113 | 168 | H | 55 | ATG | TAA | - | −10 | |
| ATP6 | - | 8104 | 8786 | 683 | H | 227 | ATG | TA- | - | 0 | ||
| Cytochrome b | Cytb | - | 14,379 | 15,519 | 1141 | H | 380 | ATG | T-- | - | 0 | |
| RNAs | Transfer RNA genes | trnF | Phe | 1 | 68 | 68 | H | - | - | - | GAA | 0 |
| trnV | Val | 1012 | 1083 | 72 | H | - | - | - | TAC | 0 | ||
| trnL | Leu | 2778 | 2851 | 74 | H | - | - | - | TAA | 0 | ||
| trnI | Ile | 3831 | 3900 | 70 | H | - | - | - | GAT | −1 | ||
| trnQ | Gln | 3900 | 3970 | 71 | L | - | - | - | TTG | −1 | ||
| trnM | Met | 3970 | 4038 | 69 | H | - | - | - | CAT | 0 | ||
| trnW | Trp | 5085 | 5155 | 71 | H | - | - | - | TCA | 1 | ||
| trnA | Ala | 5157 | 5225 | 69 | L | - | - | - | TGC | 1 | ||
| trnN | Asn | 5227 | 5299 | 73 | L | - | - | - | GTT | 38 | ||
| trnC | Cys | 5338 | 5403 | 66 | L | - | - | - | GCA | 0 | ||
| trnY | Tyr | 5404 | 5473 | 70 | L | - | - | - | GTA | 1 | ||
| trnS | Ser | 7026 | 7096 | 71 | L | - | - | - | TGA | 3 | ||
| trnD | Asp | 7100 | 7172 | 73 | H | - | - | - | GTC | 7 | ||
| trnK | Lys | 7871 | 7944 | 74 | H | - | - | - | TTT | 1 | ||
| trnG | Gly | 9572 | 9644 | 73 | H | - | - | - | TCC | 0 | ||
| trnR | Arg | 9994 | 10,062 | 69 | H | - | - | - | TCG | 0 | ||
| trnH | His | 11,734 | 11,802 | 69 | H | - | - | - | GTG | 0 | ||
| trnS | Ser | 11,803 | 11,870 | 68 | H | - | - | - | GCT | 4 | ||
| trnL | Leu | 11,875 | 11,947 | 73 | H | - | - | - | TAG | 0 | ||
| trnE | Glu | 14,305 | 14,373 | 69 | L | - | - | - | TTC | 5 | ||
| trnT | Thr | 15,520 | 15,591 | 72 | H | - | - | - | TGT | −1 | ||
| trnP | Pro | 15,591 | 15,660 | 70 | L | - | - | - | TGG | 0 | ||
| 12S rRNA | rrnS | - | 69 | 1011 | 943 | H | - | - | - | - | 0 | |
| 16S rRNA | rrnL | - | 1084 | 2777 | 1694 | H | - | - | - | - | 0 | |
| D-loop | Control region | - | - | 15,661 | 16,712 | 1052 | H | - | - | - | - | 0 |
| Amino Acid | Codon | Number | % | Fraction | Amino Acid | Codon | Number | % | Fraction |
|---|---|---|---|---|---|---|---|---|---|
| Ala | GCG | 12 | 0.316 | 0.03 | Asn | AAT | 30 | 0.789 | 0.27 |
| GCA | 75 | 1.974 | 0.21 | AAC | 83 | 2.184 | 0.73 | ||
| GCT | 70 | 1.842 | 0.19 | Pro | CCG | 8 | 0.211 | 0.04 | |
| GCC | 203 | 5.342 | 0.56 | CCA | 35 | 0.921 | 0.16 | ||
| Cys | TGT | 10 | 0.263 | 0.42 | CCT | 54 | 1.421 | 0.25 | |
| TGC | 14 | 0.368 | 0.58 | CCC | 121 | 3.184 | 0.56 | ||
| Asp | GAT | 23 | 0.605 | 0.32 | Gln | CAG | 26 | 0.684 | 0.26 |
| GAC | 50 | 1.316 | 0.68 | CAA | 74 | 1.947 | 0.74 | ||
| Glu | GAG | 30 | 0.789 | 0.30 | Arg | CGG | 15 | 0.395 | 0.20 |
