Polyculture of Pacific White Shrimp Litopenaeus vannamei (Boone) and Red Seaweed Gracilaria birdiae (Greville) under Different Densities
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boyd, C.E.; D’abramo, L.R.; Glencross, B.D.; Huyben, D.C.; Juarez, L.M.; Lockwood, G.S.; Mcnevin, A.A.; Tacon, A.G.J.; Teletchea, F.; Tomasso, J.R.; et al. Achieving sustainable aquaculture: Historical and current perspectives and future needs and challenges. J. World Aquac. Soc. 2020, 51, 578–633. [Google Scholar] [CrossRef]
- Flickinger, D.L.; Costa, G.A.; Dantas, D.P.; Proença, D.C.; David, F.S.; Durborow, R.M.; Moraes-Valenti, P.; Valenti, W.C. The budget of carbon in the farming of the Amazon river prawn and tambaqui fish in earthen pond monoculture and integrated multitrophic systems. Aquac. Rep. 2020, 17, 100340. [Google Scholar] [CrossRef]
- Wang, X.; Olsen, L.; Reitan, K.; Olsen, Y. Discharge of nutrient wastes from salmon farms: Environmental effects, and potential for integrated multi-trophic aquaculture. Aquac. Environ. Interact. 2012, 2, 267–283. [Google Scholar] [CrossRef]
- Bessa-Junior, A.P.; Flickinger, D.L.; Henry-Silva, G.G. Sedimentation rates of nutrients and particulate material in pond mariculture of shrimp (Litopenaeus vannamei) carried out with different management strategies. Aquaculture 2021, 534, 736307. [Google Scholar] [CrossRef]
- Moura, R.S.T.; Valenti, W.C.; Henry-Silva, G.G. Sustainability of Nile tilapia net-cage culture in a reservoir in a semi-arid region. Ecol. Indic. 2016, 66, 574–582. [Google Scholar] [CrossRef]
- Valenti, W.C.; Kimpara, J.M.; Preto, B.L.; Moraes-Valenti, P. Indicators of sustainability to assess aquaculture systems. Ecol. Indic. 2018, 88, 402–413. [Google Scholar] [CrossRef]
- Simão, B.R.; Silva, L.O.B.; Maia, A.S.C.; Miranda, L.C.; Azevedo, C.M.S.B. Stocking densities and feeding strategies in shrimp and tilapia polyculture in tanks. Pesqui. Agropecuária Bras. 2013, 48, 1088–1095. [Google Scholar] [CrossRef]
- Henry-Silva, G.G.; Maia, C.P.; Moura, R.S.T.; Bessa Junior, A.; Valenti, W.C. Integrated multi-trophic culture of Nile tilapia (Oreochromis niloticus) and Amazon river prawn (Macrobrachium amazonicum) in brackish water. Arq. Braileiro De Med. Veterinária E Zootec. 2015, 67, 265–273. [Google Scholar] [CrossRef]
