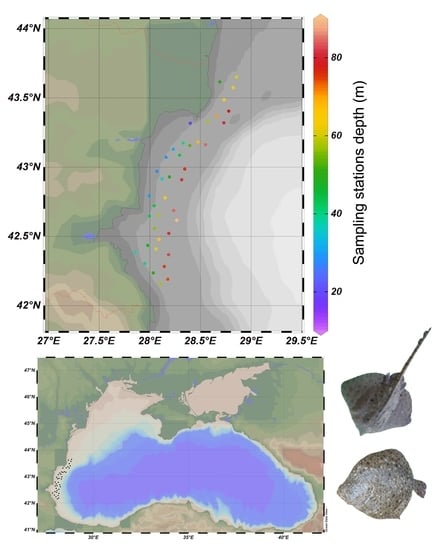
Biological Parameters and Biomass and Abundance Indices of Two Demersal Species, Turbot (Scophthalmus maximus) and Thornback Ray (Raja clavata), Estimated by a Trawl Survey in Western Black Sea
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Collection
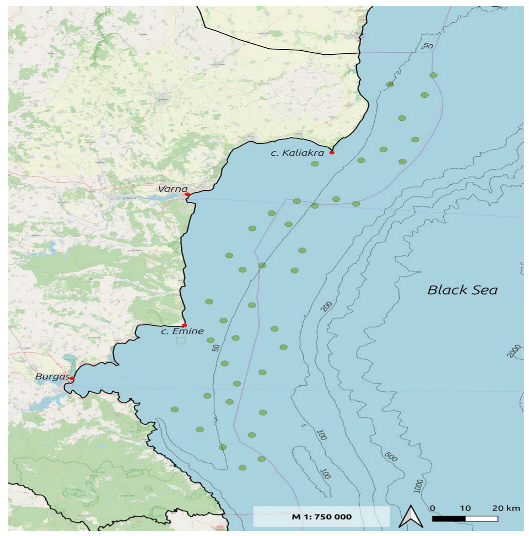
2.2. Scientific Survey
2.3. Data Analyses
3. Results
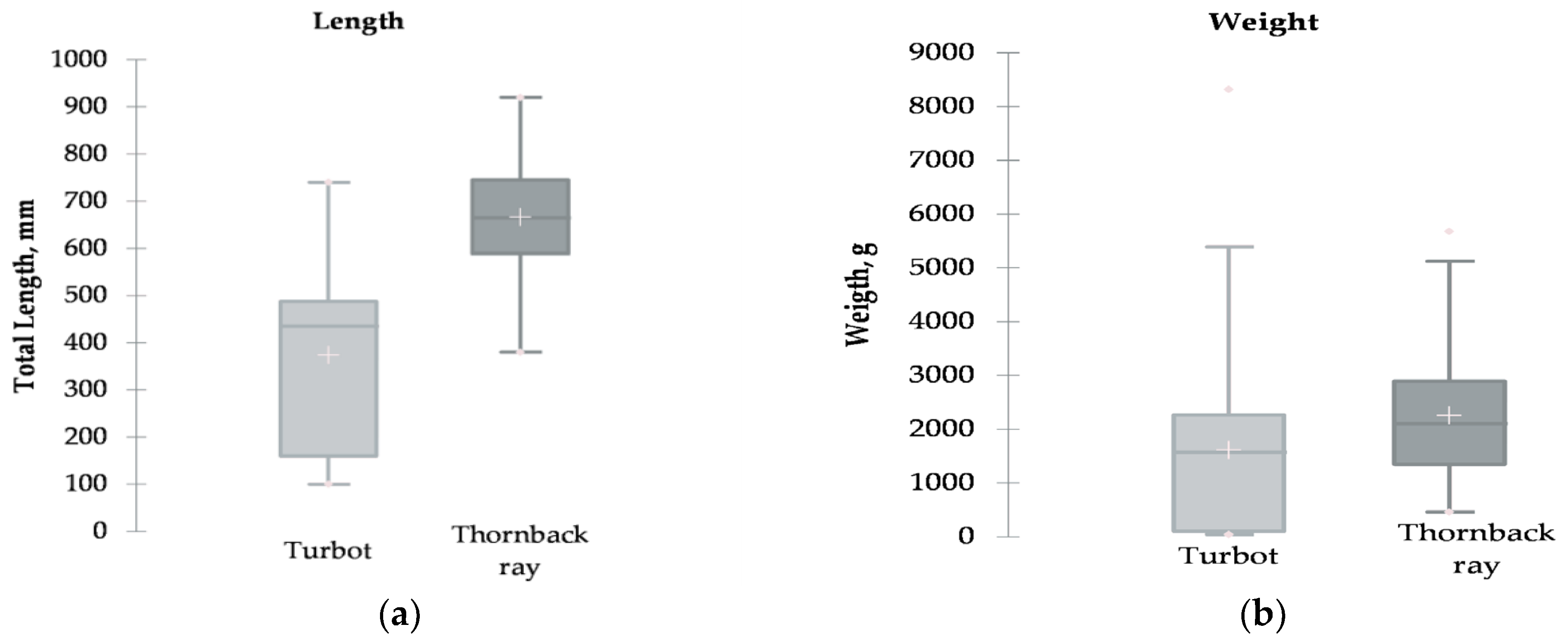
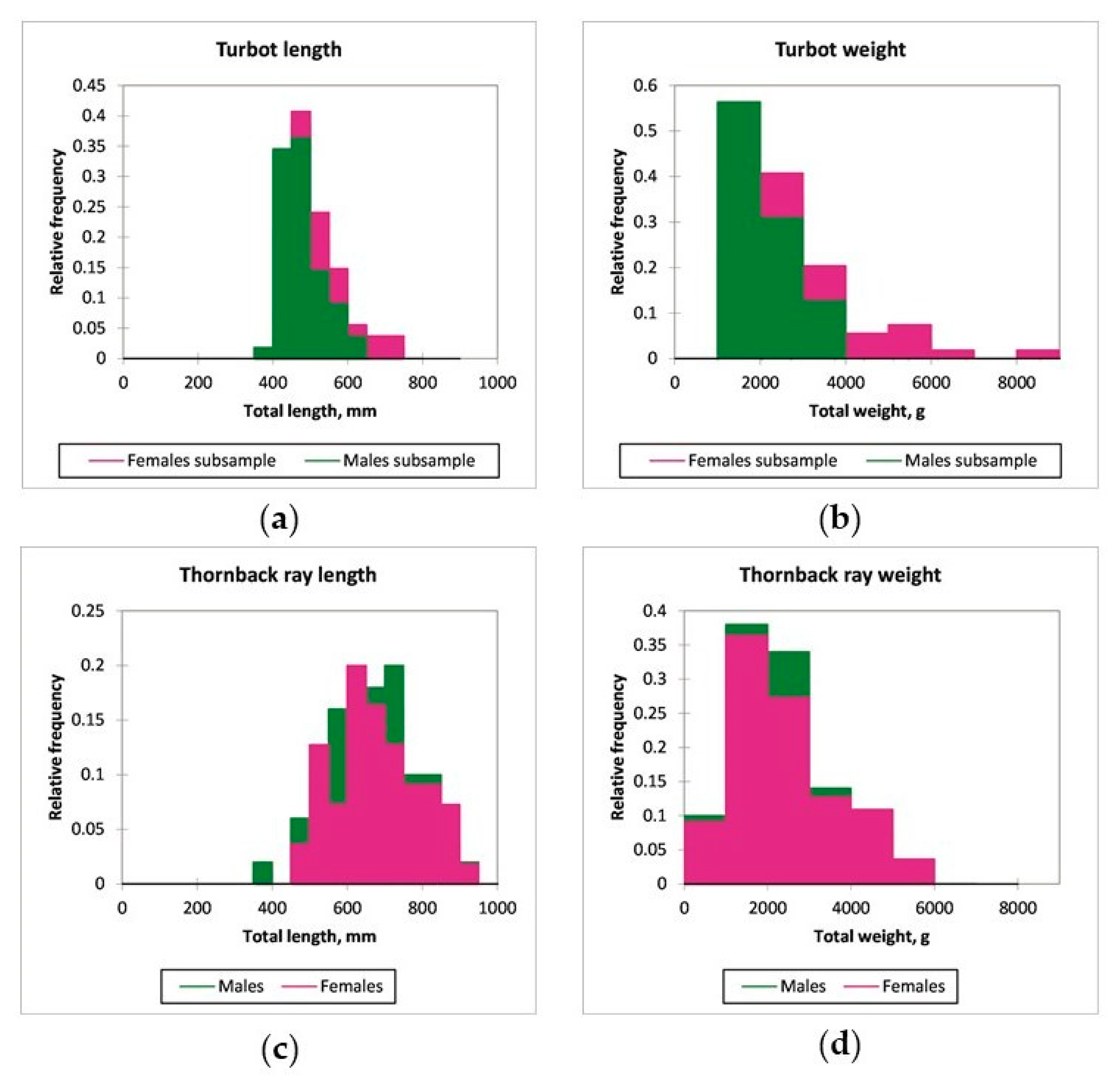
3.1. Mean Length and Weight of Turbot and Thornback Ray
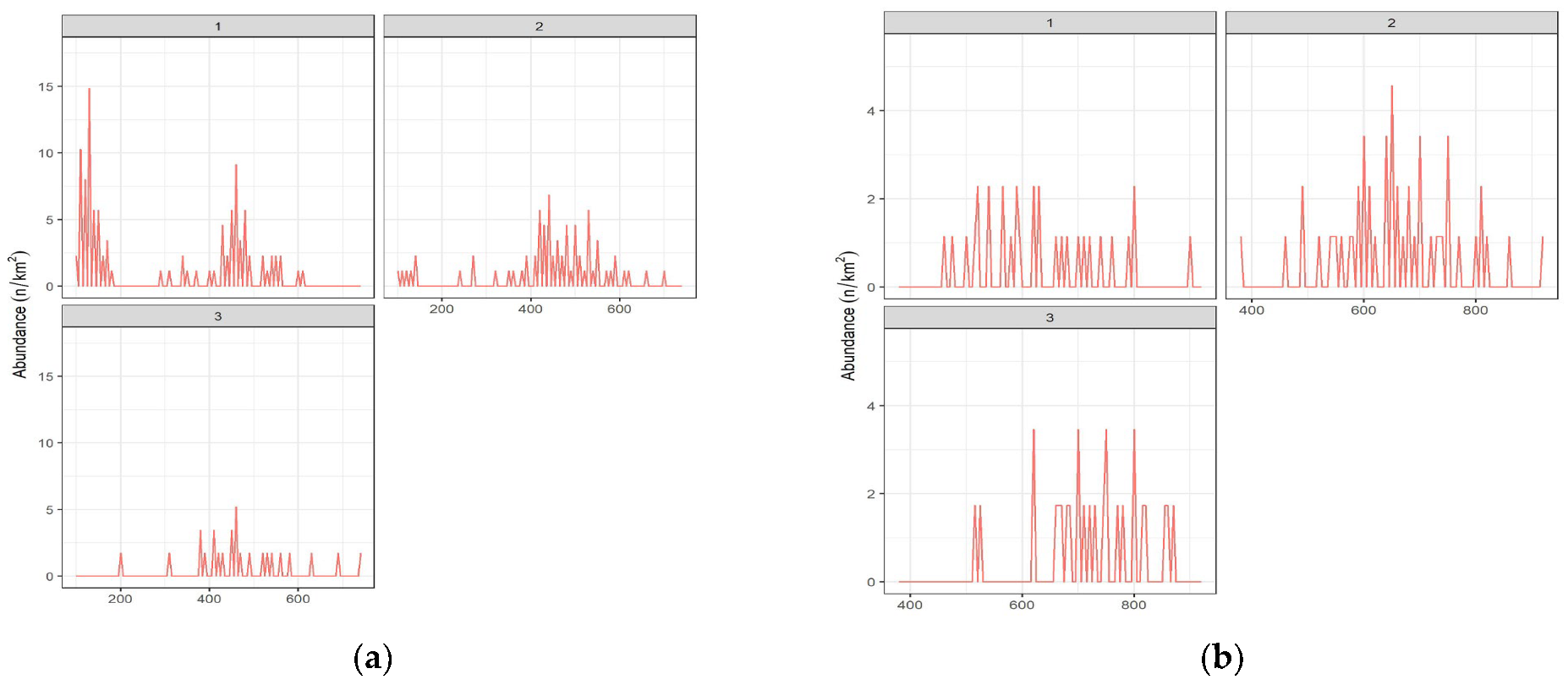
3.2. Biological Traits and Length Frequency Distribution by Depth Strata
| (a) | |||
| Biological Parameters of Turbot | Depth Stratum 1 (15–50 m) | Depth Stratum 2 (50–75 m) | Depth Stratum 3 (75–100 m) |
| Mean length (mm) | 298.84 | 440 | 473.33 |
| Mean weight (g) | 1078 | 2068 | 2440 |
| Mean abundance (ind·km−2) | 108.53 | 77.61 | 41.55 |
| Mean biomass (kg·km−2) | 116.99 | 160.45 | 101.37 |
| (b) | |||
| Biological Parameters of Thornback Ray | Depth Stratum 1 (15–50 m) | Depth Stratum 2 (50–75 m) | Depth Stratum 3 (75–100 m) |
| Mean length (mm) | 632.83 | 655.81 | 723.65 |
| Mean weight (g) | 1930 | 2128 | 2801 |
| Mean abundance (ind·km−2) | 34.27 | 55.92 | 45.01 |
| Mean biomass (kg·km−2) | 66.14 | 118.99 | 126.34 |
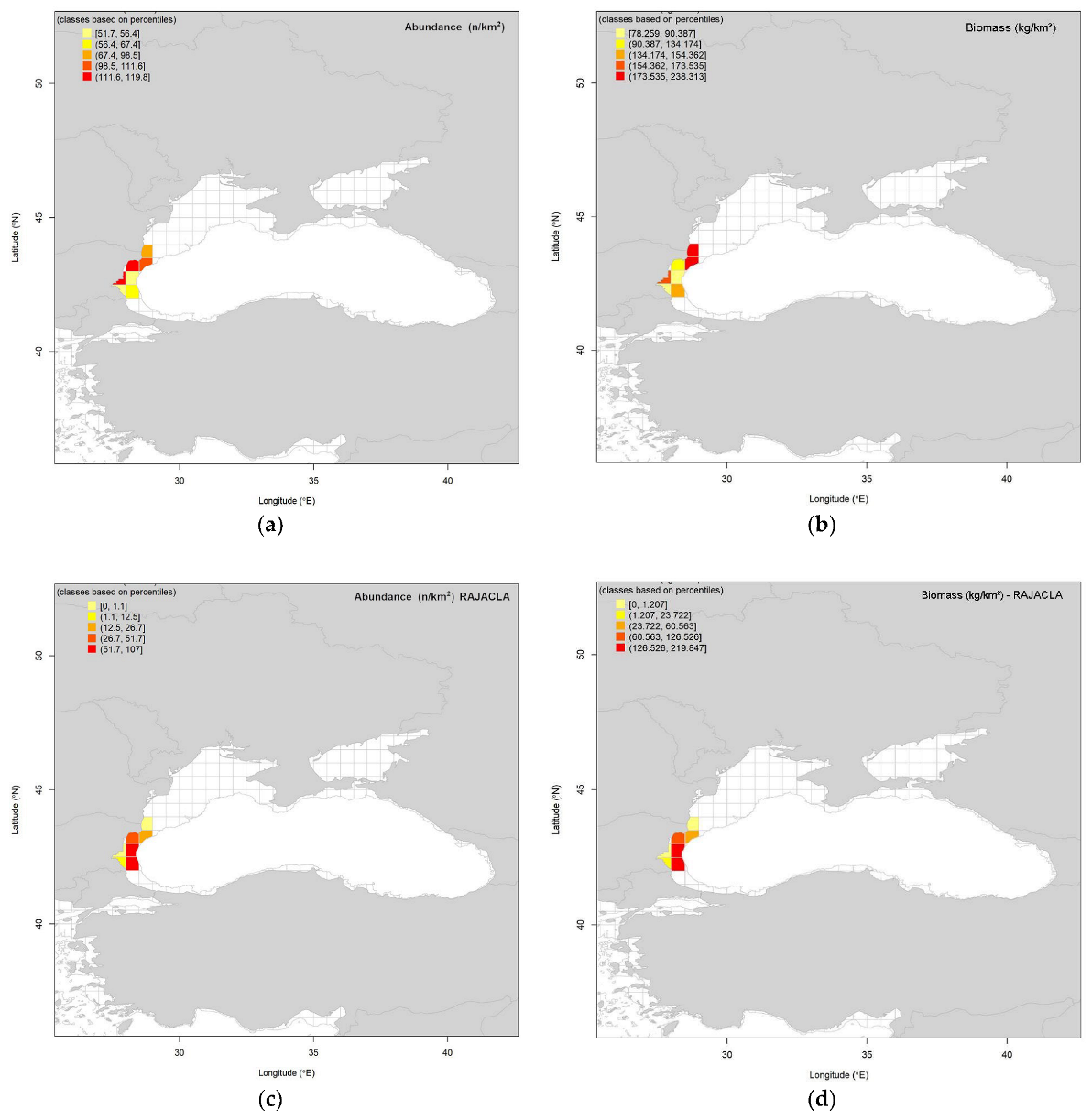
3.3. Spatial Distribution of Biomass and Abundance Indices
3.4. Length–Weight Relationships (LWRs)
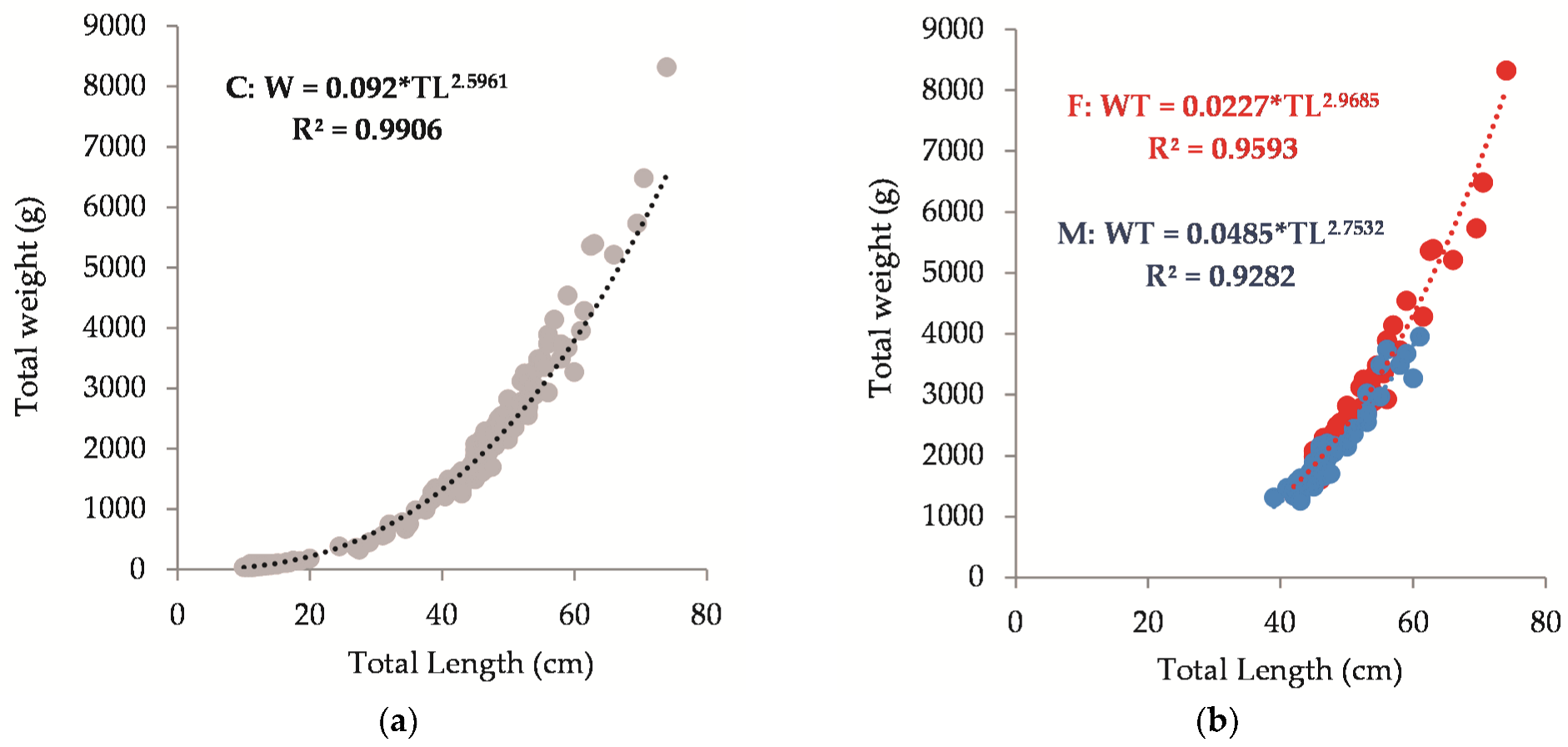
| a | b | Sex | L (cm) | R2 | n | Locality | Ref. | |
|---|---|---|---|---|---|---|---|---|
| L Min | L Max | |||||||
| 0.0044 | 3.386 | C | 50 | 75 | - | 4 | Buchan, 1981–85 | Coull et al. [42] |
| 0.0105 | 3.168 | C | 2 | 80 | 0.998 | 394 | Bay of Biscay | Dorel [43] |
| 0.0105 | 3.173 | C | 5 | 59 | 0.994 | 124 | East and West Channel | |
| 0.0168 | 2.93 | F | 3 | 48 | 0.98 | 283 | Gulf of Lion | Vianet [44] |
| 0.0218 | 2.92 | M | 3 | 45 | 0.98 | 290 | Gulf of Lion | |
