Ecological Niche and Interspecific Association of the Main Fishes in the Coastal Waters of Hainan Island, China
Abstract
:1. Introduction
2. Methods and Materials
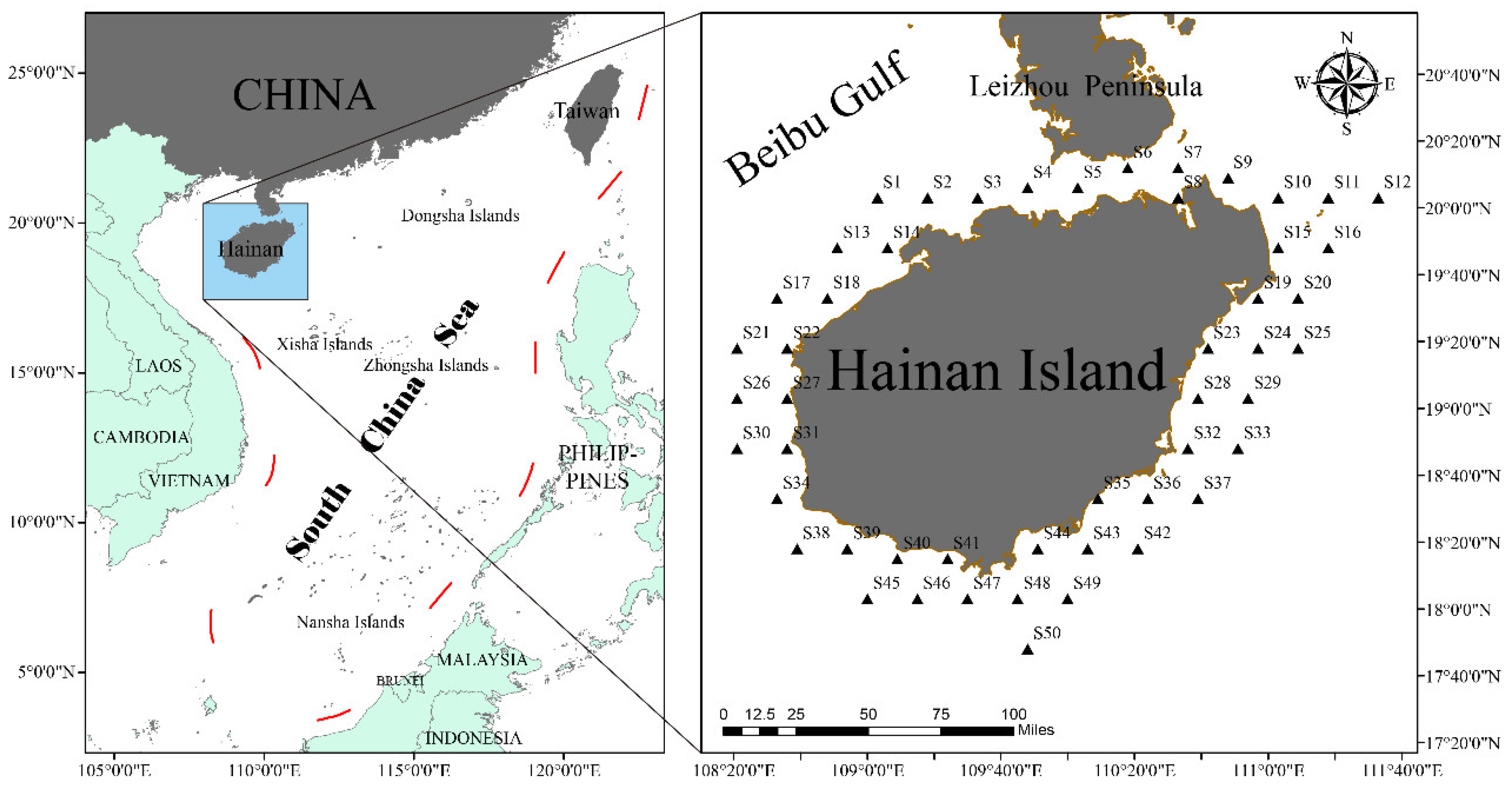
2.1. Study Area
2.2. Material Sources
2.3. Dominance Analysis
2.3.1. The Index of Relative Importance (IRI)
2.3.2. Ecological Niche
2.3.3. Interspecific Association
2.3.4. Redundancy Analysis
2.3.5. Data Processing
3. Results
3.1. Composition of Catches
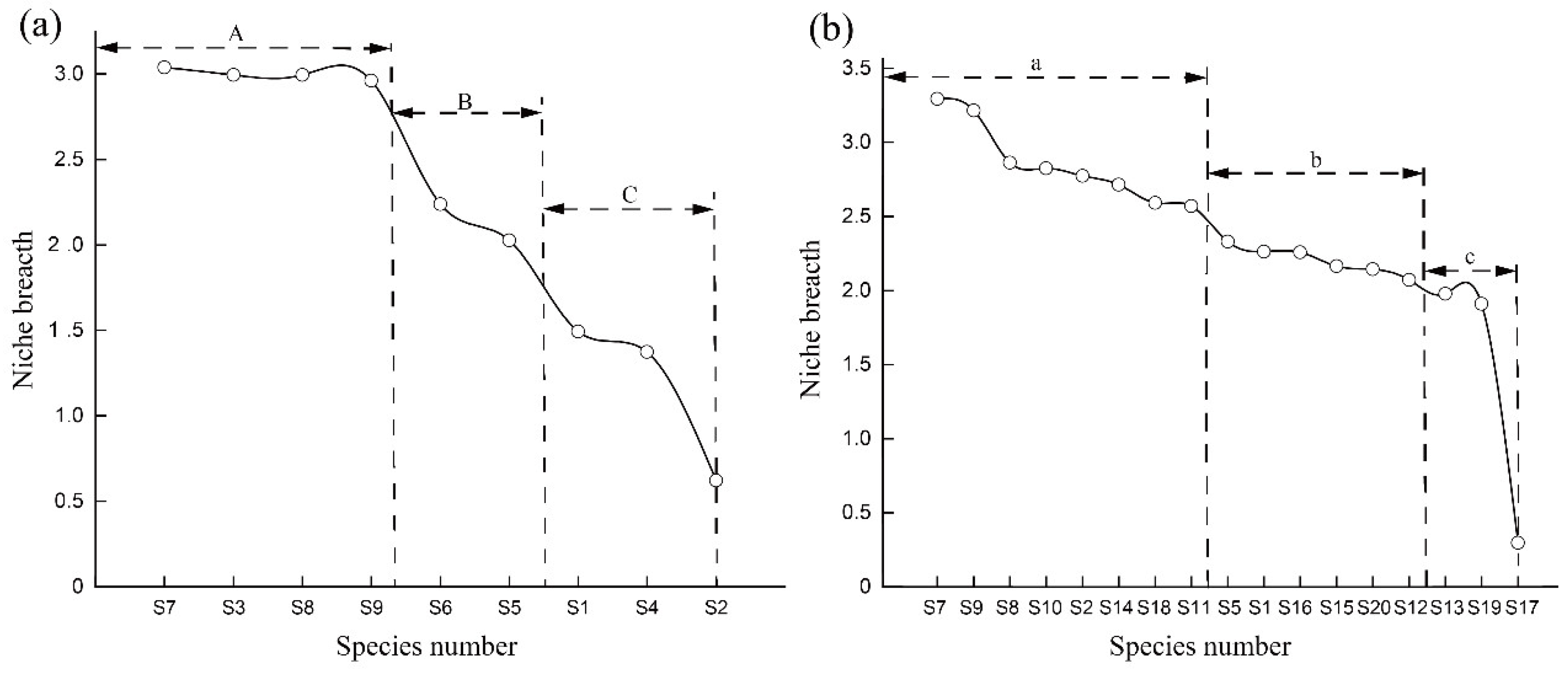
3.2. Main Species and Niche Breadth
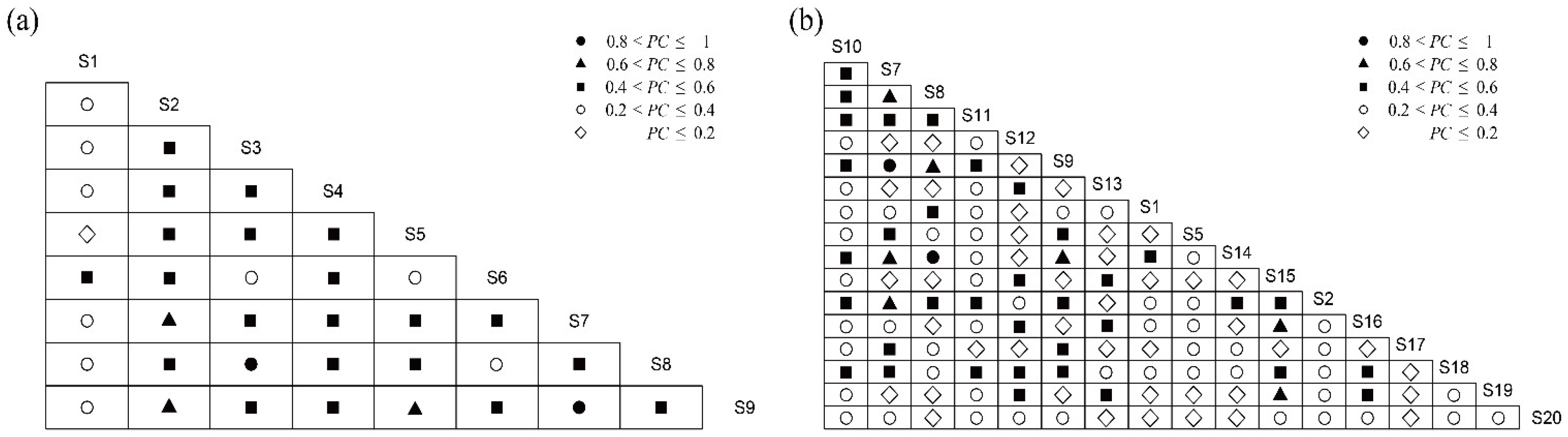
3.3. Niche Overlap
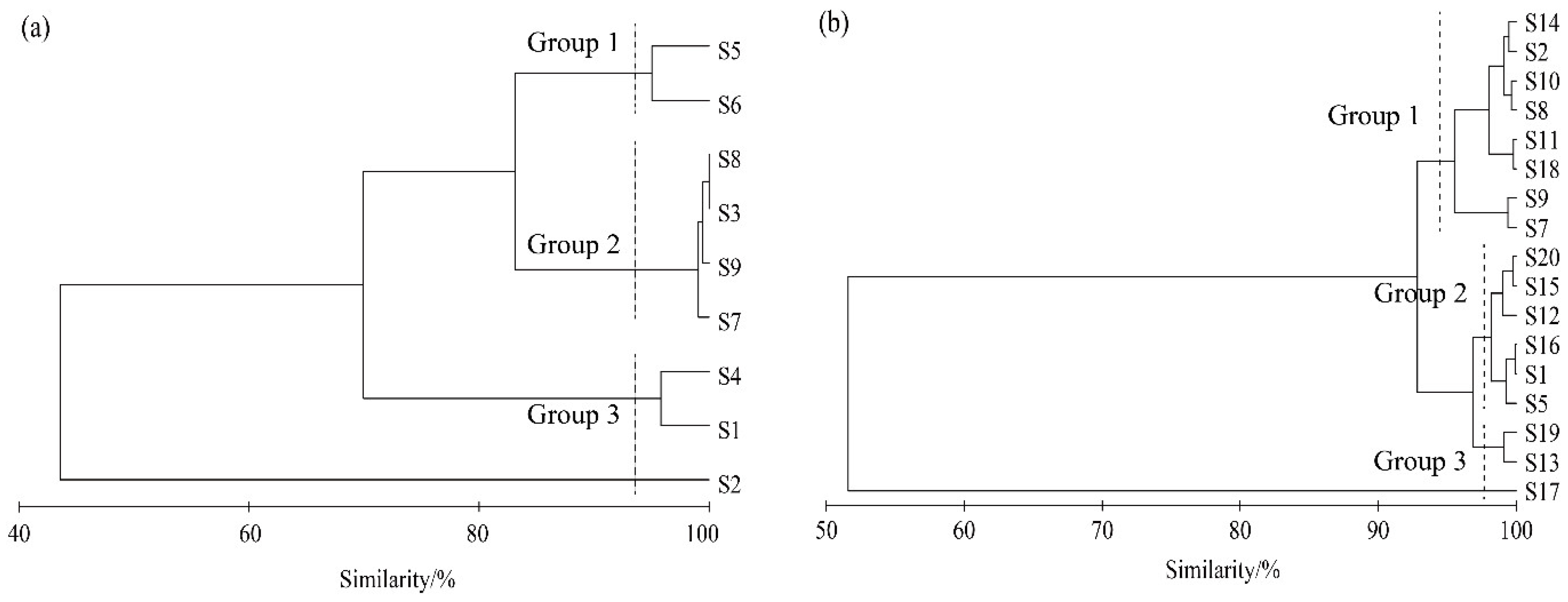
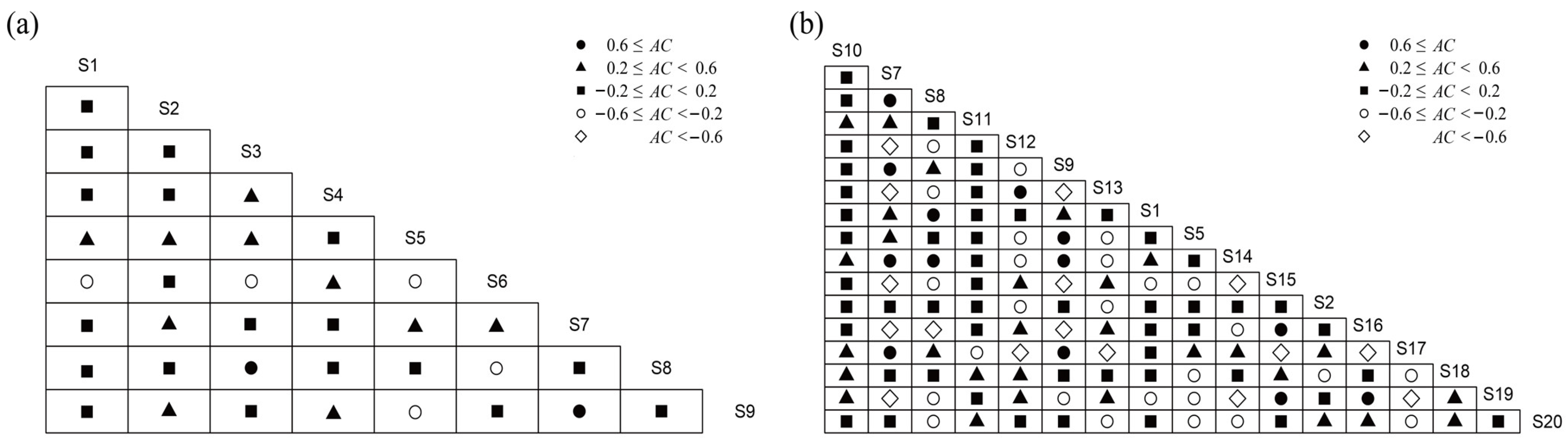
3.4. Overall Association Analysis
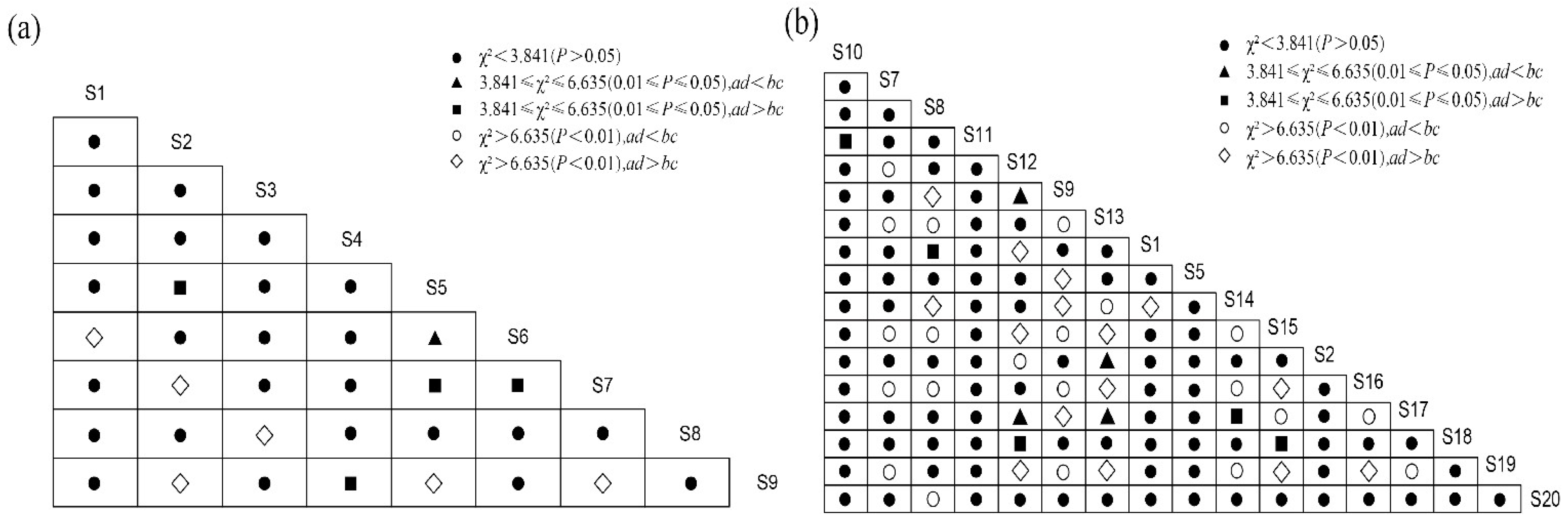
3.5. Interspecific Association Analysis
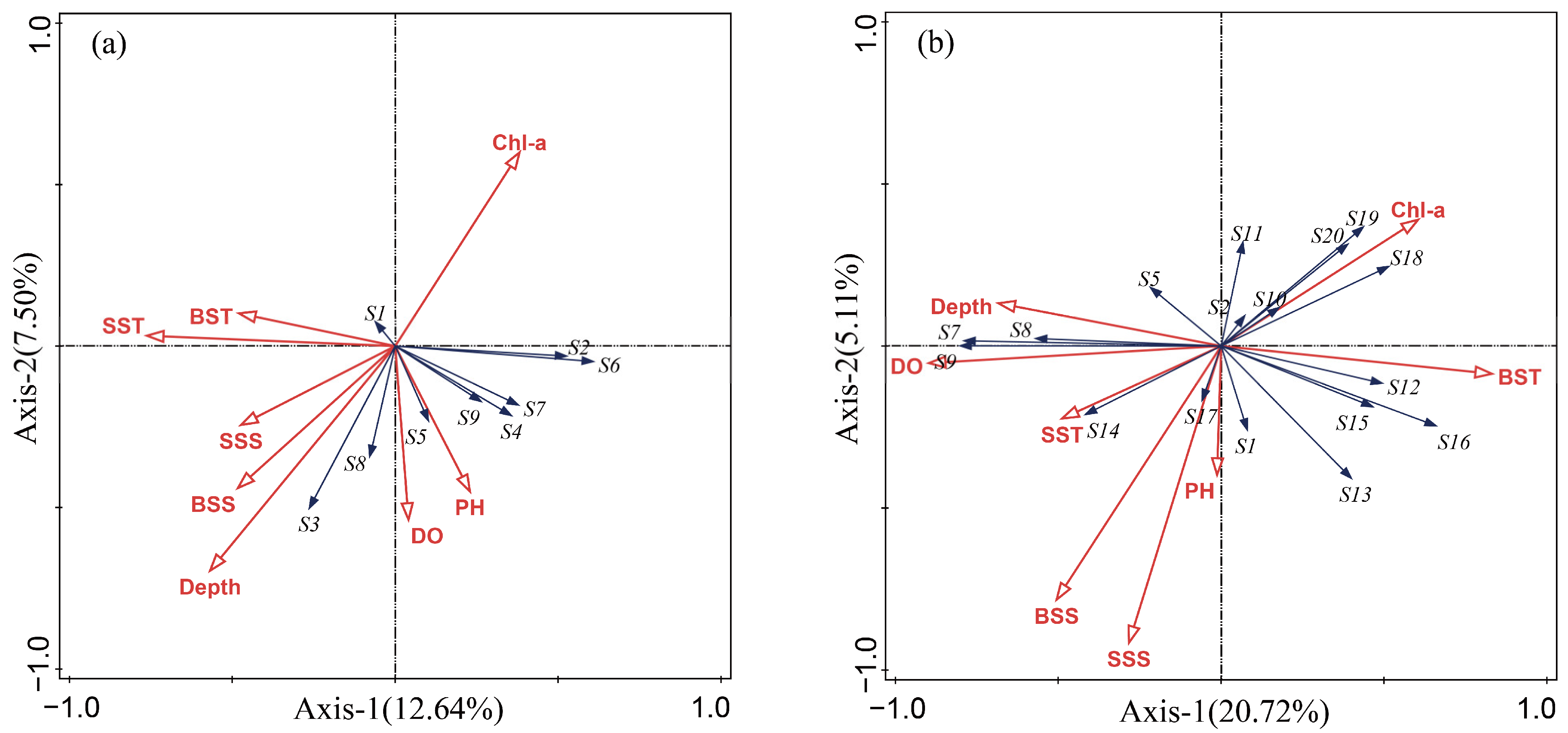
3.6. RDA Graph Analysis
4. Discussion
4.1. Niche Breadth
4.2. Niche Overlap
4.3. Overall Association and Interspecific Association
4.4. The Relationship between Main Fish Niches and Environmental Factors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Colwell, R.K.; Futuyma, D.J. On the measurement of niche breadth and overlap. Ecology 1971, 52, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Whittaker, R.H. Communities and Ecosystems, 2nd ed.; Macmillan: New York, NY, USA, 1975; pp. 39–87. ISBN 978-0024273901. [Google Scholar]