- Rodrigues, C.G.; Garcia, B.F.; Verdegem, M.; Santos, M.R.; Amorim, R.V.; Valenti, W.C. Integrated culture of Nile tilapia and Amazon river prawn in stagnant ponds, using nutrient-rich water and substrates. Aquaculture 2019, 503, 111–117. [Google Scholar] [CrossRef]
- Dantas, D.P.; Flickinger, D.L.; Costa, G.A.; Batlouni, S.R.; Moraes-Valenti, P.; Valenti, W.C. Technical feasibility of integrating Amazon river prawn culture during the first phase of tambaqui grow-out in stagnant ponds, using nutrient-rich water. Aquaculture 2020, 516, 734611. [Google Scholar] [CrossRef]
- Yokoyama, H. Growth and food source of the sea cucumber Apostichopus japonicus cultured below fish cages—Potential for integrated multi-trophic aquaculture. Aquaculture 2013, 372–375, 28–38. [Google Scholar] [CrossRef]
- Kerrigan, D.; Suckling, C.C. A meta-analysis of integrated multitrophic aquaculture: Extractive species growth is most successful within close proximity to open-water fish farms. Rev. Aquac. 2018, 10, 560–572. [Google Scholar] [CrossRef]
- Franchini, A.C.; Costa, G.A.; Pereira, S.A.; Valenti, W.C.; Moraes-Valenti, P. Improving production and diet assimilation in fish-prawn integrated aquaculture, using iliophagus species. Aquaculture 2020, 521, 735048. [Google Scholar] [CrossRef]
- Bolton, J.J.; Robertson-Andersson, D.V.; Shuuluka, D.; Kandjengo, L. Growing Ulva (Chlorophyta) in integrated systems as a commercial crop for abalone feed in South Africa: A SWOT analysis. J. Appl. Phycol. 2009, 21, 575–583. [Google Scholar] [CrossRef]
- Cruz-Suárez, L.E.; León, A.; Peña-Rodríguez, A.; Rodríguez-Peña, G.; Moll, B.; Ricque-Marie, D. Shrimp/Ulva co-culture: A sustainable alternative to diminish the need for artificial feed and improve shrimp quality. Aquaculture 2010, 301, 64–68. [Google Scholar] [CrossRef]
- Wu, H.; Kim, J.K.; Huo, Y.; Zhang, J.; He, P. Nutrient removal ability of seaweeds on Pyropia yezoensis aquaculture rafts in China’s radial sandbanks. Aquat. Bot. 2017, 137, 72–79. [Google Scholar] [CrossRef]