| 0.02102 | 2.93 | F | 3 | 25 | 0.992 | 158 | Sète, Grau-du-Roi and SaintesMarie-de-la-Mer | Robert and Vianet [45] |
| 0.0219 | 2.92 | M | 3 | 47.5 | 0.98 | 171 | Sète, Grau-du-Roi and SaintesMarie-de-la-Mer | |
| 0.011 | 3.104 | C | 25 | 79 | 0.99 | 155 | Adriatic Sea | Arneri [46] |
| 0.01508 | 3.09 | C | - | - | - | 242 | ICES | Bedfofrd [47] |
| 0.129 | 2.6564 | C | 17.5 | 68.8 | 0.952 | - | Black Sea | Anonymous [48] |
| 0.007 | 3.248 | C | 10 | 61 | 0.977 | 63 | Eastern Black Sea | Ak et al. [49] |
| 0.001 | 3.278 | C | 44.7 | 71.7 | 0.84 | 50 | BG Black Sea | Yankova [15] |
| 0.00802 | 3.26 | C | 20 | 60 | 0.982 | 101 | North Sea | Frose [50] |
| 0.0179 | 3.02 | C | 9 | 56 | 0.973 | 2953 | Baltic Sea | |
| 0.013 | 3.11 | F | - | - | - | - | Southern North Sea | Van der Hammen [41] |
| 0.022 | 2.95 | M | - | - | - | - | Southern North Sea | |
| 0.0112 | 3.1702 | F | 22.5 | 63.5 | 0.966 | 184 | North Sea | Wilhelms [51] |
| 0.0138 | 3.0988 | M | 20.5 | 56.5 | 0.952 | 221 | North Sea | |
| 0.0128 | 3.1267 | C | 4.5 | 72.5 | 0.983 | 944 | North Sea | |
| 0.0085 | 3.158 | C | 14 | 70 | 0.989 | - | Southwestern Black Sea coast | Eryilmaz and Dalyan [2] |
| 0.01 | 3.15 | C | 23.5 | 46 | 0.914 | 22 | French Catalan coast | Crec’hriou [52] |
| 0.066 | 2.688 | C | 17 | 63 | - | 74 | Northeastern Atlantic Ocean | Mahé [53] |
| 0.0147 | 3.11 | C | 28.5 | 47.9 | 0.99 | 20 | Bay of Kiel | Frose [54] |
| 0.0179 | 3.02 | C | 9 | 56 | 0.973 | 2953 | Baltic Sea | |
| 0.00802 | 3.26 | C | 20 | 60 | 0.982 | 101 | North Sea | |
| 0.000431 | 2.21 | C | - | - | BG Black Sea | STECF 13–20 [55] | ||
| 0.000011 | 3.13 | C | - | - | BG Black Sea | |||
| 0.000013 | 3.11 | F | - | - | BG Black Sea | |||
| 0.000041 | 2.78 | M | - | - | BG Black Sea | |||
| 0.000424 | 2.22 | F | - | - | BG Black Sea | |||
| 0.000022 | 2.92 | M | - | - | BG Black Sea | |||
| 0.000021 | 2.94 | F | - | - | BG Black Sea | |||
| 0.000008 | 3.17 | C | - | - | BG Black Sea | |||