- Yang, Q.W.; Liu, S.J.; Hu, C.H.; Lin, Y.B.; Zhang, B.; Luo, M.L.; Peng, H.L. Ecological Species Groups and Interspecific Association of Vegetation in Natural Recovery Process at Xiejiadian landslide after 2008 Wenchuan Earthquake. J. Mt. Sci. 2016, 13, 1609–1620. [Google Scholar] [CrossRef]
- Álvarez-Yépiz, J.C.; Búrquez, A.; Dovčiak, M. Ontogenetic Shifts in Plant–Plant Interactions in a Rare Cycad within Angiosperm Communities. Oecologia 2014, 175, 725–735. [Google Scholar] [CrossRef] [PubMed]
- Grinnell, J. The Niche-Relationships of the California Thrasher. Auk 1917, 34, 427–433. [Google Scholar] [CrossRef]
- Elton, C.S. The Animal Community. In Animal Ecology; Macmillan Co.: New York, NY, USA, 1927; pp. 50–55. [Google Scholar]
- Hutchinson, G.E. Population Studies: Animal Ecology and Demography: Concluding Remarks. Cold Spring Harb Symp. Quant. Biol. 1957, 22, 425–427. [Google Scholar] [CrossRef]
- MacArthur, R. Species Packing and Competitive Equilibrium for Many Species. Theor. Popul. Biol. 1970, 1, 1–11. [Google Scholar] [CrossRef]
