Abstract
Macrourus caml is a main by-catch in the Southern Ocean fishery and a main prey species of Antarctic toothfish Dissostichus mawsoni; it plays an important role in the Southern Ocean ecosystem. In this study, age estimation and stomach content analysis were conducted by using samples collected from the Cosmonauts Sea in 2021. The main objectives of this study were to estimate the age and diet of grenadier M. caml and explore the feeding habits of M. caml. Morphological analysis and molecular identification were conducted to determine the diet of M. caml in this study. Stomach content analysis showed that M. caml mainly fed on Malacostraca, Sagittoidea, Cnidaria and Algae, with the Malacostraca accounting for over 50%. The feeding habits of male and female M. caml were similar. The age of M. caml ranged from 9 to 19 years. Additionally, with the increase of body size, the proportion of Cnidaria was decreasing whereas the proportion of Malacostraca was increasing. The results would provide a reference for exploring the trophic level of M. caml and the food web in the Cosmonauts Sea.
1. Introduction
The Caml Grenadier, Macrourus caml, is a benthopelagic fish belonging to the family Macrouridae; it is one of the five clades among the southern hemisphere Macrourus [1,2], and it is widely distributed in the Antarctic [3]. Macrourus caml was mistaken for M. whitsoni for many years until it had been identified as a new species in 2012 [2]. Compared with M. whitsoni, M. caml has a slightly smaller and more subterminal mouth, which suggests a more benthic diet [2]. Macrourus caml mainly inhabits water from about 350 to 1660 m depth, and it is mostly captured by bottom trawls or bottom longlines [4]. Macrourus caml is a main by-catch in the toothfish fishery in the Ross Sea and is also a major prey species of Antarctic toothfish [5,6,7]. Marriott et al. found that Macrourids, including M. caml, comprised a substantial by-catch of the fishery, accounting for about 10% of the total landing from 1998 to 2004 in the Ross Sea [8]. Despite the abundance of M. caml, it is not exploited by commercial fisheries within the majority of its range [9]. In spite of being captured as by-catch, this species is clearly of commercial importance and is in need of monitoring, management and exploitation. However, little is known about its biology and ecology.
The estimation of age and growth is a critical parameter of fisheries population biology [10] and is essential for stock assessment and species management [11,12]. From 2000 to 2006, the age and growth research of grenadier in the Ross Sea was carried out by Marriott et al. [8,13]. However, in the Cosmonauts Sea, there is a lack of research. The main methods for fish age identification include calcified tissue identification, length frequency and radioactive element identification [14]. Peterson successfully used length frequency to estimate the age of fish in 1891, and this method is used by researchers [15]. However, it has many defects, such as being susceptible to different generations, water environment, fishing gears, etc., so it is mostly an auxiliary method for age identification [14]. In recent years, radioactive element identification method is employed by some researchers; for instance, Barnett et al. defined the life history of the northern Gulf of Mexico Warsaw grouper Hyporthodus nigritus through otolith radiocarbon analysis, and Sanchez et al. conducted bomb radiocarbon age validations of Warsaw grouper and snowy grouper Hyporthodus niveatus from the Gulf of Mexico [16,17]. Although it has been successfully used, it is difficult to operate, and the accuracy remains to be verified [14]. Calcified tissue identification is the most popular method, and calcified tissues include scales, otoliths and other calcified tissues (fin spines, vertebrae, etc.). The scale age of Patagonian toothfish Dissostichus eleginoides from South Georgia, determined by Cassia (1998), and Falkland Islands’ mullet Eleginops Maclovinus from Chile, determined by Brickle (2005), are consistent with their real age [18,19]. Due to the extreme climate in polar regions, most Antarctic fishes grow slowly and have a long age span, resulting in a large error in scale identification [20]. Other calcified tissues are rarely used in the age identification of Antarctic fishes due to their difficulty in obtaining and low accuracy. At present, otolith is mostly used to identify the age of Antarctic fishes because otolith growth is stable, not susceptible to external interference and relatively easy to obtain. Marriott et al. carried out an age estimation and maturity of M. whitsoni from the Ross Sea through otolith [8].