- Biswas, G.; Kumar, P.; Ghoshal, T.K.; Kailasam, M.D.; Bera, A.; Mandal, B.; Sukumaran, K.; Vijayan, K.K. Integrated multi-trophic aquaculture (IMTA) outperforms conventional polyculture with respect to environmental remediation, productivity and economic return in brackishwater ponds. Aquaculture 2020, 516, 734626. [Google Scholar] [CrossRef]
- Pereira, S.A.; Kimpara, J.M.; Valenti, W.C. Sustainability of the seaweed Hypnea pseudomusciformis farming in the tropical Southwestern Atlantic. Ecol. Indic. 2021, 121, 107101. [Google Scholar] [CrossRef]
- Cunha, M.E.; Quental-Ferreira, H.; Parejo, A.; Gamito, S.; Ribeiro, L.; Moreira, M.; Monteiro, I.; Soares, F.; Pousão-Ferreira, P. Understanding the individual role of fish, oyster, phytoplankton and macroalgae in the ecology of integrated production in earthen ponds. Aquaculture 2019, 512, 734297. [Google Scholar] [CrossRef]
- Neori, A.; Chopin, T.; Troell, M.; Buschmann, A.H.; Kraemer, G.P.; Halling, C.; Shpigel, M.; Yarish, C. Integrated aquaculture: Rationale, evolution and state of the art emphasizing seaweed biofiltration in modern mariculture. Aquaculture 2004, 231, 361–391. [Google Scholar] [CrossRef]
- Hoang, M.N.; Nguyen, P.N.; Bossier, A.M.V.E.M.; Bossier, P. The effects of two fish species mullet, Mugil cephalus, and tilapia, Oreochromis niloticus, in polyculture with white shrimp, Litopenaeus vannamei, on system performances: A comparative study. Aquac. Res. 2020, 51, 2603–2612. [Google Scholar] [CrossRef]
- Lerat, Y.; Cornish, M.L.; Critchley, A.T. Applications of algal biomass in global food and feed markets: From traditional usage to the potential for functional products. In Blue Biotechnology; Barre, S.L., Bates, S.S., Eds.; Commonwealth Secretariat: London, UK, 2018; pp. 143–189. [Google Scholar] [CrossRef]
- Abreu, M.H.; Pereira, R.; Yarish, C.; Buschmann, A.H.; Sousa-Pinto, I. IMTA with Gracilaria vermiculophylla: Productivity and nutrient removal performance of the seaweed in a land-based pilot scale system. Aquaculture 2011, 312, 77–87. [Google Scholar] [CrossRef]
- Alves, J.P.; Bessa-Junior, A.P.; Henry-Silva, G.G. Salinity tolerance of macroalgae Gracilaria birdiae. Ciência Rural. 2021, 51, 1–7. [Google Scholar] [CrossRef]