- Gao, Y.; Qian, G.Z. The Niche Analysis of Brid Community in Evergreen Broad-Left Forest in Tiantong Mountain, Zhejiang Province. Acta. Ecol. Sin. 1987, 7, 73–82. [Google Scholar]
- Liu, F.; Li, R.D. Spatial and Trophic Niches of There Species of Lizards in Desert. Acta Ecol. Sin. 1995, 15, 48–53. [Google Scholar]
- Guo, S.L. Study on Niche of Wood Plants in Mount Bei of Jinhua. Bull. Bot. Res. 1998, 18, 55–64. [Google Scholar]
- Zheng, C.X.; Li, R.G.; Jiang, J.X.; Zheng, F.W.; Wang, J.J.; Huang, X.G.; Lin, J.H. An Ecological Study on the Benthos of the Rocky Intertidal Zone in Quanzhou Bay. I. Species Diversity and its Distribution. Biodiversity Science. Chin. Biodivers. 2004, 12, 594–610. [Google Scholar]
- Zhao, Y.Q.; Zeng, J.N.; Gao, A.G.; Chen, Q.Z.; Shou, L.; Liao, Y.B.; Huang, Y.J. Niche of Macrozoobenthos in Interidal Zone of Jiaojiang Estuary. Chin. J. Appl. Ecol. 2009, 20, 1176–1183. [Google Scholar]
- Li, F.; Xu, B.Q.; Lu, Z.B.; Wang, T.T. Ecological Niche of Dominant Species of Fish Assemblages in Laizhou Bay. Acta Ecol. Sin. 2018, 38, 5195–5205. [Google Scholar]
- Han, X.F.; Wang, Y.X.; Qiu, J.J.; Zhang, M.M.; Yu, S.L.; Liang, H.; Zhang, Y.H.; Lai, H.Y.; Jiang, Y.X.; Shui, B.N. Niche and Interspecific Associations of Dominant Fishes in Southern Coastal Waters in Taizhou, China. J. Fish. 2020, 44, 621–631. [Google Scholar]