Fish feeding provides necessary nutrition and energy for their growth and reproduction [21,22]. The study of feeding ecology is one of the important methods to judge the growth status of fish and understand the migration, distribution, interspecies relationship and resource variation of fish, which is one of the basic contents of fishery biology research. Most of the diet studies on fish have been completed using direct observation of prey items, which is primarily dependent on the morphological characteristics of each prey species [23,24,25,26]. In spite of the successful application in diet studies, morphological analysis has potential limitations when used alone [27]. Morphological analysis is time-consuming and difficult. It can only determine the short-term food composition, so it is difficult to determine long-term feeding characteristics [28,29,30]. Molecular identification of prey items is now being used to complement morphological analysis [31,32,33,34]. Molecular identification can identify the food composition of fish more quickly and accurately and is not affected by the age, sex, growth stage and digestion degree of experimental subjects, which can make up for the shortcomings of traditional morphological identification [30].
So far, the information about M. caml has been limited in the Cosmonauts Sea. To define the biological and ecological characteristics of M. caml in the Cosmonauts Sea, it is essential to conduct further research about age and feeding habits on it in the Cosmonauts Sea. The specific objectives of this study were: (i) estimate age; (ii) describe the developmental stages of the gonads; and (iii) describe feeding habits. Macrourus caml is an important prey and predator in the Cosmonauts Sea. By exploring its food composition, one describes its resource use and exhibits its trophic level.
2. Materials and Methods
2.1. Sampling
Samples were collected from the Cosmonauts Sea (67°00.37′ S–67°01.30′ S, 44°15.83′ E–44°29.12′ E) by bottom longline in January 2021 during the 37th Chinese National Antarctic Research Expedition with the icebreaker R/V Xuelong 2. A total of 11 fish specimens were collected in this sampling, which were identified as M. caml by morphological analysis. The bottom longline was deployed from January 22 to January 24, with the water depth of this sampling location being about 1800 m. All individuals were measured for their weight (W, g), standard length (SL, mm), total length (TL, mm) and anal length (AL, mm); simultaneously, all the sagittal otoliths were collected from the body of fish. Gonads of males and females were dissected and observed in order to macroscopically estimate the minimum size at first maturity.
2.2. Age Determination
The sagitta of M. caml was thick and irregular, which could not be clearly observed by direct observation. Therefore, it is essential to make a sagitta section for age identification. The sagittas were cleaned and baked at 285 °C for 8 min, then embedded in epoxy resin and cured at 50 °C for 24 h [13]. The resin blocks were sectioned transversely through the otolith primordia using a diamond-edged wafering blade and polished on the cut surfaces [13]. Grind the otolith section with waterproof abrasive paper until clear bands can be observed under a microscope. The sections generally exhibited a regular pattern of translucent and opaque zones. Age determination was generated on the assumption that one opaque and one hyaline zone represent one year’s growth in the otolith. Under transmitted light, the zone of opacity was counted from core to edge along the ventral growth axis to determine the age of M. caml (Figure 1 and Figure 2).

Figure 1.
Sagittal otolith section of a M. caml aged 19 years old.

Figure 2.
Sample image of M. caml.
Each otolith section was individually estimated twice under conditions of unknown individual size. If the two readings were consistent, this reading was taken as the individual’s final age. When not consistent, a third reading was taken to determine the individual’s age. All readings were made with no prior knowledge of the fish length, sex, weight, or any previous readings.
2.3. Sexual Maturity
Gonads were collected and sexual maturity stages were determined with a 6-staged maturity scale [35], as follows:
Stage I (immature): Gonads are very small and cannot be distinguished by the naked eyes.
Stage II (immature): Gonads are small. Ovary is dully transparent and pinkish-whitish, and spermary is grayish white or grayish brown.
Stage III (maturing): Gonads are enlarged. Ovary is filled with opaque, slightly white or yellowish ovum. Spermary is grayish brown or light red and sperm cannot be squeezed out.
Stage IV (maturing): Gonads are enlarged. Ovary is orange with a large transparent ova and small white ova. Spermary is white and little sperm can be squeezed out.
Stage V (mature): Gonads are considerably enlarged. Ripe ova are visible and large and transparent. Spermary is milky white and filled with sperm.