| 0.00004 | 2.799 | M | - | - | BG Black Sea | |||
| 0.0000007 | 3.795 | F | - | - | BG Black Sea | |||
| 0.0000339 | 2.837 | C | - | - | BG Black Sea | |||
| 0.092 | 2.596 | C | 10 | 74 | 0.96 | 187 | BG Black Sea | Present study |
| 0.0227 | 2.96 | F | 42 | 74 | 0.95 | 54 | BG Black Sea | |
| 0.0485 | 2.75 | M | 39 | 61 | 0.93 | 55 | BG Black Sea | |
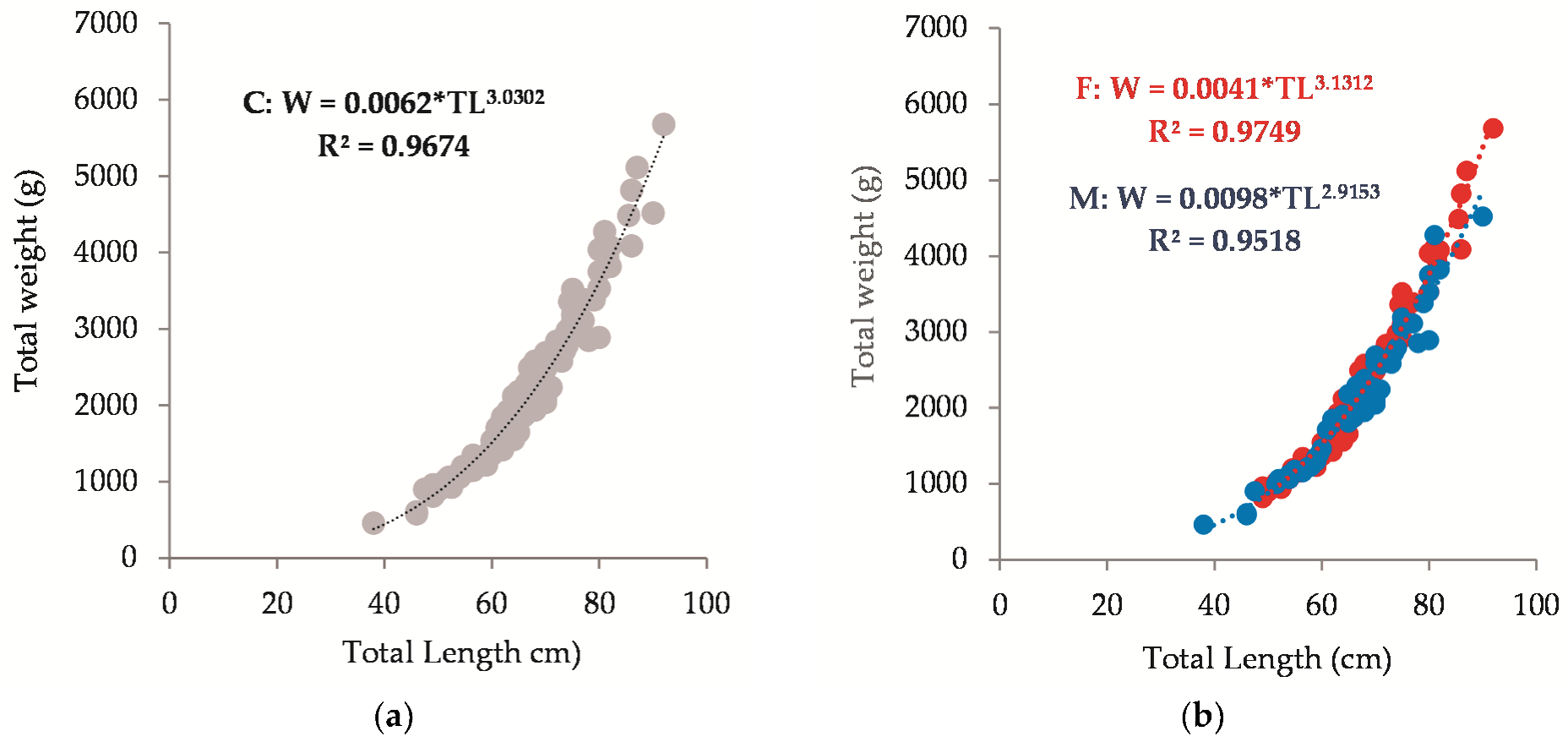
| a | b | Sex | L (cm) | R2 | n | Locality | Ref. | |
|---|---|---|---|---|---|---|---|---|
| L min | L max | |||||||
| 0.0032 | 3.19 | C | 10 | 101 | 0.998 | 960 | East and West Channel | Dorel [43] |
| 0.0032 | 3.2 | C | 11 | 98 | 0.998 | 123 | Bay of Biscay | |
| 0.0022 | 3.27 | C | 74 | (ICES division), 1986 | Bedford et al. [47] | |||
| 0.0016 | 3.29 | C | 20.5 | 99 | 0.872 | 29 | North Aegean Sea, 1999–2000 | Filiz and Mater [56] |
| 0.0016 | 3.3 | C | 20.5 | 99 | 0.94 | 37 | North Aegean Sea, 2003 (March) | |
| 0.0018 | 3.25 | F | 20.5 | 99 | 0.861 | 21 | North Aegean Sea, 1999–2000 | |
| 0.0006 | 3.56 | M | 29.7 | 67 | 0.968 | 8 | North Aegean Sea, 1999–2000 | |
| 0.0025 | 3.23 | C | 30.6 | 86.2 | 0.927 | 63 | Nazaré to St André, 1997 | Mendes et al. [40] |
| 0.0011 | 3.41 | C | Northern and central Adriatic Sea, 1996–2006 | Krstulović Sifner et al. [57] | ||||
| 0.0021 | 3.27 | C | 15.3 | 95.2 | 0.99 | 86 | Gulf of Cadiz, 2009–2011 | Torres et al. [39] |
| 0.001 | 2.3 | C | 56 | 79 | 0.86 | 24 | Black Sea, 2006–2008 | Yankova et al. [15] |
| 0.023 | 2.64 | C | 25 | 70 | 0.76 | 75 | Iskenderun Bay/2010–2011 | Başusta et al. [58] |
| 0.001 | 3.35 | C | 8.5 | 77 | 1 | 8 | Northwestern Mediterranean/2011–2013 | Barría et al. [59] |
| 0.0081 | 2.98 | C | 9.5 | 95.5 | 0.984 | 73 | North Sea/1959–2004 | Wilhelms [51] |
| 0.002 | 3.25 | C | 39 | 59 | 0.957 | 25 | French Catalan coast/2007–2010 | Crec’hriou et al. [52] |
| 0.001 | 3.29 | C | 13.2 | 90 | 0.971 | 63 | Black Sea/2009–2011 | Kasapoglu et al. [60] |