- Dai, D.X.; Yu, C.G.; Liu, H.; Yan, W.C.; Sun, B.B.; Jian, K.K.; Xin, Y.; Zhang, W.J. Niche and Interspecific Association of Major Nekton in the Sea Area to the East of the Nanji Islands. Chin. J. Appl. Ecol. 2019, 30, 4249–4258. [Google Scholar]
- Coll, M.; Libralato, S.; Tudela, S.; Palomera, I.; Pranovi, F. Ecosystem overfishing in the ocean. PLoS ONE 2008, 3, e3881. [Google Scholar] [CrossRef]
- Hogg, R.S.; Coghlan, S.M., Jr.; Zydlewski, J.; Gardner, C. Fish community response to a small-stream dam removal in a maine coastal river tributary. Trans. Am. Fish. Soc. 2015, 144, 467–479. [Google Scholar] [CrossRef]
- Teixeira, T.P.; Neves, L.M.; Araújo, F.G. Effects of a nuclear power plant thermal discharge on habitat complexity and fish community structure in Ilha Grande Bay, Brazil. Mar. Environ. Res. 2009, 68, 188–195. [Google Scholar] [CrossRef]
- Pinkas, L.M.; Oliphant, S.; Iverson, L.K. Food Habits of Albacore, Bluefin Tuna and Bonito in Californian Waters. Calif. Fish. Game 1971, 152, 1–105. [Google Scholar]
- Sun, D.R.; Li, Y.; Lin, Z.J.; Wu, Q.E.; Wang, X.H. Community Structure of Fish in the Coastal Waters of Hainan Island. J. Ocean Univ. China 2011, 41, 33–38. [Google Scholar]