| GAA | 70 | 1.842 | 0.70 | CGA | 28 | 0.737 | 0.37 | ||
| Phe | TTT | 122 | 3.211 | 0.53 | CGT | 13 | 0.342 | 0.17 | |
| TTC | 107 | 2.816 | 0.47 | CGC | 20 | 0.526 | 0.26 | ||
| Gly | GGG | 68 | 1.789 | 0.27 | Ser | AGT | 11 | 0.289 | 0.04 |
| GGA | 54 | 1.421 | 0.22 | AGC | 48 | 1.263 | 0.19 | ||
| GGT | 35 | 0.921 | 0.14 | TCG | 15 | 0.395 | 0.06 | ||
| GGC | 92 | 2.421 | 0.37 | TCA | 44 | 1.158 | 0.18 | ||
| His | CAT | 25 | 0.658 | 0.24 | TCT | 52 | 1.368 | 0.21 | |
| CAC | 81 | 2.132 | 0.76 | TCC | 78 | 2.053 | 0.31 | ||
| Ile | ATT | 127 | 3.342 | 0.49 | Thr | ACG | 27 | 0.711 | 0.09 |
| ATC | 132 | 3.474 | 0.51 | ACA | 78 | 2.053 | 0.26 | ||
| Lys | AAG | 14 | 0.368 | 0.20 | ACT | 54 | 1.421 | 0.18 | |
| AAA | 57 | 1.500 | 0.80 | ACC | 144 | 3.789 | 0.48 | ||
| Leu | TTG | 33 | 0.868 | 0.05 | Val | GTG | 34 | 0.895 | 0.15 |
| TTA | 74 | 1.947 | 0.11 | GTA | 68 | 1.789 | 0.29 | ||
| CTG | 58 | 1.526 | 0.09 | GTT | 61 | 1.605 | 0.26 | ||
| CTA | 165 | 4.342 | 0.25 | GTC | 68 | 1.789 | 0.29 | ||
| CTT | 152 | 4.000 | 0.23 | Trp | TGG | 27 | 0.711 | 0.23 | |
| CTC | 181 | 4.763 | 0.27 | TGA | 93 | 2.447 | 0.78 | ||
| Met | ATG | 74 | 19.47 | 0.50 | Tyr | TAT | 27 | 0.711 | 0.25 |
| ATA | 74 | 19.47 | 0.50 | TAC | 82 | 2.158 | 0.75 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patil, M.P.; Kim, J.-O.; Yoo, S.H.; Seo, Y.B.; Lee, Y.-J.; Kim, J.-K.; Kitamura, S.-I.; Kim, G.-D. Complete Mitogenome and Phylogenetic Analysis of a Marine Ray-Finned Fish, Alcichthys elongatus (Perciformes: Cottidae). Fishes 2023, 8, 513. https://doi.org/10.3390/fishes8100513
Patil MP, Kim J-O, Yoo SH, Seo YB, Lee Y-J, Kim J-K, Kitamura S-I, Kim G-D. Complete Mitogenome and Phylogenetic Analysis of a Marine Ray-Finned Fish, Alcichthys elongatus (Perciformes: Cottidae). Fishes. 2023; 8(10):513. https://doi.org/10.3390/fishes8100513
Chicago/Turabian StylePatil, Maheshkumar Prakash, Jong-Oh Kim, Seung Hyun Yoo, Yong Bae Seo, Yu-Jin Lee, Jin-Koo Kim, Shin-Ichi Kitamura, and Gun-Do Kim. 2023. "Complete Mitogenome and Phylogenetic Analysis of a Marine Ray-Finned Fish, Alcichthys elongatus (Perciformes: Cottidae)" Fishes 8, no. 10: 513. https://doi.org/10.3390/fishes8100513
APA StylePatil, M. P., Kim, J.-O., Yoo, S. H., Seo, Y. B., Lee, Y.-J., Kim, J.-K., Kitamura, S.-I., & Kim, G.-D. (2023). Complete Mitogenome and Phylogenetic Analysis of a Marine Ray-Finned Fish, Alcichthys elongatus (Perciformes: Cottidae). Fishes, 8(10), 513. https://doi.org/10.3390/fishes8100513