Stage VI (spent): Gonads are shortened, walls loose, flabby, empty and dark red with traces of ova or sperm.
2.4. Stomach and Intestine Content Analysis
2.4.1. Morphological Identification of Stomach and Intestine Contents
Stomach and intestine samples of M. caml cryopreserved at −80 °C were taken out and thawed at 4 °C, then dissected under aseptic conditions. Stomach and intestine contents were rinsed with water on a 500 μm steel sieve, and recognizable prey items were identified under a stereoscopic microscope to the lowest taxon possible. As the prey was digested completely, only qualitative analysis (type identification) was carried out, and quantitative analysis such as counting and weighing was not carried out.
2.4.2. Molecular Identification of Stomach and Intestine Contents
Frozen stomach and intestine contents were taken from different locations of the stomach and intestine under aseptic conditions after thawing the fish at 4 °C. Samples were stirred and put into 2 mL centrifuge tubes and then put into prepared ice boxes to ensure that the samples were at low temperature and were not easy to split and deteriorate. After the tissue was broken by the tissue crusher (70 Hz, 600 s), DNA was extracted with MasterPureTM Complete DNA and RNA Purification Kit (Cat. No.: MC85200; Lucigen, USA). PCR targeting at the 18S rDNA V8-V9 region was then performed with Q5 Hot Start High-Fidelity 2X Master Mix kit (Cat. No.: M0494S; NEB, Ipswich, MA, USA) (Table 1 and Table 2). Amplicon sequencing was performed on an Illumina Novaseq6000 sequencer, using 2 × 250 bp paired-end sequencing in accordance with the manufacturer’s instructions, at Novogene (Beijing, China).

Table 1.
PCR primers used in gene cloning and expression.

Table 2.
The reaction system of PCR amplification.
We investigated the prey at the genus level. In order to identify the prey items of M. caml, after adapter/index sequences were trimmed from the obtained raw reads using fastp v0.23.1, reads with low quality and short-read length were discarded using QIIME2 software ver. 2021.2, and then the DADA2 in QIIME2 was used to get the feature tables and sequences. The obtained paired-end contigs were clustered at 99.6% identity and assigned operational taxonomic units (OTUs) using QIIME2 software. Species annotation was based on the Silva database (release 138). The species and genus were assigned for OTUs with more than 98% and 90–98% sequence identity, respectively. The OTUs classified as M. caml were removed in further analysis.
3. Results
3.1. Size and Age
Within the samples, six individuals were male and five individuals were female. The weight of M. caml ranged from 220 g to 900 g. The average weight was 520.91 g. The standard length of M. caml ranged from 348 mm to 585 mm, and the mean standard length was 463.27 mm (Table 3). The total length ranged from 350 mm to 590 mm, and the anal length ranged from 125 mm to 190 mm (Table 3). The age value for M. caml ranged from 9 to 19 years (Table 3).

Table 3.
Biological measurements of M. caml. M and F, respectively, refer to male and female individuals. P1 to P11 refers to sample 1 to sample 11.
3.2. Feeding Habits
3.2.1. Morphological Identification of Stomach and Intestine Contents
According to the occurrence of the prey items by morphological analysis, the main prey of M. caml was Malacostraca and Polychaeta. Algae, Cephalopods, fish and some benthic organisms were also found in stomachs and intestines (Table 4). As the prey was digested completely in this study, it was difficult to identify to the species level and obtain the weight and proportion of each prey item. The index of relative importance (IRI) in the traditional morphological analysis of stomach contents can’t be calculated.

Table 4.
Prey items of M. caml are determined by morphological analyses; ‘+’ represents occurrence of the prey types. P1 to P11 refers to sample 1 to sample 11.
3.2.2. Molecular Analysis of Stomach and Intestine Contents
The Macrourus caml diet in the Cosmonauts Sea was diverse, with stomach and intestine content analysis through meta-barcoding revealing 7 phyla, 8 classes, 12 orders, 7 families, 7 genera, and 2 species. We assumed that all prey DNA recovered from the stomach and intestine was prey of M. caml. By percentage of contigs, Malacostraca made up 57.8%, and Sagittoidea made up 27.2%, whereas Cnidaria (9.1%) and Algae (5.7%) were less in the diet. Malacostraca accounted for the highest proportion of the diet (57.8%), which was dominated by Eucarida (57.3%) (Table 5).