- Zhang, Y.X.; Liu, W.; Zhao, H.L.; Wu, C.H.; Chen, M. Diversity Analysis of Nekton in the Offshore Waters of Hainan Island in Spring and Autumn. J. Fish. Res. 2021, 43, 133–142. [Google Scholar]
- Luo, Z.L.; Yang, C.P.; Wang, L.M.; Liu, Y.; Shan, B.B.; Liu, M.T.; Chen, C.; Guo, T.; Sun, D. Relationships between Fish Community Structure and Environmental Factors in the Nearshore Waters of Hainan Island, South China. Diversity 2023, 15, 901. [Google Scholar] [CrossRef]
- Search Fish Base. Available online: https://fishbase.mnhn.fr/search.php (accessed on 8 September 2023).
- Wu, H.L.; Zhong, J.S. Key to Marine and Estuarial Fishes of China; China Agriculture Press: Beijing, China, 2021; pp. 1–1437. ISBN 9787109279995. [Google Scholar]
- Chen, D.G.; Zhang, M.Z. Marine Fishes of China; China Ocean University Press: Qingdao, China, 2015; pp. 1–2196. ISBN 9787567010659. [Google Scholar]
- Shen, S.J.; Wu, G.Y. Fishes of Taiwan; National Museum of Marine Biology & Aquarium Press: Pingdong, China, 2011; pp. 1–896. ISBN 9789860298994. [Google Scholar]
- NASA Ocean Color. Available online: https://oceancolor.gsfc.nasa.gov/about/ (accessed on 8 September 2023).