- Oliveira, V.P.; Freire, F.A.M.; Soriano, E.M. Influence of depth on the growth of the seaweed Gracilaria birdiae (Rhodophyta) in a shrimp pond. Braz. J. Aquat. Sci. Technol. 2012, 16, 33–39. [Google Scholar] [CrossRef]
- Marinho-Soriano, E.; Morales, C.; Moreira, W.S.C. Cultivation of Gracilaria (Rhodophyta) in shrimp pond effluents in Brazil. Aquac. Res. 2002, 33, 1081–1086. [Google Scholar] [CrossRef]
- Brito, L.O.; Chagas, A.M.; Silva, E.P.; Soares, R.B.; Severi, W.; Gálvez, A.O. Water quality, Vibrio density and growth of Pacific white shrimp Litopenaeus vannamei (Boone) in an integrated biofloc system with red seaweed Gracilaria birdiae (Greville). Aquac. Res. 2016, 47, 940–950. [Google Scholar] [CrossRef]
- Koroleff, K. Determination of phosphorus. In Methods of Seawater Analysis, 2nd ed.; Grasshoff, K., Erhardt, M., Kremling, K., Eds.; Wiley: New York, NY, USA, 1863; pp. 125–139. [Google Scholar]
- AOAC. Official Methods of Analysis, 21th ed.; (Online); Gaithersburg, M.D., Ed.; AOAC International (Association of Analytical Communities): Rockville, MD, USA, 2019; ISBN 9780935584899. [Google Scholar]
- Jones, A.B.; Preston, N.P.; Dennison, W.C. Integrated treatment of shrimp effluent by sedimentation, oyster filtration and macroalgas absorption: A laboratory scale study. Aquaculture 2001, 193, 155–178. [Google Scholar] [CrossRef]
- Rocha, N.M.; Souza Junior, J.; Farias, W.R.L. Reuse of water in an integrated system with shrimps, sedimentation, oysters and marine macroalgae. Rev. Ciência Agronômica 2008, 39, 540. [Google Scholar]
- Raposo, D.M.T.; Oliveira, S.R.; Afonso, F.; Câmara, M.R.; Fernandes, F.O.; Marinho-Soriano, E. Performance of Shrimp Litopenaeus Vannamei and Seaweed Gracilaria Birdiae and Ulva Fasciata in an Integrated Multi-Trophic Aquaculture System; Aquaculture Europe: Trondheim, Norway, 2013. [Google Scholar]
- Fourooghifard, H.; Matinfar, A.; Mortazavi, M.S.; Roohani Ghadikolaee, K.; Mirbakhsh, M. Nitrogen and phosphorous budgets for integrated culture of white leg shrimp Litopenaeus vannamei with red seaweed Gracilaria corticata in zero water exchange system. Iran. J. Fish. Sci. 2018, 17, 471–486. [Google Scholar] [CrossRef]
- Marinho-Soriano, E.; Panucci, R.A.; Carneiro, M.A.A.; Pereira, D.C. Evaluation of Gracilaria caudata J. Agardh for bioremediation of nutrients from shrimp farming wastewater. Bioresour. Technol. 2009, 100, 6192–6198. [Google Scholar] [CrossRef]