Table 5.
List and proportions of prey items of M. caml as determined by NGS analysis. P1 to P11 refers to sample 1 to sample 11.
Macrourus caml DNA was present in every stomach, and the contigs of itself were overwhelmingly abundant, so M. caml was not included in the prey profiling. The sequences of some species that couldn’t possibly exist in the Cosmonauts Sea were also ruled out.
3.3. Feeding Habits Change with Gender
The average of the contigs quantity of male and female individuals was calculated. The diets of both were similar (p = 0.448 > 0.05, one-way ANOVA), mainly feeding on Malacostraca, Algae and Sagittoidea (Figure 3).
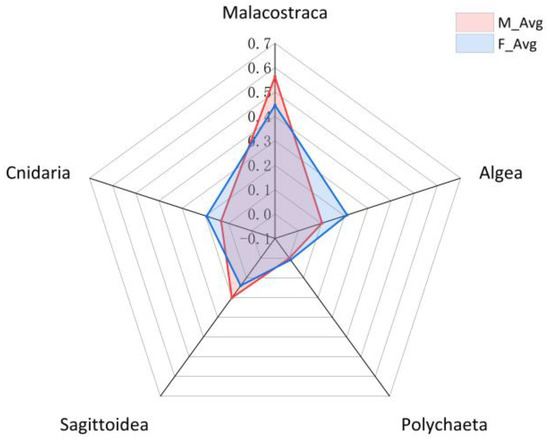
Figure 3.
Distribution of prey species of male and female samples. M_Avg and F_Avg represent the mean proportion of contigs quantity of prey of male and female M. caml.
3.4. Prey Composition Changes with a Total Length
With increasing size, the food composition of M. caml varied. According to the percentage of each prey, M. caml mainly fed on Cnidaria, Sagittoidea and Algae when TL is less than 465 mm, and with the increase of TL, the percentage of Cnidaria became less. When TL is larger than 465 mm, the diet was dominated by Malacostraca, and Cnidaria was almost absent in the prey (Figure 4).
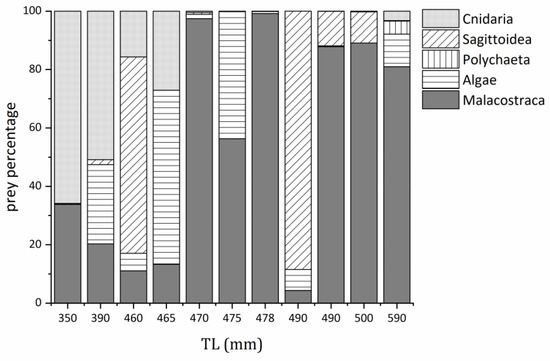
Figure 4.
Diet composition with percentage contigs of M. caml for each TL.
4. Discussion
This study provides new information on the age and feeding habits of M. caml in the Cosmonauts Sea. These data are useful to understand the growth and nutritional status of M. caml.
In this study, otolith was used to estimate the age of M. caml because otolith growth is stable, not susceptible to external interference and relatively easy to obtain. The age estimated in our study was based on the assumption that one opaque and one hyaline zone represented one year’s growth in the otolith. Although the periodicity of increment deposition has not been validated for M. caml, increment deposition has been demonstrated to occur annually in other grenadier species, supporting the assumption for M. caml [36].