- Martin, G.R.; Twigg, L.E.; Robinson, D.J. Comparison of the Diet of Feral Cats from Rural and Pastoral Western Australia. Wildl. Res. 1996, 23, 475–484. [Google Scholar] [CrossRef]
- Hart, R.K.; Calver, M.C.; Dickman, C.R. The Index of Relative Importance: An Alternative Approach to Reducing Bias in Descriptive Studies of Animal Diets. Wildl. Res. 2002, 29, 415–421. [Google Scholar] [CrossRef]
- Spies, T.A.; Franklin, J.F.; Klopsch, M. Canopy Gaps in Douglas-Fir Forests of the Cascade Mountains. Can. J. For. Res. 1990, 20, 649–658. [Google Scholar] [CrossRef]
- Shannon, C.E. A Mathematical Theory of Communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Pianka, E.R. The Structure of Lizard Communities. Annu. Rev. Ecol. Syst. 1973, 4, 53–74. [Google Scholar] [CrossRef]
- Schluter, D. A Variance Test for Detecting Species Associations, with Some Example Applications. Ecology 1984, 65, 998–1005. [Google Scholar] [CrossRef]
- Brower, J.E.; Zar, J.H.; Von Ende, C.N. Field and Laboratory Methods for General Ecology; WCB McGraw-Hill: Boston, MA, USA, 1998; Volume 4, pp. 25–51. ISBN 9780697045454. [Google Scholar]
- Dice, L.R. Measures of the Amount of Ecologic Association Between Species. Ecology 1945, 26, 297–302. [Google Scholar] [CrossRef]
- Hurlbert, S.H. A Coefficient of Interspecific Assciation. Ecology 1969, 50, 1–9. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Wang, B.S.; Li, M.G.; Zan, Q.J. An Analysis of Interspecific Associations in Secondary Succession Forest Communities in Heishiding Natural Reserve, Guangdong Province. Chin. J. Plant Ecol. 2000, 24, 332–339. [Google Scholar]
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003; pp. 1–253. ISBN 0-521-81409-X. [Google Scholar]
- Herawati, T.; Saputra, R.N.; Lili, W.; Suryadi, I.B.; Kurniawati, N.; Hedianto, D.A.; Herawati, H. The Food Habits, Niche Breadth and Niche Overlap of Fish Community in Jatigede Reservoir, West Java. In Proceedings of the 5th NA International Conference on Industrial Engineering and Operations Management Detroit, Detroit, MI, USA, 10–14 August 2020; pp. 2558–2568. [Google Scholar]
- Wathne, J.A.; Haug, T.; Lydersen, C. Prey Preference and Niche Overlap of Ringed Seals Phoca hispida and Harp Seals P. groenlandica in the Barents Sea. Mar. Ecol. Prog. Ser. 2000, 194, 233–239. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, H.C.; Jia, H.P.; Pen, M.D.J.; Liu, N.; Wei, J.J.; Zhou, B.Y. Ecological Niche and Interspecific Association of Plant Communities in Alpine Desertification Grasslands: A Case Study of Qinghai Lake Basin. Plants 2022, 11, 2724. [Google Scholar] [CrossRef] [PubMed]
- Carscadden, K.A.; Emery, N.C.; Arnillas, C.A.; Cadotte, M.W.; Afkhami, M.E.; Gravel, D.; Livingstone, S.W.; Wiens, J.J. Niche Breadth: Causes and Consequences for Ecology, Evolution, and Conservation. Q. Rev. Biol. 2020, 95, 179–214. [Google Scholar] [CrossRef]
- Gao, H.; Liu, H.P.; Wang, Q.M.; Liu, F.; Li, J.Y.; Duan, Y.T.; Zeren, L.W.; Fu, S.X.; Zhang, J.S.; Zhou, Y.; et al. Niche Analysis of the Main Fish in the Lhasa River Basin. Water 2023, 15, 860. [Google Scholar] [CrossRef]
- Hou, G.; Wang, J.R.; Chen, Z.Z.; Zhou, J.L.; Huang, W.S.; Zhang, H. Molecular and Morphological Identification and Seasonal Distribution of Eggs of Four Decapterus Fish Species in the Northern South China Sea: A Key to Conservation of Spawning Ground. Front. Mar. Sci. 2020, 7, 590564. [Google Scholar] [CrossRef]
- Xie, F.W.; Liang, J.L.; Xing, K.M. Distribution Characteristics of Zooplankton in Summer and Autumn in the Coastal Waters of Hainan Island. Coast. Eng. J. 2019, 38, 280–292. [Google Scholar]
- Han, D.Y.; Xue, Y.; Ji, L.P.; Xu, B.D.; Liu, H.; Ma, Q.Y. Trophic and Spatial Niche of Five Gobiid Fishes in Jiaozhou Bay. J. Fish. Sci. China 2013, 20, 148–156. [Google Scholar] [CrossRef]
- Jawad, L.A.; Al-Jufaili, S.M. Scale Morphology of Greater Lizardfish Saurida tumbil (Bloch, 1795) (Pisces: Synodontidae). J. Fish. Biol. 2007, 70, 1185–1212. [Google Scholar] [CrossRef]
- Thangavelu, R.; Anbarasu, M.; Zala, M.S.; Koya, M.; Sreenath, K.R.; Mojjada, S.K.; Shiju, P. Food and Feeding Habits of Commercially Important Demersal Finfishes off Veraval Coast. Indian J. Fish. 2012, 59, 77–87. [Google Scholar]
- Li, X.S.; Yu, Z.H.; Sun, S.; Jin, X.S. Ecological Niche Breadth and Niche Overlap of Dominant Species of Fish Assemblage in Yangtze River Estuary and its Adjacent Waters. Chin. J. Appl. Ecol. 2013, 24, 2353–2359. [Google Scholar]
- Pratchett, M.S.; Berumen, M.L. Interspecific Variation in Distributions and Diets of Coral Reef Butterflyfishes (Teleostei: Chaetodontidae). J. Fish. Biol. 2008, 73, 1730–1747. [Google Scholar] [CrossRef]