| 0.003 | 3.17 | C | 3 | 112 | 608 | Northeastern Atlantic Ocean/2013–2015 | Mahé et al. [53] | |
| 0.003 | 3.2 | C | 25 | 70 | 0.977 | 19 | Lebanese waters/2012–2014 | Lteif et al. [61] |
| 0.0187 | 3.01 | J | 22 | 31 | 12 | Shetland, Moray Firth, Buchan, S. Minch | Coull et al. [42] | |
| 0.0024 | 3.2 | J | 14.5 | 38.1 | 0.996 | 18 | Balearic Islands, 1995–1996 | Merella et al. [62] |
| 0.0014 | 3.36 | J | 13.7 | 54 | 0.98 | 13 | Algarve, 1996–1997 | Borges et al. [63] |
| 0.0074 | 2.87 | J | 12.2 | 70 | 0.893 | 24 | Northern Sea of Marmara, 2006–2007 (November–March) | Bok et al. [64] |
| 0.003 | 3.17 | F | 18.4 | 91.6 | 0.99 | Caernarfon Bay, north Wales, 2003 (February–September) | Whittamore et al. [65] | |
| 0.004 | 3.13 | M | 26.9 | 77.8 | 0.99 | Caernarfon Bay, north Wales, 2003 (February–September) | ||
| 0.0084 | 3.3 | F | 0.984 | 1124 | Carmarthen Bay, British Isles, 1974–1975 | Ryland et al. [38] | ||
| 0.0019 | 3.17 | M | 0.984 | 1019 | Carmarthen Bay, British Isles, 1974–1975 | |||
| 0.0019 | 3.28 | M | 11 | 85.1 | 0.981 | 256 | Eastern Adriatic Sea/1997–2001 | Pallaoro et al. [66] |
| 0.0012 | 3.39 | F | 11.4 | 105 | 0.978 | 278 | Eastern Adriatic Sea/1997–2001 | |
| 0.0015 | 3.34 | C | 11 | 105 | 0.978 | 534 | Eastern Adriatic Sea/1997–2001 | |
| 0.005 | 3 | M | 48 | 95 | 0.96 | Southeastern Black Sea, 2002–2003 | Demirhan et al. [67] | |
| 0.0003 | 3.7 | F | 34.3 | 88.2 | 0.94 | Southeastern Black Sea, 2002–2003 | ||
| 0.001 | 3.42 | C | 34.3 | 95 | 0.91 | 52 | Southeastern Black Sea | |
| 0.0007 | 3.53 | C | 11.3 | 69.5 | 0.98 | 130 | Sea of Marmara coasts/2011–2014 | Barrientos-Medina et al. [68] |
| 0.0005 | 3.6 | M | 12.8 | 69.5 | 0.98 | 66 | Sea of Marmara coasts/2011–2014 | |
| 0.0008 | 3.5 | F | 11.3 | 67.5 | 0.99 | 64 | Sea of Marmara coasts/2011–2014 | |
| 0.0016 | 3.33 | M | 11 | 81 | Mediterranean: south of Sicily and south of Malta/1990–2018 | Geraci et al. [69] | ||