Historically, Pinkerton et al. found that grenadiers (including M. caml and M. whitsoni) in the Ross Sea of the Southern Ocean mainly fed on Amphipoda (IRI: 42.8), Euphausiids (IRI: 23.2), Copepoda (IRI: 12.7), Fishes (IRI: 6.6), Mysidacea (IRI: 5.9), Isopoda (IRI: 3.3), Polychaeta (IRI: 1.6), etc. [37]. Pakhomov reported that in the Cosmonauts Sea, half of the diet of M. whitsoni (probably the mix of M. caml and M. whitsoni) consisted of krill, and the remaining half was formed by benthic crustaceans (mysids, gammarids and isopods) and fishes (myctophids and silverfish) in summer [38]. In our study, we found M. caml mainly fed on benthic animals and small plankton in January, and half of the diet was Malacostraca, which is consistent with that which was reported previously. Additionally, we found that the main prey consumed by the two sexes were the same, but there were differences in the proportions of each prey type consumed. Moreover, with the increase of body size, the food composition of M. caml varied. Apart from this, as the total length increased, the proportion of Cnidaria decreased, and the proportion of Malacostraca increased. Cnidaria was gradually replaced by Malacostraca, and Malacostraca gradually dominated the diet. This may indicate M. caml changed their feeding habits to adapt to the change in environment and its biological characteristics. The feeding conversion phenomenon is also found in the small yellow croaker Larimichthys polyactis [39]. Guo et al. found that as body length increased, the proportion of fish and shrimp in the diet gradually increased, whereas the proportion of crustaceans gradually decreased [39]. In Figure 4, the food composition of 490 mm was dominated by Sagittoidea, which seems to conflict with the conclusion that Malacostraca gradually dominated the diet. A limited number of samples may result in the accidental phenomenon. Additionally, each individual grows up in a different environment, so the occurrence of this phenomenon is reasonable. It should be noted that the food web structure may shift among seasons, so the conclusion in this study still needs more samples from different seasons to verify.
Here, two methods were used to identify stomach contents. Morphological analysis is popular because it is simple and accurate, but it also has potential limitations such as being time-consuming and difficult in morphological classification [25,26,28,29,30]. As the prey was digested completely, by only relying on the morphological classification we can hardly attain relatively accurate results. So, molecular identification is also taken to carry on the analysis. Meta-barcoding is not affected by the age, sex, growth stage and digestion extent of experimental subjects, which can make up for the shortcomings of traditional morphological identification [30].
Despite the efficiencies and veracity realized using metabarcoding for prey identification, this approach is not without defects. Through meta-barcoding, we determined that M. caml mainly fed on Malacostraca, Sagittoidea, Cnidaria and Algae. The result of morphological identification of stomach contents confirmed the veracity of molecular identification results, and it also exposed the existing defects of molecular identification. Fish and Cephalopods were common prey items from morphological analysis but were not identified by genetic methods. Additionally, similar to morphological analysis, most of the prey was not identified to the species level. The low quality of the extracted DNA and the universal primer may lead to this problem. It is unlikely that our primers could amplify all prey species. Thus, the diet presented here is not expected to be exhaustive of all taxa consumed by the M. caml. Furthermore, invertebrates proved to be difficult to identify given the potential number and diversity of available prey species inhabiting the Cosmonauts Sea and the current status of the reference databases [40]. To reduce problems like this and further enhance the resolution of sequences obtained from this method, species-specific primers could be generated to search for the presence of specific prey items that may be of concern [40,41].
Additionally, there is no current method to distinguish between prey-of-prey (i.e., items that were consumed by a prey species that the M. caml subsequently ate) and true prey, especially the algae identified through metabarcoding. However, as algae were also found in M. caml diet by morphological analysis, this circumstance likely did not affect our results.
Analysing the diet of a species is the basis of the research on the trophic level and even the food web. Even though the number of samples was limited in this study, the diet identified was useful to determine the nutrition status of M. caml and the construction of the food web in the Cosmonauts Sea.
Author Contributions
Conceptualization, P.S.; Formal analysis, S.X. and X.X.; Methodology, C.Z.; Project administration, Y.T.; Resources, J.L., S.M. and W.Z.; Writing—original draft, S.X.; Writing—review & editing, P.S., C.Z., J.L., S.M., W.Z. and Y.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the research program “Impact and Response of Antarctic Seas to Climate Change (Grant No. IRASCC 2020-2022)” from Chinese Arctic and Antarctic Administration (CAA), Ministry of Natural Resources of the People’s Republic of China.