- Ge, B.M.; Bao, Y.X.; Zheng, X.; Cheng, H.Y. The Structure of the Macrobenthic Community and Niche Analysis at a Tidal Flat of Linkun Island. Acta Ecol. Sin. 2005, 25, 245–251. [Google Scholar]
- Haukisalmi, V.; Henttonen, H. Analysing Interspecific Associations in Parasites: Alternative Methods and Effects of Sampling Heterogeneity. Oecologia 1998, 116, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.L.; Zhou, H.C.; Guo, S.C.; Huang, Z.L. Studies on the Changes in Interspecific Association of Zonal Vegetation in Dinhushan. J. Integr. Plant Biol. 1999, 41, 1239–1244. [Google Scholar]
- Su, S.J.; Liu, J.F.; He, Z.S.; Zheng, S.Q.; Hong, W.; Xu, D.W. Ecological Species Groups and Interspecific Association of Dominant Tree Species in Daiyun Mountain National Nature Reserve. J. Mt. Sci. 2015, 12, 637–646. [Google Scholar] [CrossRef]
- Ma, Y.M.; Li, Q.H.; Pan, S.P.; Liu, C.; Han, M.S.; Brancelj, A. Niche and Interspecific Associations of Pseudoanabaena limnetica–Exploring the Influencing Factors of its Succession Stage. Ecol. Indic. 2022, 138, 108806. [Google Scholar] [CrossRef]
- Forsyth, D.M.; Ramsey, D.S.; Woodford, L.P. Estimating Abundances, Densities, and Interspecific Associations in a Carnivore Community. J. Wildl. Manag. 2019, 83, 1090–1102. [Google Scholar] [CrossRef]
- Ni, Y.G.; Endler, R.; Xia, Z.; Endler, M.; Harff, J.; Gan, H.Y.; Schulz-Bull, D.E.; Waniek, J.J. The “Butterfly Delta” System of Qiongzhou Strait: Morphology, Seismic Stratigraphy and Sedimentation. Mar. Geol. 2014, 355, 361–368. [Google Scholar] [CrossRef]
- Li, X.Q.; Fairweather, L.; Wu, S.G.; Ren, J.Y.; Zhang, H.J.; Quan, X.Y.; Jiang, T.; Zhang, C.; Su, M.; He, Y.L.; et al. Morphology, Sedimentary Features and Evolution of a Large Palaeo Submarine Canyon in Qiongdongnan Basin, Northern South China Sea. J. Asian Earth Sci. 2013, 62, 685–696. [Google Scholar] [CrossRef]
- Shi, X.B.; Kohn, B.; Spencer, S.; Guo, X.W.; Li, Y.M.; Yang, X.Q.; Shi, H.C.; Gleadow, A. Cenozoic Denudation History of Southern Hainan Island, South China Sea: Constraints from Low Temperature Thermochronology. Tectonophysics 2011, 504, 100–115. [Google Scholar] [CrossRef]
- Sattler, T.; Bontadina, F.; Hirzel, A.H.; Arlettaz, R. Ecological Niche Modelling of Two Cryptic Bat Species Calls for a Reassessment of Their Conservation Status. J. Appl. Ecol. 2007, 44, 1188–1199. [Google Scholar] [CrossRef]
- Zhang, M.X.; Wang, D.X.; Kang, B.; Zhang, G.G.; Liu, P.; Du, Y.L.; Yu, F. Interspecific Associations of Dominant Plant Populations in Secondary Forest of Pinus armandii in Qinling Mountains. Sci. Silvae Sin. 2015, 51, 12–21. [Google Scholar]
- Corrêa, C.E.; Albrecht, M.P.; Hahn, N.S. Patterns of Niche Breadth and Feeding Overlap of the Fish Fauna in the Seasonal Brazilian Pantanal, Cuiabá River Basin. Neotrop. Ichthyol. 2011, 9, 637–646. [Google Scholar] [CrossRef]
- Hou, G.; Wang, J.R.; Liu, L.M.; Chen, Y.Y.; Pan, C.H.; Lin, J.B.; Zhang, H. Assemblage Structure of the Ichthyoplankton and Its Relationship With Environmental Factors in Spring and Autumn off the Pearl River Estuary. Front. Mar. Sci. 2021, 8, 732970. [Google Scholar] [CrossRef]
- Lloret-Lloret, E.; Navarro, J.; Giménez, J.; López, N.; Albo-Puigserver, M.; Pennino, M.G.; Coll, M. The Seasonal Distribution of a Highly Commercial Fish Is Related to Ontogenetic Changes in Its Feeding Strategy. Front. Mar. Sci. 2020, 7, 566686. [Google Scholar] [CrossRef]
- Li, R.H.; Liu, S.M.; Li, Y.W.; Zhang, G.L.; Ren, J.L.; Zhang, J. Nutrient Dynamics in Tropical Rivers, Lagoons, and Coastal Ecosystems of Eastern Hainan Island, South China Sea. Biogeosciences 2014, 11, 481–506. [Google Scholar] [CrossRef]
- Fang, G.H.; Wang, Y.G.; Wei, Z.X.; Fang, Y.; Qiao, F.L.; Hu, X.M. Interocean Circulation and Heat and Freshwater Budgets of the South China Sea Based on a Numerical Model. Dyn. Atmos. Oceans 2009, 47, 55–72. [Google Scholar] [CrossRef]
- Diana, A.Z.N.; Sari, L.A.; Arsad, S.; Pursetyo, K.T.; Cahyoko, Y. Monitoring of Phytoplankton Abundance and Chlorophyll-a Content in the Estuary of Banjar Kemuning River, Sidoarjo Regency, East Java. J. Ecol. Eng. 2021, 22, 29–35. [Google Scholar]
- Benedetti, F.; Jalabert, L.; Sourisseau, M.; Becker, B.; Cailliau, C.; Desnos, C.; Elineau, A.; Irisson, J.O.; Lombard, F.; Picheral, M.; et al. The Seasonal and Inter-Annual Fluctuations of Plankton Abundance and Community Structure in a North Atlantic Marine Protected Area. Front. Mar. Sci. 2019, 6, 214. [Google Scholar] [CrossRef]
- Zheng, T.; Lin, Y.S.; Cao, W.Q.; Zhang, W.J.; Zheng, L.M.; Wang, Y.J.; Yang, W.D. Ecosystem Structure and Function in Northern Beibu Gulf: Zooplankton Spatial Niche and its Differentiation. Acta Ecol. Sin. 2014, 34, 3635–3649. [Google Scholar]