- Nelson, S.G.; Glenn, E.P.; Conn, J.; Moore, M.; Walsh, T.; Akutagawa, M. Cultivation of Gracilaria parvispora Rhodophyta in shrimp-farm effluent ditches and floating cages in Hawaii: A two-phase polyculture system. Aquaculture 2001, 193, 239–248. [Google Scholar] [CrossRef]

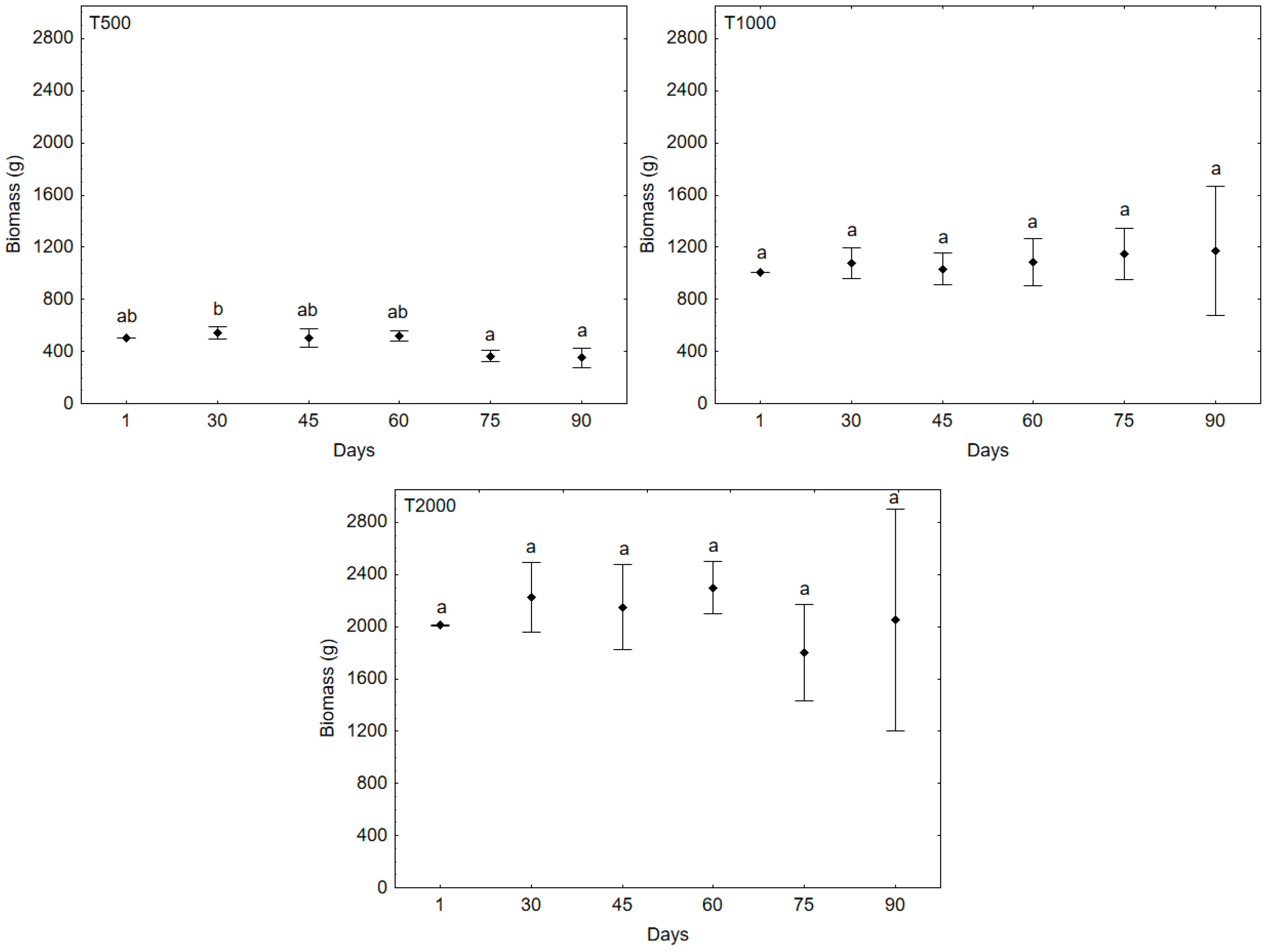
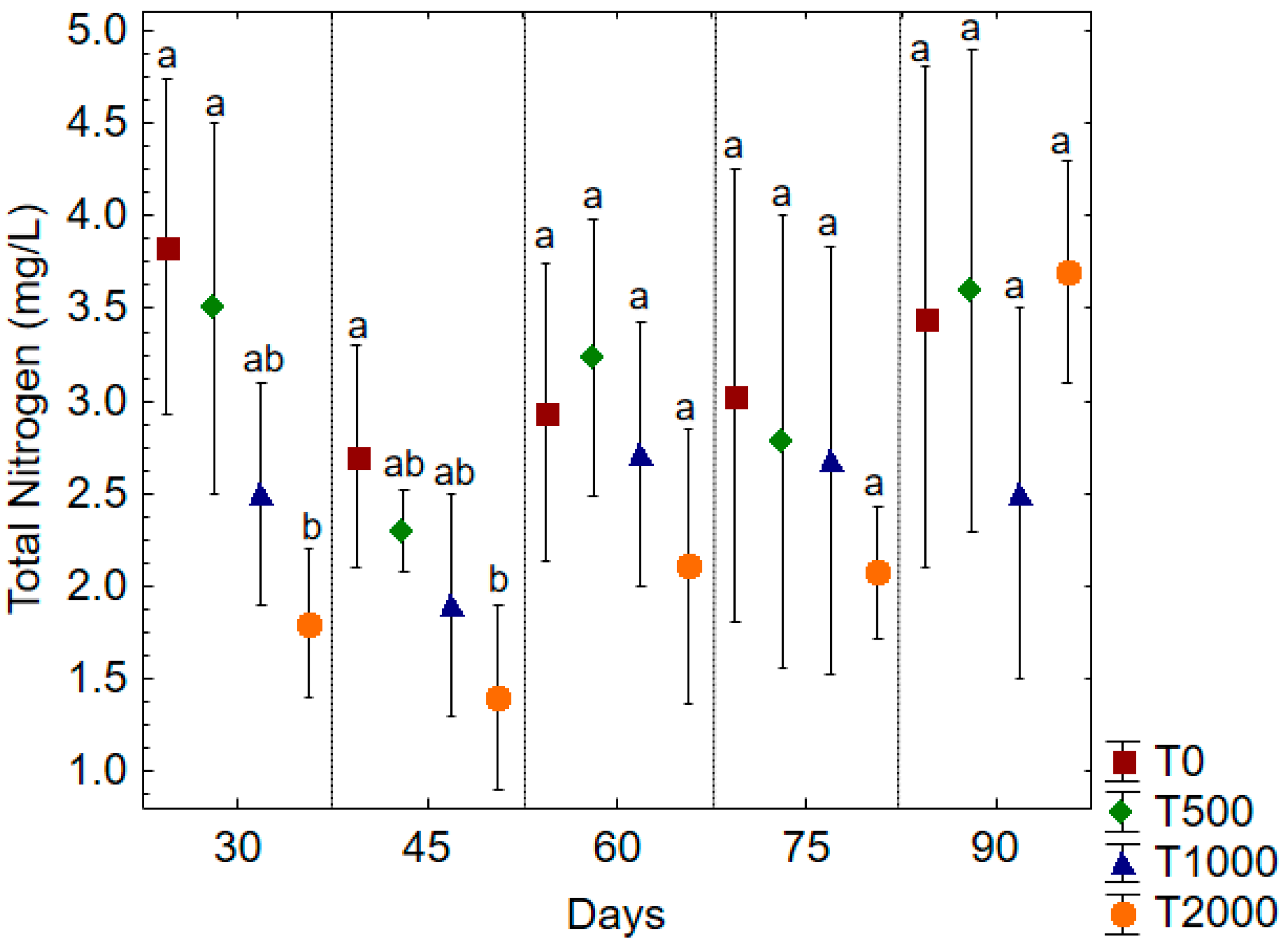

| Treatments | Temperature (°C) | pH | DO (mg/L) | Turbidity (NTU) | Transparency (cm) | Salinity |
|---|---|---|---|---|---|---|
| T0 | 29.7 ± 0.5 | 8.35 ± 0.1 | 4.4 ± 0.2 | 160.1 a ± 69.9 | 25.0 ± 2.2 | 35.0 ± 0.9 |
| T500 | 29.6 ± 0.4 | 8.33 ± 0.1 | 4.4 ± 0.1 | 152.6 a ± 63.8 | 25.5 ± 2.4 | 35.6 ± 0.8 |
| T1000 | 29.6 ± 0.6 | 8.27 ± 0.2 | 4.5 ± 0.3 | 116.8 b ± 45.9 | 28.5 ± 3.1 | 35.2 ± 1.3 |
| T2000 | 29.4 ± 0.3 | 8.28 ± 0.1 | 4.3 ± 0.2 | 108.9 b ± 59.1 | 30.7 ± 3.5 | 35.8 ± 0.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henry-Silva, G.G.; Alves, J.; Flickinger, D.; Gomes-Rebouças, R.; Bessa-Junior, A. Polyculture of Pacific White Shrimp Litopenaeus vannamei (Boone) and Red Seaweed Gracilaria birdiae (Greville) under Different Densities. Fishes 2023, 8, 54. https://doi.org/10.3390/fishes8010054
Henry-Silva GG, Alves J, Flickinger D, Gomes-Rebouças R, Bessa-Junior A. Polyculture of Pacific White Shrimp Litopenaeus vannamei (Boone) and Red Seaweed Gracilaria birdiae (Greville) under Different Densities. Fishes. 2023; 8(1):54. https://doi.org/10.3390/fishes8010054
Chicago/Turabian StyleHenry-Silva, Gustavo Gonzaga, Joseanna Alves, Dallas Flickinger, Renata Gomes-Rebouças, and Ambrosio Bessa-Junior. 2023. "Polyculture of Pacific White Shrimp Litopenaeus vannamei (Boone) and Red Seaweed Gracilaria birdiae (Greville) under Different Densities" Fishes 8, no. 1: 54. https://doi.org/10.3390/fishes8010054
APA StyleHenry-Silva, G. G., Alves, J., Flickinger, D., Gomes-Rebouças, R., & Bessa-Junior, A. (2023). Polyculture of Pacific White Shrimp Litopenaeus vannamei (Boone) and Red Seaweed Gracilaria birdiae (Greville) under Different Densities. Fishes, 8(1), 54. https://doi.org/10.3390/fishes8010054