| 0.0015 | 3.35 | C | 9.5 | 110 | Mediterranean: south of Sicily and south of Malta/1990–2018 | |||
| 0.0014 | 3.36 | F | 9.5 | 110 | Mediterranean: south of Sicily and south of Malta/1990–2018 | |||
| 0.0062 | 3.03 | C | 38 | 92 | 0.97 | 105 | BG Black Sea | Present study |
| 0.0041 | 3.13 | F | 49 | 92 | 0.97 | 55 | BG Black Sea | |
| 0.098 | 2.92 | M | 38 | 90 | 0.97 | 50 | BG Black Sea | |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aydın, I.; Şahin, T. Reproductive performance of turbot (Psetta maxima) in the southeastern Black Sea. Turk. J. Zool. 2011, 35, 109–113. [Google Scholar] [CrossRef]
- Eryilmaz, L.; Dalyan, C. Age, growth, and reproductive biology of turbot, Scophthalmus maximus (Actinopterygii: Pleuronectiformes: Scophthalmidae), from the South-Western coasts of Black Sea, Turkey. Acta Ichthyol. Et Piscat. 2015, 45, 181–188. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. FishBase. World Wide Web Electronic Publication 2023. Version (02/2023). Available online: www.fishbase.org (accessed on 21 July 2023).
- Stoyanov, S.; Georgiev, Z.; Ivanov, L.; Nikolov, P.; Kolarov, P.; Aleksandrova, K.; Karapetkova, M. Fish in the Black Sea; State Publishing House: Varna, Bulgaria, 1963; p. 101. [Google Scholar]
- Karapetkova, M.; Zhivkov, M. The Fishes of Bulgaria; Gea-Libris: Sofia, Bulgaria, 2010; p. 216. (In Bulgarian) [Google Scholar]
- Nielsen, J.G. Scophthalmidae. In Fishes of the Northeastern Atlantic and the Mediterranean; Whitehead, P.J.P., Bauchot, M.-L., Hureau, J.-C., Nielsen, J., Tortonese, E., Eds.; UNESCO: Paris, France, 1986; Volume 3, pp. 1287–1293. [Google Scholar]
- Petrova, E.; Stoykov, S.; Mihneva, V.; Valchev, S.; Penchev, P.; Tserkova, F. Bottom Trawl Surveys in the Bulgarian Black Sea Area, Autumn 2019. Report under Contract with the Executive Agency Fisheries and Aquacultures, Program for Data Collection for Fishing in 2020. p. 52. Available online: http://dcf-bulgaria.bg/2019-2/ (accessed on 21 July 2023).