Institutional Review Board Statement
The samples involved in this research were wild fish landed from a depth of over 1000 m water, and they were already dead when they landed. The process of torturing animals to painful death didn’t exist.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Acknowledgments
We acknowledge crew members on R/V Xuelong 2 and expedition scientists for their help with sample collection during the 37th Chinese National Antarctic Research Expedition. This work was supported by the research program “Impact and Response of Antarctic Seas to Climate Change (Grant No. IRASCC 2020-2022)” from Chinese Arctic and Antarctic Administration (CAA), Ministry of Natural Resources of the People’s Republic of China. Additionally, we would like to thank Ying Xue and Yehui Song for their help in the morphological analysis of stomach contents. And thank Hongan Long, Na Song and Jiao Pan for their technical support in molecular identification.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Smith, P.J.; Steinke, D.; McMillan, P.J.; Stewart, A.L.; McVeagh, S.M.; De Astarloa, J.M.D.; Welsford, D.; Ward, R.D. DNA barcoding highlights a cryptic species of grenadier Macrourus in the Southern Ocean. J. Fish Biol. 2010, 78, 355–365. [Google Scholar] [CrossRef] [PubMed]
- McMillan, P.; Iwamoto, T.; Stewart, A.; Smith, P.J. A new species of grenadier, genus Macrourus (Teleostei, Gadiformes, Macrouridae) from the southern hemisphere and a revision of the genus. Zootaxa 2012, 3165, 19. [Google Scholar] [CrossRef]
- McMillan, P.J.; Marriott, P.; Hanchet, S.M.; Fenaughty, J.M.; Mackay, E.; Sui, H.; Wei, F. Fishes of the Ross Sea region. A Field Guide to Common Species Caught in the Longline Fishery; New Zealand Aquatic Environment and Biodiversity Report, No. 134; Ministry for Primary Industries (MPI): Wellington, New Zealand, 2014; 54p.
- Daniel, M.C.; Tadashi, I.; Tomio, I.; Nadia, S. FAO Species Catalogue Vol.10 Gadiform Fishes of the World; FAO: Rome, Italy, 1990; Volume 10, pp. 238–240. [Google Scholar]
- Fenaughty, J.M.; Stevens, D.W.; Hanchet, S.M. Diet of the Antarctic toothfish (Dissostichus mawsoni) from the Ross Sea, Antarctica (CCAMLR Statistical Subarea 88.1). CCAMLR Sci. 2003, 10, 113–123. [Google Scholar]
- Stevens, D.W.; Dunn, M.R.; Pinkerton, M.H.; Forman, J.S. Diet of Antarctic Toothfish (Dissostichus mawsoni) from the Western Ross Sea, Antarctica; Document WG-FSA-12/52; CCAMLR: Hobart, Australia, 2012. [Google Scholar]
- Pinkerton, M.; McMillan, P.; Forman, J.; Marriott, P.; Horn, P.; Bury, S.; Brown, J. Distribution, morphology and ecology of Macrourus whitsoni and M. caml (Gadiformes, Macrouridae) in the Ross Sea region. CCAMLR Sci. 2013, 20, 37–61. [Google Scholar]
- Marriott, P.M.; Manning, M.J.; Horn, P.L. Age estimation and maturity of the ridge-scaled macrourid (Macrourus whitsoni) from the Ross Sea. CCAMLR Sci. 2006, 13, 291–303. [Google Scholar]
- Moore, B.R.; Parker, S.J.; Pinkerton, M.H. Otolith shape as a tool for species identification of the grenadiers Macrourus caml and M. whitsoni. Fish. Res. 2022, 253, 106370. [Google Scholar] [CrossRef]
- Van Beveren, E.; Bonhommeau, S.; Fromentin, J.-M.; Bigot, J.-L.; Bourdeix, J.-H.; Brosset, P.; Roos, D.; Saraux, C. Rapid changes in growth, condition, size and age of small pelagic fish in the Mediterranean. Mar. Biol. 2014, 161, 1809–1822. [Google Scholar] [CrossRef]