- Dong, J.; Shui, B.; Hu, C.; Shui, Y.; Du, X.; Tian, K. Niche and interspecific association of the dominant fish in the south coastal waters of Wenzhou, China. Chin. J. Appl. Ecol. 2017, 28, 1699–1706. [Google Scholar]
| Species | Dominant species | Spring | Autumn | ||
|---|---|---|---|---|---|
| IRI | Bi | IRI | Bi | ||
| S1 * | Acropoma japonicum | 3013.82 | 1.494 | 332.49 | 2.263 |
| S2 * | Decapterus maruadsi | 1475.74 | 0.621 | 200.44 | 2.773 |
| S3 | Navodon xanthopterus | 548.26 | 2.992 | ||
| S4 | Trachurus japonicus | 386.92 | 1.374 | ||
| S5 * | Upeneus japonicus | 385.01 | 2.027 | 331.61 | 2.331 |
| S6 | Psenopsis anomala | 321.09 | 2.239 | ||
| S7 * | Saurida tumbil | 217.72 | 3.039 | 646.08 | 3.293 |
| S8 * | Champsodon atridorsalis | 194.26 | 2.994 | 528.74 | 2.863 |
| S9 * | Saurida undosquamis | 147.97 | 2.962 | 380.45 | 3.216 |
| S10 | Leiognathus bindus | 730.84 | 2.824 | ||
| S11 | Leiognathus berbis | 451.21 | 2.571 | ||
| S12 | Johnius belengeri | 392.12 | 2.071 | ||
| S13 | Pennahia macrocephalus | 372.31 | 1.978 | ||
| S14 | Brachypleura novaezeelandiae | 256.09 | 2.715 | ||
| S15 | Pennahia anea | 240.42 | 2.165 | ||
| S16 | Therapon thraps | 178.53 | 2.259 | ||
| S17 | Rogadius asper | 145.78 | 0.297 | ||
| S18 | Upeneus sulphureus | 118.03 | 2.592 | ||
| S19 | Ilisha melastoma | 113.01 | 1.909 | ||
| S20 | Parargyrops edita | 108.69 | 2.143 | ||
| Species | S1 | S2 | S3 | S4 | S5 | S6 | S7 | S8 |
|---|---|---|---|---|---|---|---|---|
| S2 | <0.001 | |||||||
| S3 | 0.096 | 0.027 | ||||||
| S4 | 0.006 | 0.01 | 0.36 | |||||
| S5 | <0.001 | <0.001 | 0.17 | 0.01 | ||||
| S6 | 0.035 | 0.41 | 0.30 | 0.86 | 0.004 | |||
| S7 | 0.01 | 0.077 | 0.31 | 0.22 | 0.21 | 0.33 | ||
| S8 | 0.01 | 0.027 | 0.83 | 0.36 | 0.17 | 0.30 | 0.31 | |
| S9 | 0.038 | 0.047 | 0.32 | 0.067 | 0.27 | 0.17 | 0.91 | 0.32 |
| Species | S10 | S7 | S8 | S11 | S12 | S9 | S13 | S1 | S5 | S14 | S15 | S2 | S16 | S17 | S18 | S19 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S7 | 0.13 | |||||||||||||||
| S8 | 0.20 | 0.60 | ||||||||||||||
| S11 | 0.28 | 0.16 | 0.21 | |||||||||||||
| S12 | 0.11 | 0.002 | 0.004 | 0.17 | ||||||||||||
| S9 | 0.13 | 0.87 | 0.61 | 0.13 | 0.003 | |||||||||||
| S13 | 0.29 | 0.05 | 0.08 | 0.51 | 0.21 | 0.02 | ||||||||||
| S1 | 0.42 | 0.14 | 0.17 | 0.44 | 0.05 | 0.11 | 0.71 | |||||||||
| S5 | 0.02 | 0.34 | 0.24 | 0.10 | 0.001 | 0.49 | 0.01 | 0.07 | ||||||||
| S14 | 0.25 | 0.47 | 0.28 | 0.42 | 0.01 | 0.36 | 0.63 | 0.64 | 0.04 | |||||||
| S15 | 0.30 | 0.01 | 0.004 | 0.04 | 0.58 | 0.01 | 0.10 | 0.13 | 0.002 | 0.01 | ||||||
| S2 | 0.36 | 0.17 | 0.23 | 0.04 | 0.22 | 0.19 | 0.05 | 0.51 | 0.09 | 0.07 | 0.24 | |||||
| S16 | 0.39 | 0.02 | 0.01 | 0.08 | 0.32 | 0.01 | 0.32 | 0.03 | 0.02 | 0.005 | 0.20 | 0.13 | ||||
| S17 | 0.14 | 0.05 | 0.08 | 0.40 | 0.01 | 0.03 | 0.63 | 0.55 | 0.003 | 0.69 | 0 | 0.001 | <0.001 | |||
| S18 | 0.61 | 0.07 | 0.09 | 0.41 | 0.18 | 0.05 | 0.26 | 0.21 | 0.03 | 0.09 | 0.13 | 0.22 | 0.59 | 0.07 | ||
| S19 | 0.29 | 0.01 | 0.04 | 0.09 | 0.35 | 0.002 | 0.05 | 0.002 | <0.001 | 0.01 | 0.32 | 0.28 | 0.16 | <0.001 | 0.18 | |
| S20 | 0.21 | 0.11 | 0.01 | 0.39 | 0.04 | 0.08 | 0.03 | 0.20 | 0.08 | 0.07 | 0.08 | 0.11 | 0.05 | <0.001 | 0.21 | 0.17 |
| Season | VR | W | Inspection Result | |||
|---|---|---|---|---|---|---|
| Spring | 2.08 | 5.36 | 2.58 | 128.79 | (34.76, 67.5) | Significant positive association |
| Autumn | 3.84 | 4.08 | 1.06 | 53.11 | (34.76, 67.5) | Non-significant positive association |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, Z.; Zhou, Y.; Liu, N.; Wang, L.; Liu, Y.; Shan, B.; Liu, M.; Chen, C.; Yang, C.; Sun, D. Ecological Niche and Interspecific Association of the Main Fishes in the Coastal Waters of Hainan Island, China. Fishes 2023, 8, 511. https://doi.org/10.3390/fishes8100511
Luo Z, Zhou Y, Liu N, Wang L, Liu Y, Shan B, Liu M, Chen C, Yang C, Sun D. Ecological Niche and Interspecific Association of the Main Fishes in the Coastal Waters of Hainan Island, China. Fishes. 2023; 8(10):511. https://doi.org/10.3390/fishes8100511
Chicago/Turabian StyleLuo, Zhengli, Yanbo Zhou, Ning Liu, Liangming Wang, Yan Liu, Binbin Shan, Manting Liu, Cheng Chen, Changping Yang, and Dianrong Sun. 2023. "Ecological Niche and Interspecific Association of the Main Fishes in the Coastal Waters of Hainan Island, China" Fishes 8, no. 10: 511. https://doi.org/10.3390/fishes8100511
APA StyleLuo, Z., Zhou, Y., Liu, N., Wang, L., Liu, Y., Shan, B., Liu, M., Chen, C., Yang, C., & Sun, D. (2023). Ecological Niche and Interspecific Association of the Main Fishes in the Coastal Waters of Hainan Island, China. Fishes, 8(10), 511. https://doi.org/10.3390/fishes8100511