- Petrova, E.; Stoykov, S.; Mihneva, V.; Valchev, S.; Penchev, P.; Tserkova, F. Bottom Trawl Surveys in the Bulgarian Black Sea Area, Summer 2020. Report under Contract with the Executive Agency Fisheries and Aquacultures, Program for Data collection for fisheries in 2020. p. 53. Available online: http://dcf-bulgaria.bg/2020-2/ (accessed on 21 July 2023).
- Bradai, N.; Saidi, B.; Enajjar, S. Elasmobranchs of the Mediterranean and Black Sea: Status, ecology, and biology. Bibliographic analysis. In Studies and Reviews. General Fisheries Commission for the Mediterranean Region No. 91; FAO: Rome, Italy, 2012; p. 103. [Google Scholar]
- Bilecenoğlu, M.; Taskavak, E.; Mater, S.; Kaya, M. Checklist of Marine Fish in Turkey; Zootaxa 113; Magnolia Publishers: Auckland, New Zealand, 2002; p. 194. [Google Scholar]
- FAO. Species and Photographic Plates. In Mediterranean Skates, Rays and Chimaeras; Barone, M., Serena, F., Dimech, M., Eds.; FAO: Rome, Italy, 2018; p. 18. [Google Scholar]
- Hassan, M. Occurrence of large-eyed rabbit fish Hydrolagus mirabilis, Chimaeridae, in Syrian waters (Eastern Mediterranean). Mar. Biodivers. Rec. 2013, 6, 7. [Google Scholar] [CrossRef]
- Mahmoud, M.S.F. Deep-sea ichthyofauna from Eastern Mediterranean Sea, Egypt: Update and new records. Egypt. J. Aquat. Res. 2016, 42, 479–489. [Google Scholar]
- Serena, F. Field identification guide to the sharks and rays of the Mediterranean and Black Sea. In FAO Species Identification Guide for Fishery Purposes; 11 colour plates + egg cases; FAO: Rome, Italy, 2005; p. 97. [Google Scholar]
- Yankova, M.; Pavlov, D.; Ivanova, P.; Karpova, E.; Boltachev, A.; Öztürk, B.; Bat, L.; Oral, M.; Mgeladze, M. Marine fishes in the Black Sea: Recent conservation status. Mediterr. Mar. Sci. 2014, 15, 366–379. [Google Scholar] [CrossRef]
- Aydın, I.; Ak, O.; Polat, H.; Kucuk, E.; Hasimoglu, A.; Firidin, S.; Eroglu, O.; Kutlu, S.; Ozdemir, G.; Ture, M.; et al. Pisi Balığı (Platichthys Flesus Luscus Pallas, 1811)’nın Kültüre Alınabilirliğinin Araştırılması; TAGEM Trabzon Su Ürünleri Merkez Araştırma Enstitüsü: Trabzon, Turkiye, 2009.
- Stehmann, M.; Bürkel, D.L. Rajidae. In Fishes of the Northeastern Atlantic and the Mediterranean; Whitehead, P.J.P., Bauchot, M.L., Hureau, J.C., Nielsen, J., Tortonese, E., Eds.; United Nations Educational, Scientific, and Cultural Organization (UNESCO): Paris, France, 1984; pp. 163–196. [Google Scholar]
- Jardas, I. A contribution to our knowledge of the biology and ecology of thornback ray (Raja clavata L.) and brown ray (Raja miraletus L.) in the Adriatic. Acta Adriat. 1973, 15, 1–42. [Google Scholar]
- Walker, P.A.; Hislop, J.R.G. Sensitive skates or resilient rays? Spatial and temporal shifts in ray species composition in the central and northwestern North Sea between 1930 and the present day. ICES J. Mar. Sci. 1998, 55, 392–402. [Google Scholar] [CrossRef]
- Bingel, F.; Kideys, A.E.; Ozsoy, E.; Tugrul, S.; Basturk, O.; Oguz, T. Stock Assessment Studies for the Turkish Black Sea Coast; Nato-SSP Fisheries Final Report; METU, Institute of Marine Sciences: Erdemli, Türkiye, 1993; p. 159. [Google Scholar]
- Capture Production Statistics by Country or Areas, Species Item, and GFCM Statistical Division. 1970–2020. Available online: https://www.fao.org/gfcm/data/capture-production/en/ (accessed on 21 July 2023).
- Ellis, J.R.; Dulvy, N.K.; Serena, F. Raja clavata. The IUCN Red List of Threatened Species 2016: E.T39399A103113598. Available online: https://www.iucnredlist.org/species/39399/103113598 (accessed on 12 January 2022).
- Cardinale, M.; Chanet, B.; Martínez, P.P.; Munroe, T.A.; Nimmegeers, S.; Shlyakhov, V.; Turan, C.; Vansteenbrugge, L. Scophthalmus maximus. The IUCN Red List of Threatened Species 2021: E.T198731A144939322. Available online: https://www.iucnredlist.org/species/198731/144939322 (accessed on 14 July 2023).
- Colton, M.A.; Swearer, S.E. A comparison of two survey methods: Differences between underwater visual census and baited remote underwater video. Mar. Ecol. Prog. Ser. 2010, 400, 19–36. [Google Scholar] [CrossRef]
- Sparre, P.; Venema, S.C. Introduction to Tropical Fish Stock Assessment. Part I: Manual; FAO Fisheries Technical Paper, 306/1, rev.2, DANIDA; FAO: Rome, Italy, 1998; p. 407. ISBN 92-5-103996-8. [Google Scholar]
- Zwolinski, J.P.; Renfree, J.S.; Stierhoff, K.L.; Demer, D.A. Distribution, biomass, and demographics of coastal pelagic fishes in the California Current Ecosystem during spring 2021 based on acoustic-trawl sampling. In Series: NOAA Technical Memorandum NMFS-SWFSC; NOAA Technical Memorandum NMFS-SWFSC; Southwest Fisheries Science Center (U.S.): La Jolla, CA, USA, 2023; p. 675. [Google Scholar] [CrossRef]
- Gyanaranjan, D.; Swatipriyanka, S.; Rajesh, K.P.; Shubhadeep, G.; Jose, J.; Jayaraman, J. Modeling framework for establishing the power law between length and weight of fishes and a meta-analysis for validation of LWRs for six commercially important marine fishes from the northwestern Bay of Bengal. Fish. Res. 2023, 257, 106496. [Google Scholar] [CrossRef]
- Berkeley, S.A.; Hixon, M.A.; Larson, R.J.; Love, M.S. Fisheries sustainability via protection of age structure and spatial distribution of fish populations. Fisheries 2004, 29, 23–32. [Google Scholar] [CrossRef]
- Ottersen, G. Pronounced long-term juvenation in the spawning stock of Arc-to-Norwegian cod (Gadus morhua) and possible consequences for recruitment. Can. J. Fish. Aquat. Sci. 2008, 65, 523–534. [Google Scholar] [CrossRef]
- Paul, H.; John, R. (Eds.) Handbook of Fish Biology and Fisheries, 1st ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2002; p. 424. [Google Scholar]
- Prodanov, K.; Mikhailov, K.; Daskalov, G.; Maxim, C.; Chashchin, A.; Arkhipov, A.; Shlyakhov, V.; Ozdamar, E. Environmental management of fish resources in the Black Sea and their rational exploitation 1997. In Studies and Reviews. General Fisheries Council for the Mediterranean Region No. 68; FAO: Rome, Italy, 1997; p. 178. [Google Scholar]
- Raykov, V.; Panayotova, M.; Mikhailov, K.; Konsulova, T.; Radu, G.; Nicolaev, S.; Anton, E.; Maximov, V.; Tiganov, G.; Cristea, M.; et al. Best Practice Guideline on Scientific Surveys and Holistic Methods in the Black Sea; SRCSSMBS–Black Sea: 1 April 2013. Available online: https://www.ifrvarna.com/index.php/bg/2021-02-07-15-28-51/2021-10-03-09-01-49 (accessed on 21 July 2023).