- Ju, P.; Cheung, W.W.L.; Lu, Z.; Yang, S.; Guo, Z.; Chen, M.; Xiao, J. Age, growth, and abundance fluctuation of Jordan’s damsel, Teixeirichthys jordani (Actinopterygii: Perciformes: Pomacentridae), in the southern Taiwan Strait. Acta Ichthyol. Et Piscat. 2019, 49, 243–250. [Google Scholar] [CrossRef]
- Liao, C.S.; Li, W.; Yuan, J.; Ye, S.W.; Zhang, T.L.; Liu, J.S. Age, growth, and motality characteristics of Pelteobagrus Nitidus (sauvage et dabry) in the three gorges reservoir. Acta Hydrobiol. Sin. 2022, 46, 29–36. (In Chinese) [Google Scholar] [CrossRef]
- Marriott, P.; Horn, P.L.; McMillan, P. Species identification and age estimation for the ridge-scaled Macrourid (Macrourus whitsoni) from the Ross Sea. CCAMLR Sci. 2003, 10, 37–51. [Google Scholar]
- Xie, X.; Bao, Z.Y.; Wang, Q.Z. Advances on research and application of age determination by hard tissues in fish: A review. J. Dalian Ocean Univ. 2021, 36, 1071–1080. (In Chinese) [Google Scholar]
- Jalbani, S.; Nareio, N.T.; Jalbani, Y.M.; Lashari, I.A. Age determination of minor carp, cirrhinus reba (hamilton) from manchar lake, sindh, pakistan. Stand. Sci. Res. Essays 2014, 2, 577–580. [Google Scholar]
- Barnett, B.K.; Chanton, J.P.; Ahrens, R.; Thornton, L.; Iii, W.F.P. Life history of northern Gulf of Mexico Warsaw grouper Hyporthodus nigritus inferred from otolith radiocarbon analysis. PLoS ONE 2020, 15, e0228254. [Google Scholar] [CrossRef]
- Sanchez, P.J.; Pinsky, J.P.; Rooker, J.R. Bomb Radiocarbon Age Validation of Warsaw Grouper and Snowy Grouper. Fisheries 2019, 44, 524–533. [Google Scholar] [CrossRef]
- Cassia, M.C. Comparison of age readings from scales and otoliths of the Patagonian toothfish (Dissostichus Eleginoides) from South Georgia. CCAMLR Sci. 1998, 5, 191–203. [Google Scholar]
- Brickle, P.; Arkhipkin, A.I.; Shcherbich, Z.N. Age and growth in a temperate euryhaline notothenioid, eleginops maclovinus from the falkland islands. J. Mar. Biol. Assoc. U. K. 2005, 85, 1217–1221. [Google Scholar] [CrossRef]
- Zhu, G.P.; Wei, L. Age and growth of Antarctic fish species: A review. J. Fish. China 2017, 41, 1638–1647. (In Chinese) [Google Scholar]
- Xue, Y.; Jin, X.S.; Zhang, B.; Liang, Z.L. Diet composition and seasonal variation in feeding habits of small yellow croaker Pseudosciaena polyactis Bleeker in the central Yellow Sea. J. Fish. Sci. China 2004, 11, 237–243. (In Chinese) [Google Scholar]
- Wang, J.; Jiang, R.-J.; Hu, C.-L.; Li, Z.; Xiao, Y.; Xu, Y.-J.; He, Z.-T.; Xu, H.-X. Feeding ecology of Engraulis japonicus based on stomach contents and stable isotope. Yingyong Shengtai Xuebao 2021, 32, 2035–2044. (In Chinese) [Google Scholar] [CrossRef]
- Hynes, H.B.N. The Food of Fresh-Water Sticklebacks (Gasterosteus aculeatus and Pygosteus pungitius), with a review of methods used in studies of the food of fishes. J. Anim. Ecol. 1950, 19, 36–58. [Google Scholar] [CrossRef]
- Hyslop, E.J. Stomach contents analysis—A review of methods and their application. J. Fish Biol. 1980, 17, 411–429. [Google Scholar] [CrossRef]
- Scacco, U.; Tiralongo, F.; Mancini, E. Feeding in Deep Waters: Temporal and Size-Related Plasticity in the Diet of the Slope Predator Fish Coelorinchus caelorhincus (Risso, 1810) in the Central Tyrrhenian Sea. J. Mar. Sci. Eng. 2022, 10, 1235. [Google Scholar] [CrossRef]
- Scacco, U.; Mancini, E.; Marcucci, F.; Tiralongo, F. Microplastics in the Deep: Comparing Dietary and Plastic Ingestion Data between Two Mediterranean Bathyal Opportunistic Feeder Species, Galeus melastomus, Rafinesque, 1810 and Coelorinchus caelorhincus (Risso, 1810), through Stomach Content Analysis. J. Mar. Sci. Eng. 2022, 10, 624. [Google Scholar] [CrossRef]