- General Fisheries Commission for the Mediterranean: GFCM 2020. Available online: https://www.fao.org/gfcm/data/safs/en/ (accessed on 21 July 2023).
- Petrova, E.; Stoykov, S.; Mihneva, V.; Valchev, S.; Penchev, P.; Tserkova, F. Bottom Trawl Surveys in the Bulgarian Black Sea Area, Autumn-Winter 2020. Report under Contract with the Executive Agency Fisheries and Aquacultures, Program for Data Collection for Fisheries in 2020. p. 46. Available online: http://dcf-bulgaria.bg/2021-2/ (accessed on 21 July 2023).
- Tserkova, F.; Petrova, D.; Pavlova, E.; Stoykov, S.; Mihneva, V.; Hubenova, T.; Zaikov, A.; Hadjinikolova, L.; Terziyski, D.; Ivanova, A.; et al. Stock Assessment of Turbot (Psetta maxima) by Swept Area Method in Front of Bulgarian Black Sea Coast During Autumn—Winter 2014. Project Report for the National Agency of Fisheries and Aquaculture of Bulgaria to the National Data Collection Program for 2014. p. 56. Available online: http://dcf-bulgaria.bg/2014-2/ (accessed on 21 July 2023).
- Zupa, W.; Casciaro, L.; Bitetto, I.; Spedicato, M.T. BioIndex: R Code to Analyze Trawl Survey Data Using MEDITS File Format; Updated on March 2022. Available online: https://zenodo.org/record/6389406 (accessed on 21 July 2023).
- Karapetkova, M. State of the Fish Stock in front of the Black Sea. Fishing; Publication of the Ministry of Food Industry: Sofia, Bulgaria, 1959.
- Ryland, J.S.; Ajayi, T.O. Growth and population dynamics of three Raja species (Batoidei) in Carmarthen Bay, British Isles. J. Const. Int. Explor. Mer. 1984, 41, 111–120. [Google Scholar] [CrossRef]
- Torres, M.A.; Ramos, F.; Sobrino, I. Length-weight relationships of 76 fish species from the Gulf of Cadiz (SW Spain). Fish. Res. 2012, 127–128, 171–175. [Google Scholar] [CrossRef]
- Mendes, B.; Fonseca, P.; Campos, A. Weight-length relationships for 46 fish species of the Portugese west coast. J. Appl. Ichthyol. 2004, 20, 355–361. [Google Scholar] [CrossRef]
- Van der Hammen, T.; Poos, J.J. Data Evaluation of Data-Limited Stocks: Dab, Flounder, Witch, Lemon Sole, Brill, Turbot, and Horse Mackerel; Report No. C110/12; Institute for Marine Resources and Ecosystem Studies (IMARES): Wageningen, The Netherlands, 2012. [Google Scholar]
- Coull, K.A.; Jermyn, A.S.; Newton, A.W.; Henderson, G.I.; Hall, W.B. Length/weight relationships for 88 species of fish encountered in the North Atlantic. Scottish Fish. Res. Rep. 1989, 43, 80. [Google Scholar]
- Dorel, D. Poissons de l’Atlantique Nord-Est Relations Taille-Poids; Institut Francais de Recherche pour l’Exploitation de la Mer: Nantes, France, 1986; p. 165. [Google Scholar]
- Vianet, R.; Quignard, J.-P.; Tomasini, J.-A. Age et croissance de quatre poissons Pleuronectiformes (flet, turbot, barbue, sole) du golfe du Lion. Cybium 1989, 13, 247–258. [Google Scholar]
- Robert, F.; Vianet, R. Age and growth of Psetta maxima (Linné, 1758) and Scopthalmus rhombus (Linné, 1758) in the Gulf of Lion (Mediterranean). J. Appl. Ichthyol 1988, 4, 111–120. [Google Scholar] [CrossRef]
- Arneri, E.; Colella, S.; Giannetti, G. Age determination and growth of turbot and brill in the Adriatic Sea: Reversal of the seasonal pattern of otolith zone formation. J. Appl. Ichthyol 2001, 17, 256–261. [Google Scholar] [CrossRef]
- Bedford, B.C.; Woolner, L.E.; Jones, B.W. Length-weight relationships for commercial fish species and conversion factors for various presentations. In Ministry of Agriculture, Fisheries and Food; Directorate of Fisheries Research; Fisheries Research Data Report: 10; MAFF Fisheries Laboratory: Lowestoft, UK, 1986; p. 41. Available online: https://www.cefas.co.uk/publications/files/datarep10.pdf (accessed on 21 July 2023).
- Anonymous. Commercial Description of the Black Sea; Head Department of Navigation and Oceanography of the Ministry of Defense of USSR: Moscow, Russia, 1988; p. 139. [Google Scholar]
- Ak, O.; Kutlu, S.; Aydin, I. Length-weight relationship for 16 fish species from the Eastern Black Sea, Turkey. Turk. J. Fish. Aquat. Sci. 2009, 9, 125–126. [Google Scholar]
- Froese, R.; Sampang, A. Potential Indicators and Reference Points for Good Environmental Status of Commercially Exploited Marine Fishes and Invertebrates in the German EEZ. 17 December 2013. Available online: http://oceanrep.geomar.de/22079/ (accessed on 21 July 2023).
- Wilhelms, I. Atlas of length-weight relationships of 93 fish and crustacean species from the North Sea and the Northeast Atlantic (No. 12). In Johann Heinrich von Thünen Institute, Federal Research Institute for Rural Areas, Forestry and Fisheries; Springer: Hamburg, Germany, 2013; p. 552. [Google Scholar]
- Crec’hriou, R.; Neveu, R.; Lenfant, P. Length-weight relationship of main commercial fishes from the French Catalan coast. J. Appl. Ichthyol. 2013, 29, 1191–1192. [Google Scholar] [CrossRef]
- Mahé, K.; Bellamy, E.; Delpech, J.-P.; Lazard, C.; Salaun, M.; Vérin, Y.; Coppin, F.; Travers-Trolet, M. Evidence of a relationship between weight and total length of marine fish in the Northeastern Atlantic Ocean: Physiological, spatial, and temporal variations. J. Mar. Biol. Assoc. 2018, 98, 617–625. [Google Scholar] [CrossRef]
- Froese, R.; Flindt, F.; Meyer, E.; Meyer, J.; Egerland, O. Untersuchung zum Laichverhalten des Dorsches in der Kieler Bucht im Frühjahr 2020. Available online: https://www.fishbase.de/rfroese/LaichDorsch2020.pdf (accessed on 1 October 2020).
- Scientific, Technical and Economic Committee for Fisheries (STECF): Black Sea Assessments. Available online: https://stecf.jrc.ec.europa.eu/reports/medbs?p_p_id=101_INSTANCE_y1bW&p_p_lifecycle=0&p_p_state=normal&p_p_mode=view&p_p_col_id=column-2&p_p_col_pos=1&p_p_col_count=2&_101_INSTANCE_y1bW_delta=20&_101_INSTANCE_y1bW_keywords=&_101_INSTANCE_y1bW_advancedSearch=false&_101_INSTANCE_y1bW_andOperator=true&p_r_p_564233524_resetCur=false&_101_INSTANCE_y1bW_cur=3 (accessed on 21 July 2023).
- Filiz, H.; Mater, S. A preliminary study on length-weight relationships for seven elesmobranch species from North Aegean Sea, Turkey. Ege J. Fish. Aquat. Sci. 2002, 19, 401–409. [Google Scholar]
- Krstulović Sifner, S.; Vrgoć, N.; Isajlović, V.; Dadić, I.; Peharda, M.; Piccinetti, C. Long-term changes in distribution and demographic composition of thornback ray, Raja clavata, in the northern and central Adriatic Sea. J. Appl. Ichthyol. 2009, 25 (Suppl. S1), 40–46. [Google Scholar] [CrossRef]
- Başusta, A.; Başusta, N.; Sulikowski, J.A.; Driggers, W.B., III; Demirhan, S.A.; Çiçek, E. Length–weight relationships for nine species of batoids from the Iskenderun Bay, Turkey. J. Appl. Ichthyol. 2012, 28, 850–851. [Google Scholar] [CrossRef]
- Barría, C.; Navarro, J.; Coll, M.; Fernancez-Arcaya, U.; Sáez-Liante, R. Morphological parameters of abundant and threatened chondrichthyans in the northwestern Mediterranean Sea. J. Appl. Ichthyol. 2015, 31, 114–119. [Google Scholar] [CrossRef]
- Kasapoglu, N.; Duzgunes, E. Length-weight relationships of marine species caught by five gears from the Black Sea. Mediterr. Mar. Sci. 2013, 15, 95–100. [Google Scholar] [CrossRef][Green Version]
- Lteif, M.; Mouawad, R.; Jemaa, S.; Khalaf, G.; Lenfant, P.; Verdoit-Jarraya, M. The length-weight relationships of three sharks and five batoids in the Lebanese marine waters, eastern Mediterranean. Egypt. J. Aquat. Res. 2016, 42, 475–477. [Google Scholar] [CrossRef]
- Merella, P.; Quetglas, A.; Alemany, F.; Carbonell, A. Length-weight relationship of fishes and cephalopods from the Balearic Islands (western Mediterranean). Naga ICLARM Q. 1997, 20, 66–68. [Google Scholar]
- Borges, T.C.; Olim, S.; Erzini, K. Weight-length relationship for fish species discarded in commercial fisheries of the Algarve (southern Portugal). J. Appl. Ichthyol. 2003, 19, 394–396. [Google Scholar] [CrossRef]
- Bok, T.D.; Gokturk, D.; Kahraman, A.E.; Alicli, T.Z.; Acun, T.; Ates, C. Length-weight relationships of 34 fish species from the Sea of Marmara, Turkey. J. Anim. Vet. Adv. 2011, 10, 3037–3042. [Google Scholar]
- Whittamore, J.M.; McCarthy, I.D. The population biology of the thornback ray Raja clavata in Caernafon Bay, north Wales. J. Mar. Biol. Assoc. 2005, 85, 1089–1094. [Google Scholar] [CrossRef]
- Pallaoro, A.; Jardas, I.; Santic, M. Weight-length relationships for 11 chondrichthyan species in the eastern Adriatic Sea. Cybium 2005, 29, 93–96. [Google Scholar]
- Demirhan, S.A.; Engin, S.; Seyhan, K.; Akamca, E. Some biological aspectsof thorn back ray (Raja clavata L.,1758) in the southeastern Black Sea. Turk. J. Fish. Aquat. Sci. 2005, 5, 75–83. [Google Scholar]
- Barrientos-Medina, R.C.; Avilés-Torres, S.; Navarro-Alberto, J.A. Length-weight relationships of eight fish species from a small Caribbean coastal lagoon in Mexico. Acta Ichthyol. Piscat. 2013, 43, 71–74. [Google Scholar] [CrossRef]
- Geraci, M.L.; Ragonese, S.; Scannella, D.; Falsone, F.; Gancitano, V.; Mifsud, J.; Gambin, M.; Said, A.; Vitale, S. Batoid abundances, spatial distribution, and life history traits in the Strait of Sicily (Central Mediterranean Sea): Bridging a knowledge gap through three decades of survey. Animals 2021, 11, 2189. [Google Scholar] [CrossRef] [PubMed]
- Popova, V.P. Methods for evaluating the state of turbot stocks in the Black Sea. Tr. Vses. Nauchno. Issled. Inst. Morzk. Rybn. Khoz. Okeanogr. 1967, 62, 197–200. [Google Scholar]
- Panayotova, M.; Raykov, V.; Todorova, V. Turbot (Psetta maxima L.) abundance indices and stock dynamics off Bulgarian Black Sea coast during the period 2006–2009. Acta Zool. Bulg. 2012, 64, 85–91. [Google Scholar]
- Mutlu, E.; Deval, M.C.; De Meo, I.; Saygu, İ.; Miglietta, C. Spatiotemporal density and ecology of batoids (Elasmobranchii) along a Turkish shelf-upper slope of the Mediterranean Sea during years 2009–2015. Thalass. Int. J. Mar. Sci. 2022, 38, 57–69. [Google Scholar] [CrossRef]
- Demirhan, S.A.; Seyhan, K.; Başusta, N. Dietary overlap in spiny dogfish (Squalus acanthias) and thorn back ray (Raja clavata) in the southeastern Black Sea. Ekoloji 2007, 16, 1–8. [Google Scholar]
- Saglam, H.; Bascinar, N.S. Feeding ecology of thorn back ray (Raja clavata Linnaeus, 1758) on the Turkish coast of the southeastern Black Sea. Mar. Res. 2008, 4, 451–457. [Google Scholar]
- Saglam, H.; Ak, O. Reproductive biology of Raja clavata (Elasmobranchii:Rajidae) from the southern Black Sea coast of Turkey. Helgol. Mar. Res. 2012, 66, 117–126. [Google Scholar] [CrossRef][Green Version]
- Froese, R. Cube law, condition factor, and weight–length relationships: History, metaanalysis and recommendations. J. Appl. Ichthyol. 2006, 22, 241–253. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tserkova, F.M.; Mihneva, V.V.; Petrova-Pavlova, E.P. Biological Parameters and Biomass and Abundance Indices of Two Demersal Species, Turbot (Scophthalmus maximus) and Thornback Ray (Raja clavata), Estimated by a Trawl Survey in Western Black Sea. Fishes 2023, 8, 400. https://doi.org/10.3390/fishes8080400
Tserkova FM, Mihneva VV, Petrova-Pavlova EP. Biological Parameters and Biomass and Abundance Indices of Two Demersal Species, Turbot (Scophthalmus maximus) and Thornback Ray (Raja clavata), Estimated by a Trawl Survey in Western Black Sea. Fishes. 2023; 8(8):400. https://doi.org/10.3390/fishes8080400
Chicago/Turabian StyleTserkova, Feriha M., Vesselina V. Mihneva, and Elitsa P. Petrova-Pavlova. 2023. "Biological Parameters and Biomass and Abundance Indices of Two Demersal Species, Turbot (Scophthalmus maximus) and Thornback Ray (Raja clavata), Estimated by a Trawl Survey in Western Black Sea" Fishes 8, no. 8: 400. https://doi.org/10.3390/fishes8080400
APA StyleTserkova, F. M., Mihneva, V. V., & Petrova-Pavlova, E. P. (2023). Biological Parameters and Biomass and Abundance Indices of Two Demersal Species, Turbot (Scophthalmus maximus) and Thornback Ray (Raja clavata), Estimated by a Trawl Survey in Western Black Sea. Fishes, 8(8), 400. https://doi.org/10.3390/fishes8080400