- Saraswati, E.; Perdhana, G.O. Analysis of stomach content of Nemipterus japonicus from the Blimbingsari waters, Banyuwangi, East Java. IOP Conf. Ser. Earth Environ. Sci. 2020, 404, 012019. [Google Scholar] [CrossRef]
- Hashimoto, R. Investigation of feeding habits and variation of inhabiting depths with cod (Gadus macrocephalus) distributed on the northeastern fishing ground in Japan. Bull. Tohoku Reg. Fish. Res. Lab. 1974, 33, 51–67. [Google Scholar]
- Mata-Sotres, J.A.; Moyano, F.J.; Martínez-Rodríguez, G.; Yúfera, M. Daily rhythms of digestive enzyme activity and gene expression in gilthead seabream (Sparus aurata) during ontogeny. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 197, 43–51. [Google Scholar] [CrossRef]
- Xi, X.Q.; Bao, B.L.; Zhang, S.Y. Application of DNA barcoding in species analysis of fish stomach content. J. Shanghai Ocean Univ. 2015, 24, 203–210. (In Chinese) [Google Scholar]
- Carreon-Martinez, L.; Johnson, T.B.; Ludsin, S.A.; Heath, D.D. Utilization of stomach content DNA to determine diet diversity in piscivorous fishes. J. Fish Biol. 2011, 78, 1170–1182. [Google Scholar] [CrossRef]
- Paquin, M.M.; Buckley, T.W.; Hibpshman, R.E.; Canino, M.F. DNA-based identification methods of prey fish from stomach contents of 12 species of eastern North Pacific groundfish. Deep. Sea Res. Part I: Oceanogr. Res. Pap. 2013, 85, 110–117. [Google Scholar] [CrossRef]
- Symondson, W.O.C. Molecular identification of prey in predator diets. Mol. Ecol. 2002, 11, 627–641. [Google Scholar] [CrossRef]
- Leray, M.; Meyer, C.; Mills, S. Metabarcoding dietary analysis of coral dwelling predatory fish demonstrates the minor contribution of coral mutualists to their highly partitioned, generalist diet. Peer J. 2015, 3, e1047. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.P.; Ye, Z.J.; Xue, Y.; Zhang, C.; Xu, B.D.; Zhang, C.L.; Wang, J.; Mu, X.X. Fishery Resource Biology; China Agriculture Press: Beijing, China, 2020. [Google Scholar]
- Gordon, J.D.M.; Swan, S.C. Validation of age readings from otoliths of juvenile roundnose grenadier, Coryphaenoides rupestris, a deep-water macrourid fish. J. Fish Biol. 1996, 49, 289–297. [Google Scholar] [CrossRef]
- Pinkerton, M.H.; Forman, J.; Stevens, D.W.; Bury, S.J.; Brown, J. Diet and trophic niche of Macrourus spp. (Gadiformes, Macrouridae) in the Ross Sea region of the Southern Ocean. J. Ichthyol. 2012, 52, 787–799. [Google Scholar] [CrossRef]
- Pakhomov, E. Feeding and exploitation of the food supply by demersal fishes in the Antarctic part of the Indian Ocean. J. Ichthyol. 1997, 37, 360–380. [Google Scholar]
- Guo, B.; Zhang, B.; Jin, X.S. Diet composition and ontogenetic variation in feeding habits of juvenile small yellow croaker Pseudosciaena polyactis Bleeker in the Yellow Sea. J. Fish. Sci. China 2010, 17, 289–297. [Google Scholar]
- Harms-Tuohy, C.; Schizas, N.; Appeldoorn, R. Use of DNA metabarcoding for stomach content analysis in the invasive lionfish Pterois volitans in Puerto Rico. Mar. Ecol. Prog. Ser. 2016, 558, 181–191. [Google Scholar] [CrossRef]
- Pompanon, F.; Deagle, B.E.; Symondson, W.O.C.; Brown, D.S.; Jarman, S.N.; Taberlet, P. Who is eating what: Diet assessment using next generation sequencing. Mol. Ecol. 2012, 21, 1931–1950. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).